Enantioselective amino acid recognition using acyclic thiourea receptors
Abstract
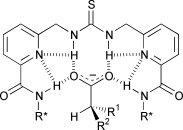
A series of acyclic thiourea derivatives, designed to create a cleft with four hydrogen bond donors suitable for carboxylate recognition, have been prepared, and their ability to bind to N-protected amino acid carboxylate salts has been investigated. The crystal structure of one of the

 Please wait while we load your content...
Please wait while we load your content...