Optimisation of the allylsilane approach to C-10 deoxo carba analogues of dihydroartemisinin: synthesis and in vitroantimalarial activity of new, metabolically stable C-10 analogues
Abstract
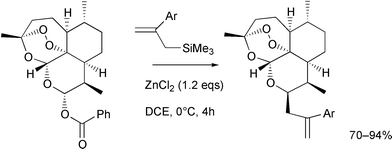
An optimised protocol has been developed for the coupling of dihydroartemisinin benzoate with a range of aromatic allylsilanes to provide a number of new C-10 deoxo derivatives (11a–11g) in yields ranging from 70 to 94%. These compounds were up to ten times more potent than

 Please wait while we load your content...
Please wait while we load your content...