Open Access Article
Open Access ArticleInterpretation of the adsorption process of toxic Cd2+ removal by modified sweet potato residue
Yu Gao a,
Zhuolin Yia,
Jinling Wangb,
Fan Dingc,
Yang Fanga,
Anping Dua,
Yijia Jianga,
Hai Zhao*a and
Yanling Jin*a
a,
Zhuolin Yia,
Jinling Wangb,
Fan Dingc,
Yang Fanga,
Anping Dua,
Yijia Jianga,
Hai Zhao*a and
Yanling Jin*a
aCAS Key Laboratory of Environmental and Applied Microbiology, Environmental Microbiology Key Laboratory of Sichuan Province, Chengdu Institute of Biology, Chinese Academy of Sciences, Chengdu 610041, China. E-mail: zhaohai@cib.ac.cn; jinyl@cib.ac.cn
bCollege of Life Science and Biotechnology, Mianyang Teachers' College, Mianyang 621000, China
cCrop Characteristic Resources Creation and Utilization Key Laboratory of Sichuan Province, Mianyang Academy of Agricultural Sciences, Mianyang 621023, China
First published on 2nd January 2024
Abstract
Cadmium (Cd) is a common and toxic non-essential heavy metal that must be effectively treated to reduce its threat to the environment and public health. Adsorption with an adsorbent, such as agricultural waste, is widely used to remove heavy metals from wastewater. Sweet potato, the sixth most abundant food crop worldwide, produces a large amount of waste during postharvest processing that could be used as an economic adsorbent. In this study, the feasibility of using sweet potato residue (SPR) as an adsorbent for Cd2+ adsorption was assessed. To enhance the removal rate, SPR was modified with NaOH, and the effects of the modification and adsorption conditions on the removal of Cd2+ from wastewater were investigated. The results showed that modified sweet potato residue (MSPR) could be adapted to various pH and temperatures of simulated wastewater, implying its potential for multi-faceted application. Under optimized conditions, the removal of Cd2+ by MSPR was up to 98.94% with a maximum adsorption capacity of 19.81 mg g−1. Further investigation showed that the MSPR exhibited rich functional groups, a loose surface, and a mesoporous structure, resulting in advantageous characteristics for the adsorption of Cd2+. In addition, the MSPR adsorbed Cd2+ by complexation, ion exchange, and precipitation during a monolayer chemisorption adsorption process. This work demonstrates a sustainable and environment friendly strategy for Cd2+ removal from wastewater and a simple approach for the preparation of MSPR and also revealed the adsorption mechanism of Cd2+ by MSPR, thus providing a suitable adsorbent and strategy for the removal of other heavy metals.
1. Introduction
Cadmium (Cd) is a common and toxic non-essential heavy metal, which is mostly found in industrial wastewater because of its numerous applications in manufacturing, construction, and the chemical industry.1 Cd2+ contamination impairs the activity of soil enzymes,2 interferes with water uptake, reduces photosynthesis, inhibits the root growth of plants,3 and can lead to liver lesions, kidney diseases, osteoporosis, and various types of cancer.4 Removing Cd from wastewater is therefore critical to meet effluent standards before it can be discharged.5Various physical, chemical, and/or biological approaches have been developed to remove heavy metals from wastewater, such as photocatalysis, electrochemical treatment, ion exchange, coagulation/flocculation, nanofiltration, and adsorption.6 Among the reported approaches, adsorption, including physical and chemical adsorption processes, has been considered as one of the easiest and most cost-effective techniques because of its strong adaptability in removing various heavy metals from aqueous solutions with simple operation.7 Therefore, adsorption processes have been widely applied, especially in developing countries.8 Many adsorbents have been used for Cd2+ adsorption, such as activated carbon, fly ash, bio-adsorbents, algae, and nanoparticles.9–13 However, the adsorption capacity of these adsorbents is usually low, while the complex modification of adsorption materials increases the cost of the adsorbents. Therefore, the search for low-cost adsorbents with practical utility has recently attracted much attention.
Agricultural wastes have been used as a kind of low-cost biomaterial and are promising bio-adsorbents for Cd2+ removal because they contain many functional groups, such as carboxyl, hydroxyl, and sulfhydryl groups, which can be effectively combined with Cd2+.11 Before being used as bio-adsorbents for Cd2+ removal, agricultural wastes are often modified to improve their selectivity and adsorption performance. Various modification technologies for biomaterials have been explored, such as high-temperature and high-pressure treatment, material mixing, and alkali treatment,14,15 among which, direct alkali modification is considered simpler, safer, and more energy efficient. Hsua et al. reported that treating Ti–7.5Mo with NaOH resulted in better bioactive properties and exhibited a nanoscale porous network structure.16
Sweet potato (SP) is the sixth most abundant food crop worldwide and is rich in nutrients, including starch, crude fiber, flavonoids, and carotene.17 The average annual yield of SP in the world from 2016 to 2020 was 91.12 million tons, and the annual production in China was approximately 51.54 million tons, accounting for 56.56% of the world's SP yield.18 SP has a high starch content and is mostly used for starch production. However, 4.5–5.0 tons of sweet potato residue (SPR) are generated while producing 1 ton of starch,19 which is not only a waste of resources but also harmful to the environment. SRP contains starch and other nutrients, which are easily used by microorganisms, thereafter resulting in spoilage and odor. SPR contains a large amount of useful component, that can be further used, such as lignin, cellulose, hemicellulose, protein, and pectin, which makes it suitable for wastewater treatment. For example, protein isolated from SPR was used as a frother to remove trace crystal violet dye by foaming.20 Arachchige et al. showed that SPR modified with high hydrostatic pressure-assisted pectinase could effectively adsorb Pb2+ in wastewater.19
In this study, the adsorption capacity of SPR for Cd2+ was investigated for the first time. The SPR was modified with NaOH under optimized conditions. Then, the factors that affect the adsorption performance were evaluated. Finally, the intrinsic adsorption mechanism was elucidated by structure characterization, adsorption kinetics, and isotherms. This study demonstrates a new strategy for cost-effective Cd2+ removal with SPR, as well as presenting a method for the alkali modification of SPR to improve the Cd2+ adsorption, and revealing the mechanism of Cd2+ removal by the alkali-modified SPR. This provides a new idea for the utilization of SPR and the treatment of wastewater contaminated with heavy metals.
