Influence of antioxidant rich fresh vegetable juices on starch induced postprandial hyperglycemia in rats†
Ashok K.
Tiwari
*,
K. Srikanth
Reddy
,
Janani
Radhakrishnan
,
D. Anand
Kumar
,
Amtul
Zehra
,
Sachin B.
Agawane
and
K.
Madhusudana
Pharmacology Division, Indian Institute of Chemical Technology (CSIR), Hyderabad, 500607, India. E-mail: tiwari@iict.res.in; astiwari@yahoo.com; Fax: +91-040-27193189; Tel: +91-040-27191617
First published on 26th August 2011
Abstract
This research analyzed the major chemical components and multiple antioxidant activities present in the fresh juice of eight vegetables, and studied their influence on starch induced postprandial glycemia in rats. A SDS-PAGE based protein fingerprint of each vegetable juice was also prepared. The yields of juice, chemical components like total proteins, total polyphenols, total flavonoids, total anthocyanins and free radicals like the ABTS˙+ cation, DPPH, H2O2, scavenging activities and reducing properties for NBT and FeCl3 showed wide variations. Vegetable juice from brinjal ranked first in displaying total antioxidant capacity. Pretreatment of rats with vegetable juices moderated starch induced postprandial glycemia. The fresh juice from the vegetables ridge gourd, bottle gourd, ash gourd and chayote significantly mitigated postprandial hyperglycemic excursion. Total polyphenol concentrations present in vegetable juices positively influenced ABTS˙+ scavenging activity and total antioxidant capacity. However, NBT reducing activity of juices was positively affected by total protein concentration. Contrarily, however, high polyphenol content in vegetable juice was observed to adversely affect the postprandial antihyperglycemic activity of vegetable juices. This is the first report exploring antihyperglycemic activity in these vegetable juices and highlights the possible adverse influence of high polyphenol content on the antihyperglycemic activity of the vegetable juices.
Introduction
Diabetes mellitus is one of the most common chronic diseases in nearly all countries and continues to increase in number and significance, as changing life styles lead to reduced physical activity and increased obesity.1 Excessive ingestion of calorie-dense, easily digestible foods causes abnormal surges in postprandial blood glucose and triglycerides.2–4 Postprandial hyperglycemia (PPHG) has been established as one of the earliest detectable abnormalities expressed in ensuing diabetes mellitus,5 a better predictor of the progression of diabetes6 and cardiovascular disease (CVD), and an important contributing factor to the development of atherosclerosis7 and oxidative stress.8 The higher carbohydrate content in the diet of south Asians9–11 have been held responsible for the increased incidence of hyperglycemia12 and may explain higher PPHG in this particular ethnic population in the world.13,14Postprandial glucose excursion (PPGE) has also been held directly responsible for the ensuing increase in free radical generation.15 Hyperglycemic spikes, even in non-diabetic individuals, have been shown to markedly increase free radical generation.16 The overt generation of free radicals and consequent oxidative stress has recently been recognized as a major pathophysiological link between CVD and diabetes17 and also in the development of diabetic complications.18 Therefore, mitigation of postprandial hyperglycemic excursion and scavenging of excessive free radicals leading to the consequent reduction in oxidative stress holds promise in reducing the risks responsible for the development of diabetes, CVD and diabetic complications.
While promising pharmacological approaches to the normalization of post-prandial metabolic disturbances are evolving,15 and currently available medicines are struggling to win the battle of glucose control,19 it is being seen that resorting to drug therapy for an epidemic caused by a maladaptive diet is less rational than simply realigning eating habits with physiological needs.20 Unfortunately, however, there is glaring absence of research on lifestyle interventions to prevent or reverse diabetes.19
The awareness for inclusion of fresh fruits and vegetables in human diet is mounting.21,22 One of the major health benefits from the higher intake of fruits and vegetables has been ascribed to the presence of variety of potential biological antioxidants.23,24 Recently, however, the health benefits of polyphenol-rich dietary supplements,25 dietary antioxidant supplements,26 as well as isolated antioxidant compounds from natural resources,27 has raised suspicions owing to their prooxidative effects at high concentrations or their potential to react with beneficial concentrations of indigenously produced free radicals normally present at physiological conditions and required for optimal cellular functioning.27 In addition, the timing28 and order of their consumption with a meal is also being questioned.29 It is increasingly being realized that antioxidants when taken on an empty stomach may affect appetite and when consumed along with the diet may affect satiety.28 Similarly, some antioxidant rich fruits have been alleged to induce hyperlipidemia30 and antioxidant-rich fractions of some of the food grains has been observed to exacerbate starch induced postprandial hyperglycemia.31
Therefore, it becomes pertinent to identify fruits and vegetables that possess potent free radical scavenging antioxidant activities however, do not adversely affect blood glucose level. Recently, there has been a large increase in the number of vegetable juices, which have become commercially available; conversely, publications detailing the antioxidant capacity of vegetable juices are sparse.24 Similarly, scientific data on the influence of such vegetable juices on other metabolic parameters are lacking.