2. Materials and methods
2.1 Material preparation
The fresh SPR was collected from Jinmei Ltd (Sichuan, China), which had been separated with starch from sweet potato Xushu 22, and immediately stored at 4 °C. The cadmium chloride (CdCl2, Tianjin Jinbei Fine Chemical Co., Ltd, China) was dried at 105 °C for 2 h and dissolved to the target concentration to use as simulated cadmium-containing wastewater. Sodium hydroxide (NaOH) was purchased from Chengdu Cologne Chemical Co., Ltd, China.2.2 Experimental design
MSPR (0.3 g) was added to a 50 mL centrifuge tube containing 50 mg L−1 Cd2+ and mixed for 4 h. After adsorption, the simulated wastewater was collected and the concentration of Cd2+ in the water was measured by atomic adsorption spectrometry (iCE™ 3400 AAS, Thermo Scientific™, USA) to determine the optimal modification conditions for the next experiment.
To investigate the effect of the adsorbent amount and Cd2+ initial concentrations on the removal rate, a series of MSPR amounts (0.1, 0.2, 0.3, 0.4, and 0.5 g) were added to 50 mL of simulated wastewater (Cd2+, 50 mg L−1), and the Cd2+ concentrations were set to 10, 30, 50, 70, and 90 mg L−1 and mixed with 0.3 g MSPR for 4 h, respectively.
2.3 Adsorption isotherms and kinetics
An equilibrium adsorption study was performed by placing 0.3 g of MSPR in 50 mL of simulated wastewater with different initial Cd2+ concentrations (10, 30, 50, 70, and 90 mg L−1) for 4 h at 25 °C. The amount of adsorbed Cd2+ (qe, mg g−1) was calculated using eqn (1).
 | (1) |
Different contact times were used to investigate the adsorption kinetics. First, 50 mL of simulated wastewater (50 mg L−1, Cd2+) was contacted with 0.3 g MSPR in 50 mL centrifuge tubes at 25 °C. Samples were taken at 30 min intervals to determine the changes in the Cd2+ concentration during a contact time of 6 h. The variation in Cd2+ adsorption, qt (mg g−1), was calculated using eqn (2).
 | (2) |
The details of the Langmuir and Freundlich equations and the pseudo-first/second-order equations are shown in Table 1.21–25
| Adsorption model | Original form equation | Linearized form equation |
|---|---|---|
| a q is the amount of Cd2+ adsorbed per specific amount of adsorbent (mg g−1).b qm and qe are the saturation adsorption amount and equilibrium adsorption amount, respectively (mg g−1).c kL and kF are the Langmuir and Freundlich equilibrium constants, respectively.d C is the equilibrium concentration of Cd2+ (mg L−1).e 1/n is the adsorption index.f qt is the amount of adsorption at time t (mg g−1).g k1 is the pseudo-first-order constant (min−1).h k2 is the pseudo-second-order constant (g (mg min−1)). | ||
| Langmuir |  |
 |
| Freundlich | q = kcF·C1/ne |  |
| Pseudo-first order | qft = qbe(1 − e−kg1t) |  |
| Pseudo-second order | 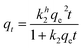 |
 |
2.4 Structural characterization
SPR, MSPR, and adsorbed MSPR (AMSPR) were collected and dried. The adsorption mechanism was further investigated by analyzing the physical and chemical properties of the three materials.To evaluate the Brunauer–Emmet–Teller (BET)-specific surface area and pore-size distribution, the samples were degassed by vacuum to remove any impurity gas adsorbed on the surface. The degassing temperature was set to 120 °C, and the degassing time was 6 h. Then the samples were weighed and placed in liquid nitrogen, and the nitrogen adsorption capacity of the samples was determined at different pressure points set in advance to obtain the adsorption isotherm using a dedicated fully automatic specific surface and porosity analyzer BET system (Nova 4000e, Quantachrome, USA). The BET and Barrett–Joyner–Halenda (BJH) models were used to calculate the specific surface area and pore-size distribution.
Fourier-transform infrared spectroscopy (FTIR, Nicolet 6700, Thermo Scientific, USA) was used to investigate the influence of the functional groups on the adsorption process. First, 1–2 mg sample and 200 mg of KBr were mixed into a mold to form transparent flakes using an oil pressure machine. The prepared flakes were subjected to FTIR analysis and the test conditions were set at 4000–400 cm−1 wavelength, scanning 32 times, and resolution of 4 cm−1 to determine the transmittance.