The aims of the present study were to analyze various chemical components and free radical scavenging antioxidant activities in the fresh juice of some common and readily available vegetables and evaluate their influence on the blood glucose level of normal rats under starch induced postprandial hyperglycemia. This research also provides an electrophoretic protein fingerprint of individual vegetable juices as a quality control tool for standardization.
Materials and methods
Chemicals
2,2′-Azinobis(3-ethyl benzthiazoline-6-sulphonic acid) liquid substrate (ABTS), AzBTS microwell enhancer solution, ABTS diammonium salt, 2,2-diphenyl-1-picrylhydrazyl (DPPH), hydrogen peroxide (H2O2) solution, Folin–Ciocalteu phenol reagent, bovine serum albumin (BSA), nitro blue tetrazolium (NBT), trolox, and gallic acid were obtained from Sigma-Aldrich chemicals (St Louis, MO). Other chemicals of analytical grade were purchased from Merck Limited (Mumbai, India), S.D. Fine Chemicals Ltd (Mumbai, India).Vegetables and preparation of juice
Fruits of eight vegetables (Fig.1), namely ridge gourd (Luffa acutangula L. Roxb), bottle gourd (Lagenaria siceraria Molina Standl.), cucumber (Cucumis melo var. utilissimus Duthie & Fuller), brinjal (Solanum melongena L.), ash gourd (Benincasa hispida Thunb.Cogn.), yellow pumpkin (Cucurbita maxima Duchesne), snake gourd (Trichosanthes cucumerina L.), and chayote (Sechium edule L.), were purchased from local vegetable markets of the city Hyderabad (India). Vegetables were washed with water and cut into pieces. Juice was obtained by grinding a weighed amount of vegetable pieces with a food grade grinder and squeezed by the maximum amount with clean muslin cloth. Juices were centrifuged at 5000 rpm, 30 min at room temperature to get a transparent supernatant and used for the study as fresh. | ||
| Fig. 1 Images of the vegetables selected in this study. | ||
Total protein estimation
Protein content in the vegetable juices was determined using Bradford's dye.32 Briefly 10 μL of juice was mixed with 240 μL of 1× Bradford reagent and absorbance was read at 595nm on a BioTek synergy4 multi-mode microplate reader (BioTek Instruments, Inc. Winooski, VT, USA). Protein concentration (mg mL−1) was expressed applying a BSA standard curve (y = 0.719x + 0.016, R2 = 0.977).Determination of total polyphenolic content
Total polyphenolic content in the juices was measured using Folin-Ciocalteu reagent.33 Briefly, 25 μL of the fresh juice was diluted with 2.5mL of distilled deionised water followed by addition of 250 μL of Folin–Ciocalteu reagent (1 M) and 250 μL of Na2CO3 (20%, w/v). After incubation for 60 min at room temperature absorbance was measured spectrophotometrically at 765 nm (BioTek synergy4 multi-mode microplate reader, BioTek Instruments, Inc Winooski, VT, USA). Quantification was performed with respect to the standard curve of gallic acid (y = 0.719x + 0.059, R2 = 0.999). Results were expressed as milligrams of gallic acid equivalent (GAE) per mL of the extract.Determination of total flavonoids
Total flavonoid content in the samples was measured by mixing equal volume of vegetable juice with 2% AlCl3·6H2O in a 96 well plate, as described by Hsieh et al.34 Absorbance was recorded at 430 nm spectrophotometrically (BioTek synergy4 multi-mode microplate reader, BioTek Instruments, Inc Winooski, VT, USA). Flavonoid content in the juices was determined with respect to the standard curve of the flavonoid rutin (y = 5.224x + 0.037, R2 = 0.998). Results were expressed as milligrams of rutin equivalent (RE) per mL of the extract.Determination of total anthocyanins
Presence of anthocyanins in vegetable juices was determined as described by Giusti et al.35 Juices were mixed directly with equal volumes of 25 mM potassium chloride solution (pH 1.0) and 0.4 M sodium acetate buffer (pH 4.5). Absorbance was measured at 510 and 700 nm (Perkin Elmer precisely, Lambda 25,UV/VIS spectrometer, Massachusetts, USA). Data were expressed as milligrams of anthocyanins per 100 mL of juice using a molar extinction coefficient of 26900, molecular weight of 449.2, and an absorbance of A = [(A510 − A700) pH 1.0 − (A510 − A700) pH 4.5].ABTS˙+ scavenging assay
Scavenging of the ABTS˙+ cation was performed as described by Walker and Everette36 with suitable modifications. Briefly, 100 mL stock solution of ABTS˙+(0.5 mM) was prepared by addition of 1 mL potassium persulfate (6.89 mM PBS, pH 8.0). The mixture was stored in the dark for 16 h. 10 μL of various dilutions of juice was mixed with 190 μL of ABTS˙+ in a 96-well microplate. Absorbance of decolorized ABTS˙+ was measured at 734 nm after 15 min incubation in the dark on a BioTek synergy4 multi-mode microplate reader. For each test sample a separate blank sample (devoid of ABTS˙+) was used for background subtraction. The percentage of ABTS˙+ scavenging by juices was obtained in terms of Trolox equivalent concentration (y = 8.7606x + 1.1074, R2 = 0.9951). Suitable regression analysis was applied for calculation of SC50.Scavenging of DPPH free radical