To measure and analyze the surface morphology and chemical composition, SPR, MSPR, and AMSPR were analyzed as per the following methods. Since SPR is a non-conductive material, the material was sprayed with platinum (Pt) as a pretreatment for the scanning electron microscopy (SEM, Sigma 300, ZEISS, Germany) and energy dispersive X-ray (EDAX, Gemini 300, ZEISS, Germany) analysis. The samples were directly pasted to the conductive adhesive, and sprayed by a sputter coater (SC7620, Quorum, UK) with Pt for 45 s, at a current of 10 mA. Subsequently, SEM and EDAX was used to assess the sample morphology and for the energy spectrum mapping, where the accelerating voltage was 3 kV for the topography photography, 15 kV for energy spectrum mapping, using a secondary electron detector (SE2).
2.5 Data analysis
All the treatments were performed in triplicate. Microsoft Excel 16.53, IBM SPSS 22 and origin 2021 software were used to analyze the data.3. Results and discussion
3.1 Effect of the modification conditions on Cd2+ removal
NaOH can de-polymerize lignin and hydrolyze hemicellulose to expose cellulose. Breaking the covalent relationship between the lignocellulose components by NaOH solution can affect the pore structure of cellulose. In addition, NaOH can also alter the chemical and mechanical properties of SPR, revealing functional groups like –OH.26–28Temperature can affect the hydrolysis of starch and lignocellulose in SPR under NaOH treatment.29 As shown in Fig. 1, the removal rate of MSPR for Cd2+ reached the highest values of 94.78%, 95.82%, and 95.58%, under 20 °C, 30 °C, and 40 °C, respectively, while the removal rate for the unmodified SPR was 82.67%. The highest removal rate of MSPR was obtained at 30 °C, which was 13.51% higher than that of SPR. Similar trends were also found for different NaOH concentrations and different adsorbent sizes. The effect of 30 °C modification was generally better than that of the other two temperatures, and also superior to the unmodified SPR. Therefore, 30 °C was the temperature chosen for the further adsorption experiments.
 | ||
| Fig. 1 The effects of temperature and NaOH concentration on the adsorption rates with (a) adsorbent size 300 μm, (b) adsorbent size 150 μm, and (c) adsorbent size 100 μm. | ||
The NaOH concentration normally has a significant effect on the modification of biomaterials.30 Under the optimal temperature of 30 °C, when using a particle size of 300 μm, 1.5 M NaOH was the best modification condition for Cd2+ removal, and its removal rate was 94.59% (Fig. 1(a)); whereas when particle sizes of 150 and 100 μm were used, the removal rates of SPR treated with 1.5 M and 2.0 M NaOH were quite similar with no significant difference (p = 0.41, >0.05 and p = 0.95, >0.05), showing the higher values of 95.12% and 95.83%, and 95.52% and 95.48% respectively, than the other conditions (Fig. 1(b) and (c)). In short, at different particle sizes and 30 °C, 1.5 M and 2.0 M NaOH, especially 1.5 M NaOH, endowed SPR with better adsorption than the other conditions. Therefore, based on both the removal rate and cost considerations, 1.5 M was finally chosen as the modification concentration.
The rate of ion exchange in solution is generally controlled by the rate of ion diffusion within the adsorbent particle. A decrease in the average particle size of the adsorbent increases the surface area. Overall, the removal rate of MSPR at 300 μm particle size was lower than that of the other two particle sizes. However, the removal rates of the 100 μm and 150 μm sized particles under 1.5 M NaOH at 30 °C were similar with no significant differences (p = 0.144, >0.05). In addition, the smaller the particle size, the lower the material yield, and the higher the energy consumption for shredding. Therefore, 150 μm was chosen as the most suitable particle size.
After comprehensively consideration, SPRs modified with 1.5 M NaOH at 30 °C and a particle size of 150 μm were selected as the optimal adsorbent for the next experiments.
3.2 Characterization of the adsorbents
The nitrogen adsorption–desorption isotherm is usually divided into six categories. It can be seen from Fig. 2(a) that the three materials were similar, with typical capillary condensation followed by hysteresis loops,39 belonging to the IV-type isotherm. The IV-type isotherm is mainly found in mesoporous materials. There was an overlap between N2 adsorption and desorption in Fig. 2(a). One of the reasons for this phenomenon may be that the orifices of SPR, a carbon material, shrink directly after adsorbing gas, resulting in a hard desorption of the gas from SPR, and this shrinkage together with the hard desorption can easily cause the overlap. The nitrogen adsorption–desorption isotherm indicated that SPR, MSPR, and AMSPR were mesoporous materials. Mesoporous materials are promising adsorbents because they have many pores (between 2–50 nm), and the adsorption sites are uniformly distributed on the internal and external surfaces.40 The BJH adsorption results showed that the pore diameters in SPR, MSPR, and AMSPR were 3.475, 3.107, and 3.479 nm, respectively, all of which were in the mesoporous range. Both the pore-size determination and nitrogen adsorption–desorption isotherms showed that the MSPR is a mesoporous material that can be used as an effective adsorbent.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (aromatic lignin rings), –CO (carboxylic acids), and other alcohols, ethers, and phenol.11,41 The abundant functional groups are advantageous for interactions with metal ions, such as covalent, complexation, hydrogen bonding, or other electrostatic interactions in solution.
C (aromatic lignin rings), –CO (carboxylic acids), and other alcohols, ethers, and phenol.11,41 The abundant functional groups are advantageous for interactions with metal ions, such as covalent, complexation, hydrogen bonding, or other electrostatic interactions in solution.Compared to unmodified SPR, MSPR displayed a clear peak in its FTIR spectrum at 2137 cm−1 (Fig. 2(b)), corresponding to triple bond and cumulative double bond regions, such as –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C, –C
C, –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N, –C
N, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and –C
C, and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. These groups may interact with Cd2+ and remove Cd2+ from the water. Meanwhile, all three samples displayed a broad absorption peak at 3427 cm−1, corresponding to the –OH groups. After modification, the peak was stronger than that of the unmodified SPR, indicating that NaOH modification might increase the amount and change the role of the functional groups. The –CH stretching of the methyl and methylene groups always occurred in the adsorbent at about 2932 cm−1, which was characteristic of lignin, cellulose, and hemicellulose. The peak at 1654 cm−1 corresponded to the –COOH functional group, while the –CH2 or O–H functional groups appeared at 1430 cm−1. It was obvious that the –COOH stretching was enhanced significantly after NaOH modification. The peaks at 1000 cm−1 were assigned to the stretching vibrations of C–O in lignin, hemicellulose, and cellulose. Moreover, the peak at 600 cm−1 corresponded to the –CH of the amine group, with the mechanical vibration strength of MSPR significantly stronger than that of SPR. In the 500–800 cm−1 range, the functional groups of the starch or other sugars could have undergone chemical reactions.42 The adsorbent had abundant –OH, –COOH, and –CH groups, which provide more adsorption sites.
O. These groups may interact with Cd2+ and remove Cd2+ from the water. Meanwhile, all three samples displayed a broad absorption peak at 3427 cm−1, corresponding to the –OH groups. After modification, the peak was stronger than that of the unmodified SPR, indicating that NaOH modification might increase the amount and change the role of the functional groups. The –CH stretching of the methyl and methylene groups always occurred in the adsorbent at about 2932 cm−1, which was characteristic of lignin, cellulose, and hemicellulose. The peak at 1654 cm−1 corresponded to the –COOH functional group, while the –CH2 or O–H functional groups appeared at 1430 cm−1. It was obvious that the –COOH stretching was enhanced significantly after NaOH modification. The peaks at 1000 cm−1 were assigned to the stretching vibrations of C–O in lignin, hemicellulose, and cellulose. Moreover, the peak at 600 cm−1 corresponded to the –CH of the amine group, with the mechanical vibration strength of MSPR significantly stronger than that of SPR. In the 500–800 cm−1 range, the functional groups of the starch or other sugars could have undergone chemical reactions.42 The adsorbent had abundant –OH, –COOH, and –CH groups, which provide more adsorption sites.
After modification, the absorption peaks all increased to varying degrees when compared with the raw material, which could improve the interaction with Cd2+ and ultimately increase the removal rate. Compared with materials that were not adsorbed, the vibrational peak of the –OH group was somewhat attenuated, indicating that Cd2+ had reacted with the –OH group. After adsorbing Cd2+ from the simulated wastewater, the peak intensities of MSPR at 3427 and 2932 cm−1 were shifted because Cd2+ was exchanged with H+ in the –OH and –CH2 or –CH3, and the carbonyl group could also be complexed with Cd2+. In short, the change in the functional group type and content may be an important reason for the significant improvement of the MSPR adsorption ability.
 | ||
| Fig. 3 SEM images of SPR under (a) 500× magnification and (b) 2000× magnification. (c) SEM images showing Cd2+ distribution on AMSPR. The red dots represent Cd2+ . | ||
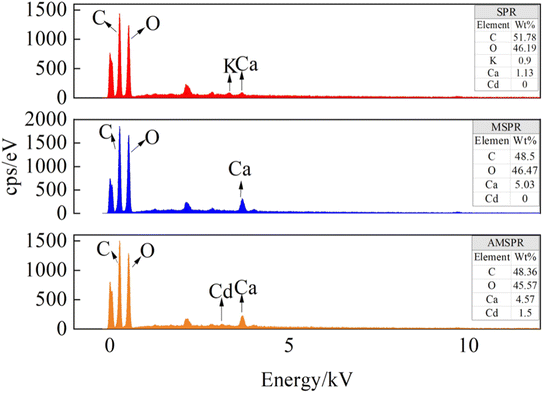
The EDAX spectra and elemental compositions of the materials are shown in Fig. 4. SPR, MSPR, and AMSPR were mainly composed of C and O elements and a small number of metal ions. The SPR and MSPR had no characteristic peak associated with Cd2+, showing no presence of Cd2+ before adsorption. Whereas, the EDAX spectra showed a characteristic peak associated with Cd2+ (around 1.5%) for the AMSPR, confirming the presence of Cd2+. After adsorption, Ca2+ decreased and Cd2+ increased on the surface of AMSPR, which could be due to the exchange of Ca2+ with Cd2+ and the simultaneous occupation by Cd2+ of the adsorption site of Ca2+. This was also observed in a study by Liu et al.,44 in which synthetic thiol-functionalized mesoporous calcium silicate was used to remove Cd2+, and Ca2+ was exchanged with Cd2+, which made a large contribution to Cd2+ removal. In another study, He et al. investigated the mechanism of adsorption of Cd2+ on silica–calcium phosphate hybrid nanoparticles and found that ion exchange between Cd2+ and Ca2+ in the material improved the adsorption capacity of the material.45 Our results show that the adsorption patterns presented in the above-mentioned synthetic materials also existed in agricultural waste.