The assay for the scavenging of free radical DPPH was done as reported earlier.37 Briefly, in a 96-well microplate, 25 μL of various dilutions of fresh vegetable juice, 100 μL of tris-HCl buffer (0.1 M, pH 7.4), and 125 μL DPPH solution (0.5 mM in methanol) were added. The reaction mixture was shaken well. DPPH decolorisation was recorded (517 nm) on a BioTek synergy4 multi-mode microplate reader after 30 min incubation in the dark. The percentage of DPPH scavenging by juices was obtained in terms of ascorbic acid equivalent concentration (y = 0.8688x + 12.179, R2 = 0.999). Suitable regression analysis was applied for calculation of SC50.Nitroblue tetrazolium reducing assay
Nitroblue tetrazolium (NBT) reducing activity as a measure of the presence of ascorbic acid38 in vegetable juices was also determined. Briefly, in a 96-well plate containing 100 μL phosphate buffer (50 mM, pH 10) and an equal quantity of NBT (1 mM, prepared in the same buffer), 50 μL of various dilutions of vegetable juices were mixed and incubated for 15 min. A blank with every juice sample in the absence of NBT was run to correct the background absorbance. The reduction of NBT was measured at 560 nm using a BioTek synergy4 multimode microplate reader (BioTek Instruments Inc, Winooski, VT, USA). The percentage of NBT reduction by vegetable juices was obtained in terms of ascorbic acid equivalent concentration [y = 21.471ln(x) − 6.8001, R2 = 0.971]. Suitable regression analysis was applied to obtain RC50 values.Ferric chloride reducing activity
FeCl3 reducing activity39 was modified to suit 96 well plate reading. In Eppendorf tubes 100 μL of various dilutions of vegetable juices were mixed with 100 μL of phosphate buffer and incubated with 100 μL of 1% potassium ferricyanide at 50 °C for 20 min. The reaction was terminated by the addition of 10% TCA and centrifuged (Heraeus, Biofuge stratos, Kendro Laboratory Products, Sollentum Germany) at 3000 rpm for 10 min. 100 μL of supernatant was transferred to 96 well micro plates. Further, 100 μL of distilled water and 20 μL of 0.1% FeCl3 was added and mixed well. Absorbance was measured at 700 nm on a BioTek synergy4 multi-mode microplate reader (BioTek Instruments Inc, USA).H2O2 scavenging assay
The determination of H2O2 scavenging activity was performed as described by Wettasinghe and Shahidi40 with slight modifications. 40 μL of fresh juice was mixed with 1.2 mL of H2O2 solution (21.5 mM H2O2 in 0.1 M phosphate buffer (pH 7.4)). Absorbance values (230 nm) of the reaction mixture were recorded at 0 min and then at every 10th min up to 30 min on a Perkin Elmer precisely, Lambda 25,UV/VIS spectrometer (USA). For each test sample a separate blank sample (devoid of H2O2) was also used for background subtraction. The percentage of H2O2 scavenging was calculated by applying following formula [(Absorbancecontrol − Absorbancetest)/Absorbancecontrol] × 100.Total antioxidant capacity
The total antioxidant capacity of vegetable juices was determined by ranking their SC50 and RC50 values. The lowest value was ranked 1st and rest followed the order. Values of ABTS˙+, DPPH˙, NBT and FeCl3 were taken for ranking. The mean of the rank was obtained and juice with rank order 1st was considered highly potent.SDS-PAGE analysis of vegetable juice protein
Finely chopped vegetable pieces were soaked overnight in ice cold 0.1 mM Tris-HCl (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; w/v) containing PMSF (5 mM). Soaked pieces were homogenized using a mortar and pestle in cold conditions. During this process PMSF (1 mM) was again added to prevent protein degradation. The homogenate was centrifuged (Heraeus, Biofuge stratos, Kendro Laboratory Products, Sollentum Germany) for 20 min at 15
1; w/v) containing PMSF (5 mM). Soaked pieces were homogenized using a mortar and pestle in cold conditions. During this process PMSF (1 mM) was again added to prevent protein degradation. The homogenate was centrifuged (Heraeus, Biofuge stratos, Kendro Laboratory Products, Sollentum Germany) for 20 min at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g (4 °C). Crude protein precipitate were separated and the total protein content was measured.32
000g (4 °C). Crude protein precipitate were separated and the total protein content was measured.32
20 μL equal concentration protein samples were mixed with appropriate volume of 2× SDS loading buffer containing 0.5 M Tris-HCl (pH 6.8), 10% SDS, glycerol, 2-mercaptoethanol and bromophenol blue and heated for 5 min in boiling water. 20 μl of samples were separated on 12% SDS-PAGE (Bio-Rad mini Protein gel apparatus) along with a molecular weight marker. The gel was allowed to run in 1× Tris-glycine buffer (10× buffer – 250 mM Tris base, 1.92 M glycine, 1% SDS), at a constant voltage of 100 V. Gels were stained in 0.5% Coomassie brilliant blue (250 R) solution for an hour and de-stained several times with fresh methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid
acetic acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (50
water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40) solution until appropriate staining of the gel was reached. Destained gels were photographed using a BioDoc-It™ Imaging System, UV Transilluminator UVP (Cambridge UK).