3.3 Effects of the adsorption conditions on Cd2+ removal
In short, the removal rates of MSPR under different pH, temperature, and initial concentration of treatment conditions were always more than 90%. Besides, the removal of Cd2+ by MSPR was a fast process, and 90% of the Cd2+ in water could be removed with 1 h.
3.4 Adsorption isotherms and kinetics
Fig. 6(a) and Table 2 show the fitting results of the Langmuir and Freundlich models, respectively.
 | ||
| Fig. 6 (a) Langmuir and Freundlich equilibrium isotherm modeling for Cd2+ adsorption by MSPR and (b) pseudo-first/second-order kinetic plot of the MSPR. | ||
| Model | Parameter | Modified sweet potato residue |
|---|---|---|
| Langmuir | qm (mg g−1) | 17.53 |
| kL (mg L−1) | 0.35 | |
| R2 | 0.9914 | |
| Freundlich | n | 2.07 |
| kF | 4.83 | |
| R2 | 0.9847 | |
| Pseudo-first order | qm (mg g−1) | 7.82 |
| k1 (mg L−1) | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 945 945 |
|
| R2 | 0.8382 | |
| Pseudo-second order | qm | 8.01 |
| k2 | 7393.47 | |
| R2 | 0.9957 |
As shown in Table 2, the R2 of the Langmuir isotherm (0.9914) was larger than that of the Freundlich isotherm (0.9847), which was a better fit for this adsorption curve. The Langmuir adsorption equation is defined as a single-layer adsorption isotherm in which the chemisorption and adsorption vacancies reach equilibrium on a surface with a uniform energy distribution. This fitting results showed that the adsorption process of Cd2+ by MSPR was single-layer surface adsorption; all the adsorption sites were the same, and there was no interaction between the Cd2+ ions, which was a characteristic of chemical adsorption. Also, kL is the equilibrium constant of adsorption, and a larger value represents a stronger adsorption capacity. In a study on the removal of Cd2+ from contaminated wastewater with poplar sawdust, the highest kL was 0.017.53 In another study, the kL of an activated carbon/zirconium oxide composite was 0.059.47 The kL obtained in this study was 0.348, which was greater than that of the common materials used to adsorb Cd2+, indicating that the potential ability of MSPR to adsorb Cd2+ was stronger.
The pseudo-first-order and pseudo-second-order fitting results are shown in Fig. 6(b) and Table 2. In terms of the curve fitting and R2, the kinetics of Cd2+ adsorption by MSPR was better fitted with the pseudo-second-order model (R2 values of 0.996) than the pseudo-first-order model (R2 values of 0.838) (Table 2). The pseudo-second-order rate expression is used to describe chemisorption involving valency forces through the sharing or exchange of electrons between the adsorbent and the adsorbate as covalent forces and ion exchange, which are assumed to occur via chemisorption. In the study of the adsorption of Cd2+ by iron/manganese binary metal oxide-biochar nanocomposites, the R2 of the pseudo-second-order fitting equation was found to be greater than the first, which indicated it belonged to chemical adsorption, less than physical adsorption.54 The higher R2 of the pseudo-second-order model indicated that MSPR in contact with Cd2+ is a chemical adsorption process, and the adsorption rate is determined by the concentration of Cd2+ and the amount of adsorbent.
Based on the results of both the adsorption isotherms and kinetics, we could deduce that the adsorption of Cd2+ by MSPR was a process dominated by chemical adsorption.
4. Conclusion
Our research suggested a possibility of using MSPR as an economical adsorbent for Cd2+ removal from wastewater. The optimum modification conditions were determined as follows: a concentration of 1.5 M NaOH, a reaction temperature of 30 °C, and a particle size of 150 μm. The removal rate of Cd2+ increased with the contact time and the amount of adsorbent added. MSPR could adapt well to a wide pH range and adsorption temperatures, with a highest removal rate of 98.94% and an adsorption capacity of up to 19.81 mg g−1. The MSPR had rich functional groups, a loose surface, and a mesoporous structure, as determined by BET, FTIR, and SEM analyses. The EDAX results showed that AMSPR exhibited distinct Cd2+-related peaks. The adsorption kinetics and isotherms analyses showed good agreement with the Langmuir model and pseudo-second-order model, indicating that the process of adsorption of Cd2+ by MSPR could be characterized by chemical adsorption in single molecular layers. This study demonstrates that MSPR can effectively adsorb Cd2+. Using MSPR, an abundant agricultural waste, to remove Cd2+ from wastewater is beneficial to reduce the cost of wastewater treatment and relieve environmental pollution brought about by waste discharge from sweet potato processing.Data availability
Data can be required to the corresponding author.Author contributions
Yu Gao: writing—original draft, processing data, and analysis. Zhuolin Yi: writing—review and editing. Jinling Wang: validation, software. Fan Ding: conceptualization. Yang Fang: project administration. Anping Du: investigation. Yijia Jiang: methodology. Hai Zhao: funding acquisition. Yanling Jin: upervision, visualization, methodology, writing—review. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was financially supported by the earmarked fund for CARS-10-Sweetpotato (CARS-10-GW24), the Sichuan Science and Technology Program (2022YFN0043), and CAS “Light of West China” Program (2020XBZG_XBQNXZ_A_001).References
- Q. Li, G. Liu, L. Qi, H. Wang, Z. Ye and Q. Zhao, Heavy metal-contained wastewater in China: Discharge, management and treatment, Sci. Total Environ., 2022, 808, 152091, DOI:10.1016/j.scitotenv.2021.152091.
- J. Liu, Y. Li, D. Li, Y. Wang and S. Wei, The burden of coronary heart disease and stroke attributable to dietary cadmium exposure in Chinese adults, 2017, Sci. Total Environ., 2022, 825, 153997, DOI:10.1016/j.wri.2014.07.003.
- M. T. Hayat, M. Nauman, N. Nazir, S. Ali and N. Bangash, Cadmium Toxicity and Tolerance in Plants, 2019, pp. 163–183 Search PubMed.
- G. Genchi, M. S. Sinicropi, G. Lauria, A. Carocci and A. Catalano, The Effects of Cadmium Toxicity, Int. J. Environ. Res. Public Health, 2020, 17, 3782, DOI:10.3390/ijerph17113782.
- M. Kumar, A. Kushwaha, L. Goswami, A. K. Singh and M. Sikandar, A review on advances and mechanism for the phycoremediation of cadmium contaminated wastewater, Clean. Eng. Technol., 2021, 5, 100288, DOI:10.1016/j.clet.2021.100288.
- T. A. Saleh, M. Mustaqeem and M. Khaled, Water treatment technologies in removing heavy metal ions from wastewater: A review, Environ. Nanotechnol., Monit. Manage., 2022, 17, 100617, DOI:10.1016/j.enmm.2021.100617.
- S. Rajendran, A. K. Priya, P. S. Kumar, T. K. A. Hoang, K. Sekar, K. Y. Chong, K. S. Khoo, H. S. Ng and P. L. Show, A critical and recent developments on adsorption technique for removal of heavy metals from wastewater-A review, Chemosphere, 2022, 303, 135146, DOI:10.1016/j.chemosphere.2022.135146.
- Y. Zhan, Z. Ye, L. Wang, L. Yang and Y. Li, Removal of Cd (II) by polystyrene-base chelating resins: adsorption properties and experiences of industrial wastewater treatment, Desalin. Water Treat., 2013, 52(34–36), 6481–6491, DOI:10.1080/19443994.2013.821032.
- R. Leyva-Ramos, J. R. Rangel-Mendez, J. Mendoza-Barron, L. Fuentes-Rubio and R. M. Guerrero-Coronado, Adsorption of cadmium(II) from aqueous solution onto activated carbon, Water Sci. Technol., 1997, 35(7), 205–211, DOI:10.1016/S0273-1223(97)00132-7.
- B. Gabriela, L. Nicoleta, C. Horia, C. Gabriela, B. Roxana-Dana, B. Daniel, F. Lidia and H. Maria, Performance assessment of five adsorbents based on fly ash for removal of cadmium ions, J. Mol. Liq., 2021, 333(333), 115932, DOI:10.1016/j.molliq.2021.115932.
- M. M. Kwikima, S. Mateso and Y. Chebude, Potentials of agricultural wastes as the ultimate alternative adsorbent for cadmium removal from wastewater. A review, Sci. Afr., 2021, 13, e00934, DOI:10.1016/j.sciaf.2021.e00934.
- V. Jayakumar, S. Govindaradjane, P. Senthil Kumar, N. Rajamohan and M. Rajasimman, Sustainable removal of cadmium from contaminated water using green alga - Optimization, characterization and modeling studies, Environ. Res., 2021, 199, 111364, DOI:10.1016/j.envres.2021.111364.
- K. Hachem, D. Bokov, M. D. Farahani, B. Mehdizade and A. A. Kazemzadeh Farizhandi, Ultrasound-assisted adsorption of dyes and cadmium ion from aqueous solutions by ZnAl2O4 nanoparticles, Mater. Chem. Phys., 2022, 276, 125398, DOI:10.1016/j.matchemphys.2021.125398.
- G. Sharma and M. Naushad, Adsorptive removal of noxious cadmium ions from aqueous medium using activated carbon/zirconium oxide composite: Isotherm and kinetic modelling, J. Mol. Liq., 2020, 310, 113025, DOI:10.1016/j.molliq.2020.113025.
- R. F. Susanti, R. G. R. Wiratmadja, H. Kristianto, A. A. Arie and A. Nugroho, Synthesis of high surface area activated carbon derived from cocoa pods husk by hydrothermal carbonization and chemical activation using zinc chloride as activating agent, Mater. Today: Proc., 2022, 63, S55–S60, DOI:10.1016/j.matpr.2022.01.042.
- H. C. Hsua, S. K. Hsu, S. C. Wu, Y. H. Hung and W. F. Ho, Surface modification of nanotubular anodized Ti–7.5Mo alloy using NaOH treatment for biomedical application, Thin Solid Films, 2020, 710, 138273, DOI:10.1016/j.tsf.2020.138273.
- M. K. Alam, A comprehensive review of sweet potato (Ipomoea batatas [L.] Lam): Revisiting the associated health benefits, Trends Food Sci. Technol., 2021, 115, 512–529, DOI:10.1016/j.tifs.2021.07.001.
- FAO, Food and Agriculture Organization of the United Nations, 2021, (Accessed 2016 2022) Search PubMed.
- M. P. M. Arachchige, T. Mu and M. Ma, Effect of high hydrostatic pressure-assisted pectinase modification on the Pb2+ adsorption capacity of pectin isolated from sweet potato residue, Chemosphere, 2021, 262, 128102, DOI:10.1016/j.chemosphere.2020.128102.