40) solution until appropriate staining of the gel was reached. Destained gels were photographed using a BioDoc-It™ Imaging System, UV Transilluminator UVP (Cambridge UK).
Animal experiments
Animal experiments were performed using adult Wistar rats of either sex (180–220 g body weight). Institutional Animal Ethical Committee (CPCSEA Reg. No.97/1999, Government of India) approval for the experimental protocol was obtained. All the experiments with live animals were performed in compliance with the relevant laws and institutional guidelines. Experiments were performed as reported earlier.41 All the animals were kept for overnight fasting. The next day forenoon blood was collected from the retro orbital plexus in EDTA-containing tubes. Plasma glucose levels for the basal (‘0’ h) value were measured by the glucose-oxidase test method using an auto-blood analyzer instrument (Bayer EXPRESS PLUS, NY, USA). Rats were divided into various groups (six rats in each group) viz. control, test groups.An individual vegetable's juice (fresh juice, at a random dose of 7.5 mL kg−1 body weight) to respective groups of animals was administered orally through gastric intubation. The control group of animals was treated with normal saline. Fifteen minutes after treatment, animals were fed with soluble-starch dissolved in normal saline at a dose of 2 g kg−1 body weight. Thereafter, blood was collected at the intervals of 30, 60, 90, and 120th minute post-starch feeding. Plasma was separated out for glucose measurement as described above. The two-hour postprandial glycemic load (AUC0–120 minutes) was calculated following trapezoidal rules.41
Statistical analysis
All the data were expressed as mean ± SD. The Pearson correlation coefficient (r) and p-value were used to obtain correlation and significance among free radical scavenging activities, chemical compositions and AUC0–120 minutes. One way ANOVA followed by Dunnett's multiple comparison test was applied to compare difference in animal study groups. The criterion for statistical significance was p < 0.05. Statistical analyses were performed by using GraphPadPRISM®Version 5.01(GraphPad Software, Inc. California, USA).Results
Chemical components, free radicals scavenging activities and correlations
Table 1 presents data of chemical components analyzed in vegetable juices. The yield of juice from eight studied vegetables varied between 38 to 64% (mL/100 g). Snake gourd, ash gourd and bottle gourd were found to be better juicing vegetables than others. Ridge gourd was observed to be a protein-rich vegetable, whereas cucumber and snake gourd showed less protein content than other vegetables. In brinjal the highest polyphenol content was observed, whereas ash gourd represented a polyphenol poor vegetable. Total flavonoid content in yellow pumpkin, bottle gourd and ridge gourd was observed to be higher than other vegetables. In our analysis, total anthocyanins could be detected in yellow pumpkin (2.93 ± 0.01% w/w) only.| Vegetables | % Yield of juice (v/w; mL/100 g) | Total Protein (mg mL−1) | Total Polyphenols (mg GAE/mL) | Total Flavonoids (mg RE/mL) |
|---|---|---|---|---|
| a Values represent mean ± Standard Deviations (SD), % yield was calculated on five occasions, total protein concentrations were measured on 10 occasions, and total polyphenol and flavonoid concentrations were measured on two occasions. GAE; gallic acid equivalent, RE; rutin equivalent. All the analysis each time was performed in triplicate. | ||||
| Ridge gourd | 38.8 ± 2.5 | 0.54 ± 0.17 | 0.16 ± 0.01 | 0.40 ± 0.02 |
| Bottle gourd | 57.8 ± 5.2 | 0.34 ± 0.18 | 0.18 ± 0.03 | 0.60 ± 0.06 |
| Cucumber | 39.7 ± 1.6 | 0.25 ± 0.16 | 0.31 ± 0.01 | 0.03 ± 0.02 |
| Brinjal | 40.2 ± 11.3 | 0.40 ± 0.22 | 0.87 ± 0.09 | 0.10 ± 0.01 |
| Ash gourd | 60.6 ± 13.9 | 0.37 ± 0.21 | 0.08 ± 0.01 | 0.01 ± 0.01 |
| Yellow pumpkin | 48.6 ± 5.9 | 0.39 ± 0.30 | 0.28 ± 0.01 | 0.61 ± 0.02 |
| Snake gourd | 64.4 ± 4.0 | 0.26 ± 0.12 | 0.13 ± 0.01 | 0.01 ± 0.01 |
| Chayote | 54.0 ± 9.8 | 0.35 ± 0.14 | 0.14 ± 0.02 | 0.02 ± 0.01 |
Wide differences in the various free radical scavenging potentials of vegetable juices were observed in this study (Table 2). Though all the juices were more potent in scavenging ABTS˙+ cation than the reference compound trolox, brinjal and bottle gourd were twenty times more potent than trolox. Potent DPPH radical scavenging activity could be observed only in brinjal, ridge gourd, cucumber, and bottle gourd. Here too, brinjal displayed a twice as high potency as the reference compound ascorbic acid. Similarly, brinjal displayed more potency in reducing either NBT or the FeCl3. The NBT reducing power of snake gourd and cucumber was similar to the ascorbic acid while the other juices displayed more reducing power than ascorbic acid except ash gourd. All the vegetables juices were observed to be more potent than the reference compound ascorbic acid in reducing FeCl3. In the order of their total antioxidant capacity ranking, brinjal was observed to be more potent than other vegetables juices in scavenging various free radicals.