- N. Hu, K. Zhang, Y. Zhao, Z. Zhang and H. Li, Flotation-based dye removal system: Sweet potato protein fabricated from agro-industrial waste as a collector and frother, J. Cleaner Prod., 2020, 269, 122121, DOI:10.1016/j.jclepro.2020.122121.
- M. S. iban, B. Radetic, Z. Kevrešan and M. Klašnja, Adsorption of heavy metals from electroplating wastewater by wood sawdust, Bioresour. Technol., 2007, 98(2), 402–409, DOI:10.1016/j.biortech.2005.12.014.
- K. L. Tan and B. H. Hameed, Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions, J. Taiwan Inst. Chem. Eng., 2017, 74, 25–48, DOI:10.1016/j.jtice.2017.01.024.
- M. A. Al-Ghouti and D. A. Da'ana, Guidelines for the use and interpretation of adsorption isotherm models: A review, J. Hazard. Mater., 2020, 393, 122383, DOI:10.1016/j.jhazmat.2020.122383.
- J. Wang and X. Guo, Adsorption kinetic models: Physical meanings, applications, and solving methods, J. Hazard. Mater., 2020, 390, 122156, DOI:10.1016/j.jhazmat.2020.122156.
- P. Su, X. Gao, J. Zhang, R. Djellabi, B. Yang, Q. Wu and Z. Wen, Enhancing the adsorption function of biochar by mechanochemical graphitization for organic pollutant removal, Front. Environ. Sci. Eng., 2021, 15(6), 130, DOI:10.1007/s11783-021-1418-2.
- S. N. Jain and P. R. Gogate, NaOH-treated dead leaves of Ficus racemosa as an efficient biosorbent for Acid Blue 25 removal, Int. J. Environ. Sci. Technol., 2016, 14(3), 531–542, DOI:10.1007/s13762-016-1160-7.
- S. D. Ashrafi, H. Kamani and A. H. Mahvi, The optimization study of direct red 81 and methylene blue adsorption on NaOH-modified rice husk, Desalin. Water Treat., 2014, 57(2), 738–746, DOI:10.1080/19443994.2014.979329.
- S. Chakraborty, S. Chowdhury and P. Das Saha, Adsorption of Crystal Violet from aqueous solution onto NaOH-modified rice husk, Carbohydr. Polym., 2011, 86(4), 1533–1541, DOI:10.1016/j.carbpol.2011.06.058.
- B. Tatiana and N. Patrick, Cellulose in NaOH–water based solvents: a review, Cellulose, 2015, 23(1), 5–55, DOI:10.1007/s10570-015-0779-8.
- C. Wang, J. Yu, K. Feng, H. Guo and L. Wang, Alkali treatment to transform natural clinoptilolite into zeolite Na-P: Influence of NaOH concentration, J. Phys. Chem. Solids, 2022, 168, 110827, DOI:10.1016/j.jpcs.2022.110827.
- Y. L. d O. Salomón, J. Georgin, D. S. P. Franco, M. S. Netto, P. Grassi, D. G. A. Piccilli, M. L. S. Oliveira and G. L. Dotto, Powdered biosorbent from pecan pericarp (Carya illinoensis) as an efficient material to uptake methyl violet 2B from effluents in batch and column operations, Adv. Powder Technol., 2020, 31(7), 2843–2852, DOI:10.1016/j.apt.2020.05.004.
- S. Wan, Z. Ma, Y. Xue, M. Ma, S. Xu, L. Qian and Q. Zhang, Sorption of Lead(II), Cadmium(II), and Copper(II) Ions from Aqueous Solutions Using Tea Waste, Ind. Eng. Chem. Res., 2014, 53(9), 3629–3635, DOI:10.1021/ie402510s.
- V. d S. Lacerda, J. B. L. p. Sotelo, A. C. Guimaraes, S. H. n. Navarro, M. S. n B. scones, L. M. N. Gracia, P. M. n. Ramos and J. s M. n. Gil, Rhodamine B removal with activated carbons obtained from lignocellulosic waste, J. Environ. Manage., 2015, 155, 67–76, DOI:10.1016/j.jenvman.2015.03.007.
- R. Shwetharani, A. Poojashree, G. R. Balakrishna and M. S. Jyothi, La activated high surface area titania float for the adsorption of Pb(ii) from aqueous media, New J. Chem., 2018, 42(2), 1067–1077, 10.1039/C7NJ03358C.
- M. Abdouss, A. M. Shoushtari, A. M. Simakani, S. Akbari and A. Haji, Citric Acid-Modified Acrylic Micro and Nanofibers for Removal of Heavy Metal Ions from Aqueous Media, 11th World Filtration Congress, 2012 Search PubMed.
- L. Mao, Y. Wu, W. Zhang and Q. Huang, The reuse of waste glass for enhancement of heavy metals immobilization during the introduction of galvanized sludge in brick manufacturing, J. Environ. Manage., 2019, 231, 780–787, DOI:10.1016/j.jenvman.2018.10.120.
- J. Ponce, J. o G. d S. Andrade, L. N. d. Santos, M. K. Bulla, B. C. B. Barros, N. H. Silvia Luciana Favaroc, W. Caetano and V. R. Batistela, Alkali pretreated sugarcane bagasse, rice husk and corn husk wastes as lignocellulosic biosorbents for dyes, Carbohydr. Polym. Technol. Appl., 2021, 2, 100061, DOI:10.1016/j.carpta.2021.100061.
- J. W. Shima, S. J. Parkb and S. K. Ryu, Effect of modification with HNO3 and NaOH on metal adsorption by pitch-based activated carbon fibers, Carbon, 2001, 39(11), 1635–1642, DOI:10.1016/S0008-6223(00)00290-6.
- H. Zhang, H. Zhao, S. Mu, J. Cai, Y. Xiang, J. Wang and J. Hong, Surface relaxation and permeability of cement pastes with hydrophobic agent: Combining 1H NMR and BET, Constr. Build. Mater., 2021, 311, 125264, DOI:10.1016/j.conbuildmat.2021.125264.
- Y. He, Y. Hao, J. Shen, C. Wang and Y. Wei, Removal of adsorption sites on the external surface of mesoporous adsorbent for eliminating the interference of proteins in enrichment of phosphopeptides/nucleotides, Anal. Chim. Acta, 2021, 1178, 338849, DOI:10.1016/j.aca.2021.338849.
- A. V. r. Filho,L. V. Tholozan, E. O. d. Silva, L. Meili, A. R. F. d. Almeida and G. S. d Rosa, Biomass-Derived Materials for Environmental Applications, 2022, pp. 243–266 Search PubMed.
- C. LanChai, F. Yang, J. Y. Ling, C. Qian, Z. Y. Gui, X. Yao and Z. Hai, Biosorption of Cd2+by untreated dried powder of duckweedLemna aequinoctialis, Desalin. Water Treat., 2013, 53(1), 183–194, DOI:10.1080/19443994.2013.839399.
- A. Akinterinwa, E. Oladele, A. Adebayo, O. Ajayi, O. O. Iyanu and E. Gurgur, Cross-linked-substituted (esterified/etherified) starch derivatives as aqueous heavy metal ion adsorbent: a review, Water Sci. Technol., 2020, 82(1), 1–26 CAS . https://iwaponline.com/wst/article-pdf/doi/10.2166/wst.2020.332/713230/wst2020332.
- L. Liu, T. Li, G. Yang, Y. Wang, A. Tang and Y. Ling, Synthesis of thiol-functionalized mesoporous calcium silicate and its adsorption characteristics for heavy metal ions, J. Environ. Chem. Eng., 2017, 5(6), 6201–6215, DOI:10.1016/j.jece.2017.11.046.
- Y. He, L. Luo, S. Liang, M. Long and H. Xu, Synthesis of mesoporous silica-calcium phosphate hybrid nanoparticles and their potential as efficient adsorbent for cadmium ions removal from aqueous solution, J. Colloid Interface Sci., 2018, 525, 126–135, DOI:10.1016/j.jcis.2018.04.037.
- M. Kavand, P. Eslami and L. Razeh, The adsorption of cadmium and lead ions from the synthesis wastewater with the activated carbon: Optimization of the single and binary systems, J. Water Process. Eng., 2020, 34, 101151, DOI:10.1016/j.jwpe.2020.101151.
- A. Akisawa, A. Tamogami, N. Takeda, M. Nakayama and T. Natsui, Effect of Adsorbent on the Performance of Double Effect Adsorption Refrigeration Cycle with Adsorption Heat Recovery, Int. J. Refrig., 2022, 141, 21–30, DOI:10.1016/j.ijrefrig.2022.06.012.
- E. Kelechi, E. O. Afamefuna, K. A. Kenneth and J. A. Ojo, Thermodynamic study of the adsorption of Cd2+ and Ni2+ onto chitosan – Silica hybrid aerogel from aqueous solution, Results Chem., 2023, 5, 100730, DOI:10.1016/j.rechem.2022.100730.
- B. Y. Z. Hiew, L. Y. Lee, X. J. Lee, S. Thangalazhy-Gopakumar and S. Gan, Utilisation of environmentally friendly okara-based biosorbent for cadmium(II) removal, Environ. Sci. Pollut. Res., 2020, 28(30), 40608–40622, DOI:10.1007/s11356-020-09594-3.
- X. Li, Y. Huang, X. Liang, L. Huang, L. Wei, X. Zheng, H. A. Albert, Q. Huang, Z. Liu and Z. Li, Characterization of biochars from woody agricultural wastes and sorption behavior comparison of cadmium and atrazine, Biochar, 2022, 4(1), 27, DOI:10.1007/s42773-022-00132-7.
- X. Guo, S. Tang, Y. Song and J. Nan, Adsorptive removal of Ni2+ and Cd2+ from wastewater using a green longan hull adsorbent, Adsorpt. Sci. Technol., 2017, 36(1–2), 762–773, DOI:10.1177/0263617417722254.
- H. Çelebi, G. Gök and O. Gök, Adsorption capability of brewed tea waste in waters containing toxic lead(II), cadmium (II), nickel (II), and zinc(II) heavy metal ions, Sci. Rep., 2020, 10(1), 17570, DOI:10.1038/s41598-020-74553-4.
- S. Cheng, Y. Liu, B. Xing, X. Qin, C. Zhang and H. Xia, Lead and cadmium clean removal from wastewater by sustainable biochar derived from poplar saw dust, J. Cleaner Prod., 2021, 314, 128074, DOI:10.1016/j.jclepro.2021.128074.
- J. Qu, N. Che, G. Niu, L. Liu, C. Li and Y. Liu, Iron/manganese binary metal oxide-biochar nano-composites with high adsorption capacities of Cd2+: Preparation and adsorption mechanisms, J. Water Process. Eng., 2023, 51, 103332, DOI:10.1016/j.jwpe.2022.103332.
| This journal is © The Royal Society of Chemistry 2024 |