| Vegetable | ABTS˙+ | DPPH˙ | NBT | FeCl3 | Total Antioxidant Capacity (Rank) |
|---|---|---|---|---|---|
| a Values represent (mean ± Standard Deviations, N = 3) SC50 μM Trolox equivalent/mL for ABTS˙+, SC50, μM ascorbic acid equivalent/mL for DPPH˙ and RC50 μM ascorbic acid equivalent ascorbic acid equivalent/mL for NBT and FeCl3 reduction. SC50; scavenging concentration 50%, RC50; reducing concentration 50%. ND; not detected. | |||||
| Ridge gourd | 6.62 ± 0.29 | 33.47 ± 3.20 | 4.02 ± 0.21 | 1.38 ± 0.01 | 3.8 |
| Bottle gourd | 2.68 ± 0.05 | 44.96 ± 2.09 | 7.86 ± 0.27 | 0.40 ± 0.01 | 3.5 |
| Cucumber | 6.71 ± 0.08 | 33.67 ± 0.98 | 14.06 ± 1.07 | 0.23 ± 0.01 | 4.3 |
| Brinjal | 2.27 ± 0.08 | 26.70 ± 2.87 | 0.72 ± 0.34 | 0.19 ± 0.01 | 1 |
| Ash gourd | 12.27 ± 0.57 | ND | ND | 1.76 ± 0.04 | 7 |
| Yellow pumpkin | 5.11 ± 0.31 | ND | 1.49 ± 0.06 | 1.02 ± 0.17 | 3.8 |
| Snake gourd | 6.93 ± 0.55 | ND | 13.21 ± 0.07 | 2.09 ± 0.35 | 6.3 |
| Chayote | 11.81 ± 0.48 | ND | 6.67 ± 0.42 | 0.61 ± 0.05 | 5 |
| Trolox | 22.29 ± 0.01 | ||||
| Ascorbic acid | 43.73 ± 0.04 | 13.56 ± 0.14 | 5.10 ± 0.02 |
It was interesting, however, to note that chayote, followed by yellow pumpkin and snake gourd, scavenged H2O2 more potently than other vegetable juices but brinjal, followed by bottle gourd, could not display H2O2 scavenging activity (Fig. 2).
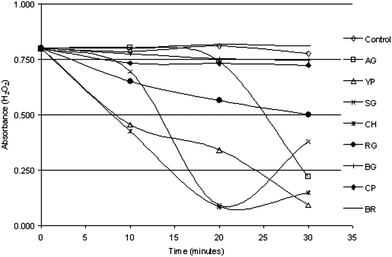 | ||
| Fig. 2 Kinetics of H2O2 scavenging by vegetable juices. 40 μl of fresh juice was applied in this study. Values represent the mean of triplicate observations. The control represents H2O2 absorbance without vegetable juice. Ash gourd (AG), yellow pumpkin (YP), snake gourd (SG), chayote (CH), ridge gourd (RG), bottle gourd (BG), cucumber (CP) and brinjal (BR). | ||
It was observed that ABTS˙+ cation scavenging activity was significantly (p, 0.05) correlated with total polyphenol content in the vegetable juices (Fig. 3A). The higher the total polyphenol content, the lower the SC50 for scavenging the ABTS˙+ cation. Similarly, higher total-antioxidant capacity rank orders could be ascribed to a higher total polyphenol content in the vegetable juices (Fig. 3A). Although we could not observe significant correlation with the total polyphenol content in the juice with other free radicals like DPPH scavenging, NBT and FeCl3 reducing activity, a highly significant (p < 0.02 correlation between total protein content and NBT reducing activity was observed in our study (Fig. 3B). The correlation between total flavonoid content in the vegetable juices could not reach the desired (p < 0.05) degree of significance (Pearson r = −0.557, p < 0.07) for ABTS˙+ cation scavenging.
![Correlations between antioxidant activity and total polyphenol content. [A] Closed circles [●] SC50 value of ABTS˙+ scavenging and open circles [○] represents rank total antioxidant capacity. The lower the SC50 or RC50 value on the y-axis, the higher the antioxidant potency. Rank total antioxidant capacity represents the cumulative mean of SC50 and RC50 values for a juice on different free radical models studied. The lower the rank number on the y-axis; the higher the total antioxidant capacity. Pearson r = (−)0.616, p < 0.05, R2 = 0.3794 for ABTS˙+. Pearson r = (−)0.8336, p < 0.01, R2 = 0.6923 for rank total antioxidant capacity. [B] Correlation between NBT reduction and total protein concentration in vegetable juices. Pearson r = (−)0.7608, p < 0.02, R2 = 0.5789. GAE; gallic acid equivalent, RC50; reducing concentration 50%.](/image/article/2011/FO/c1fo10093a/c1fo10093a-f3.gif) | ||
| Fig. 3 Correlations between antioxidant activity and total polyphenol content. [A] Closed circles [●] SC50 value of ABTS˙+ scavenging and open circles [○] represents rank total antioxidant capacity. The lower the SC50 or RC50 value on the y-axis, the higher the antioxidant potency. Rank total antioxidant capacity represents the cumulative mean of SC50 and RC50 values for a juice on different free radical models studied. The lower the rank number on the y-axis; the higher the total antioxidant capacity. Pearson r = (−)0.616, p < 0.05, R2 = 0.3794 for ABTS˙+. Pearson r = (−)0.8336, p < 0.01, R2 = 0.6923 for rank total antioxidant capacity. [B] Correlation between NBT reduction and total protein concentration in vegetable juices. Pearson r = (−)0.7608, p < 0.02, R2 = 0.5789. GAE; gallic acid equivalent, RC50; reducing concentration 50%. | ||
Effect of pretreatment of vegetable juice on starch induced postprandial glycemic excursion in rats
The two-hour postprandial glycemic load (AUC0–120 minutes) induced by starch feeding to the overnight fasted rats is presented in Fig. 4. It was observed that the pretreatment of rats with fresh vegetable juice moderated starch induced postprandial glycemic excursion. The juice of vegetables like ridge gourd, bottle gourd, ash gourd and chayote, however, significantly (p < 0.05) mitigated starch induced postprandial glycemic excursion. The moderation of starch induced postprandial glycemia in rats by brinjal juice was the lowest amongst the studied vegetable juices. We observed in our study a significant (p < 0.01) positive correlation between total polyphenol content in the vegetable juices and two-hour postprandial glycemic load (Fig. 5A) which shows that higher polyphenol contents may adversely affect postprandial antihyperglycemic activity of vegetable juices. These observations show that polyphenols may not be responsible for the antihyperglycemic effect exerted by the vegetable juices. On the other hand, a negative correlation pattern with higher protein concentration and AUC0–120 minute was noticed in our study (Fig. 5B). It appears, therefore, that the high protein concentration along with other macro/micro nutrients present in the vegetable juices other than polyphenols may be responsible for exhibiting antihyperglycemic activity.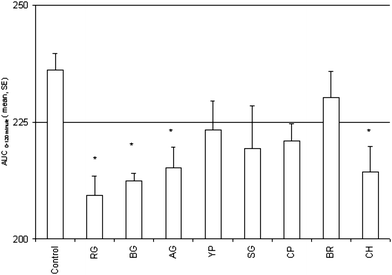 | ||
| Fig. 4 Starch induced postprandial glycemia (AUC0–120 minute)) and effect of vegetable juices in normal Wistar rats. One-way ANOVA followed by Dunnett's Multiple Comparison Test was applied to find differences between control and vegetable juices group of animals. Degree of significance (*) P < 0.05 when compared with control. Values represent mean ± Standard error, N = 6. Ash gourd (AG), yellow pumpkin (YP), snake gourd (SG), chayote (CH), ridge gourd (RG), bottle gourd (BG), cucumber (CP) and brinjal (BR). | ||
![Correlation between total polyphenols [A], and total protein [B] with postprandial glycemia (AUC0–120 minute). [A] Pearson r = 0.813, p < 0.01, R2 = 0.6603, [B] Pearson r = (−)0.2977, p = 0.2370, R2 = 0.0886.](/image/article/2011/FO/c1fo10093a/c1fo10093a-f5.gif) | ||
| Fig. 5 Correlation between total polyphenols [A], and total protein [B] with postprandial glycemia (AUC0–120 minute). [A] Pearson r = 0.813, p < 0.01, R2 = 0.6603, [B] Pearson r = (−)0.2977, p = 0.2370, R2 = 0.0886. | ||
SDS-PAGE protein fingerprint of vegetable juices
The SDS-polyacrylamide gel electrophoregram (Fig. 6) displayed unique and distinct protein band patterns for each vegetable juice. The lesser protein bands in cucumber and snake gourd may be due to less protein concentration in these vegetable juices. Yellow pumpkin presented clear multiple bands, however; ash gourd displayed a condensed heavy protein band close to 66.2 kDa. Juice of all the vegetables showed a clear matching protein band between 45.0 kDa to 66.2 kDa. However, the difference in protein bands and unique fingerprint for each vegetable juice presents their different identity.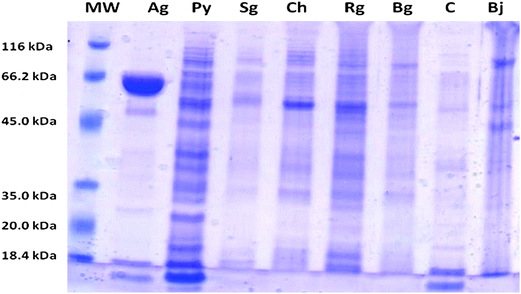 | ||
| Fig. 6 The SDS-PAGE protein fingerprint of individual vegetable's juice. Molecular weight marker (MW), Ash gourd (Ag), yellow pumpkin (Py), Snake gourd (Sg), Chayote (Ch), ridge gourd (Rg), Bottle gourd (Bg), cucumber (C) and brinjal (Bj). | ||
Discussion
In a biological system, multiple free radicals and oxidant systems have been identified and hence it has been equipped with multiple sources of antioxidants. The oxidants and antioxidants have different chemical and physical characteristics,42 it has been proposed that an individual antioxidant may, in some cases, act by multiple mechanisms in a single system,43 by a different single mechanism depending on the reaction system or may respond in a different manner to different radicals or oxidant sources.42 Because of these multiplicities involved in the characteristics as well as the mechanism of antioxidants, no single assay can optimally reflect the true characteristics of antioxidants in a mixture;24 therefore, we selected several free radical models and studied the radical scavenging capacity of vegetable juices.ABTS˙+ is soluble in both aqueous as well as organic solvents, hence has been used in multiple media to determine both the hydrophilic and lipophilic antioxidant capacity of plant materials.44DPPH˙ is an organic nitrogen radical and has been used to assess the reducing ability of antioxidants.42 It was observed that brinjal and bottle gourd's juice displayed the most potent ABTS˙+ scavenging activity, chayote and ash gourd being the less potent. However, only brinjal, ridge gourd, cucumber and bottle gourd's juice could reduce DPPH˙ (Table 2). The differences between the activities observed for scavenging these two free radicals may be due to the fact that many polyphenolic compounds with low redox potentials can react with ABTS˙+, however, they may react slowly or even be inert to DPPH˙ scavenging due to the steric inaccessibility.42 Such differences have also been observed by other researchers.24 This may be the reason some vegetable juices in this study could not display DPPH scavenging activity. The ferric chloride (FeCl3) reduction screening method measures the reduction of ferric ions into ferrous and detects a compound's reducing power. Therefore, the FeCl3 reduction method has been identified as a reasonable screen for identification of antioxidants that may possess the ability to maintain redox status in cells or tissues.42 Reducing power appears to be related to the degree of hydroxylation and extent of conjugation in polyphenols.45 Brinjal and cucumber juice displayed the most potent FeCl3 reducing activity (Table 2). NBT has been applied as a reagent for visual detection of ascorbic acid in plant leaves.38Ascorbic acid is a potent antioxidant and cellular reductant. Brinjal juice displayed the most potent NBT reducing activity; however ash gourd juice could not display this activity (Table 2).
Although vegetable juices may serve as a good source of many biologically active antioxidants,46 publications detailing the antioxidant capacity of vegetable juices are sparse.24 Consideration of the SC50 or RC50 value of individual vegetable juices and their chemical components (Table 1) makes it difficult to rank them in the order of their potency due to wide variations in activity levels. Therefore, in order to give an appropriate rank to a vegetable's juice in our study for one's overall antioxidant capacity, we ranked vegetables for each parameter depending on their SC50 or RC50 value. The lower the value of SC50 or RC50, the more potent the activity. Therefore the lowest SC50 or RC50 value possessing juice in each column was given rank one (Table 2). It was observed that brinjal possess the maximum total antioxidant capacity followed by bottle gourd, ridge gourd, yellow pumpkin, cucumber, chayote and snake gourd (Table 2). The total polyphenols displayed a significant correlation (p < 0.05) with the SC50 value of ABTS˙+ and with the rank total antioxidant capacity (p < 0.01) of vegetable juices (Fig. 3A). A significant correlation with total protein content and NBT reduction (p < 0.02) was also observed in our study (Fig. 3B).
H2O2 at micromolar concentrations is poorly reactive, however, it generates a hydroxyl radical (˙OH) in the presence of metal ions and oxygen. Therefore, H2O2 scavenging processes are important in living organisms.31,47 We observed that yellow pumpkin and chayote scavenged H2O2 more potently over the time than other juices. In our study, brinjal, cucumber and bottle gourd could not display H2O2 scavenging activity (Fig. 2).
A few vegetables like bitter gourd, ivy gourd and cabbage, have been claimed to possess antidiabetic activity.48 However, these vegetables are not juicy. Among these vegetables, bitter gourd has been extensively studied.49 Despite being potent antidiabetic vegetable, its bitter taste mars public preference and the risk of hypoglycemia warrants its cautious use.49 It is important to mention here that none of the vegetables selected in this study are bitter in taste.
Our study observed that pretreatment of rats with ridge gourd, bottle gourd, ash gourd and chayote juice significantly (p < 0.05) mitigated starch induced postprandial hyperglycemic excursion (Fig. 4). Analysis of the correlation between total polyphenol content and AUC0–120 minute revealed a significant positive correlation (p < 0.01) between total polyphenol content and glycemic excursion in animals (Fig. 5A). A negative non-significant correlation (Pearson r = −0.298, p < 0.237) between total protein content and AUC0–120 minute was also observed in this study (Fig. 5B).
High polyphenol concentration was responsible for high antioxidant activities in vegetable juices, the observation that high polyphenol contents may adversely affect postprandial glycemia in animals warrants their cautious use and selection of vegetable juice for hyperglycemic individuals. This study finds concurrence with earlier observation that antioxidant rich fruits30 or antioxidant rich food grain concentrates31 may adversely affect other metabolic parameters, like blood lipids and glucose levels. Although the effects of total or individual polyphenols on metabolic disorders is not very clear, a recent study observed that their consumption in high doses may affect adversely other physiological processes and warrants that more is not always better.50 Our study further highlights the timing of the consumption of antioxidant rich materials,28 as the eating order of vegetables with a meal has been reported to affect postprandial glycemic status in Japanese patients with type II diabetes.29
One of the major problems associated with natural products is the lack of the availability of suitable standardization tool. Lack of this tool increases malpractice of adulterations and also erroneous identification of a natural material. Based on molecular characteristics of electrophoretic protein fingerprints, it has become possible to gather information about genetic variations, taxonomic relationship, phylogenetic diversity and even identification of sub-species of a plant material, including vegetables.51–53 The unique banding pattern of the protein electrophoregram of a vegetable juice (Fig. 6) could therefore serve as an important supplemental tool that can provide passport data for its identification. This tool may serve the purpose of correct identification as well as proper standardization of vegetables and their juices.
This research shows that, regardless of variations in chemical compositions, free radical scavenging activities and total antioxidant capacity, vegetable juices may serve as a rich source of biological antioxidants. Our study reports, for the first time, potent antioxidant activities and significant postprandial antihyperglycemic activity in the juice of the vegetables ridge gourd, bottle gourd, ash gourd and chayote. Furthermore, we also provide the electrophoretic protein fingerprint of each vegetable juice that may serve the purpose of the correct identification of vegetables and standardization of the juice. The observations that total polyphenol content in vegetable juices finds significant correlation with an increase in total antioxidant capacity but adversely affect postprandial antihyperglycemic activity of the vegetable juices, warrants further research to revisit and define potential risk and health benefits of natural polyphenols. The most significant advantage of this research is that the juice of the reported vegetables possessing potent antioxidant activity and antihyperglycemic properties can be prepared at home by the common people and may serve as a readily available preventive measure for diet induced postprandial hyperglycemic excursion.
Acknowledgements
The authors thank Dr J. S. Yadav, Director, IICT, Hyderabad, for constant support, encouragements and personal support to carry out this research work. The authors also thank honorable reviewers for their constructive comments and suggestions in modifying this research manuscript. This research was supported financially in part by project grant NWP-004 (CSIR-New Delhi). The authors declare no conflict of interest, financial or otherwise.References
- J. E. Shaw, R. A. Sicree and P. Z Zimmet, Diabetes Res. Clin. Pract., 2010, 87, 4–14 CrossRef CAS.
- S. Blum, M. Aviram, A. Ben-Amotz and Y. Levy, Ann. Nutr. Metab., 2006, 50, 20–24 CrossRef CAS.
- A. Ceriello, R. Assaloni, R. D. Ros, A. Maier, L. Piconi, L. Quagliaro, K. Espositi and D. Giugliano, Circulation, 2005, 111, 2518–2524 CrossRef CAS.
- F. Jakulj, K. Zernicke, S. L. Bacon, L. E. VanWielingen, B. L. Key, S. G. West and T. S. Campbell, J. Nutr., 2007, 137, 935–939 CAS.
- J. E. Gerich, Horm. Metab. Res., 1996, 28, 404–412 CrossRef CAS.
- J. Davidson, Diabetes Care, 2003, 26, 1919–1921 CrossRef.
- A. Ceriello, Diabetes Metab. Res. Rev., 2000, 16, 125–132 CrossRef CAS.
- A. Ceriello, Diabetes Vasc. Dis. Res., 2008, 5, 260–268 CrossRef.
- M. L. Burden, A. Samanta, D. Spalding and A. C. Burde, Pract. Diabetes Int., 1994, 11, 208–211 CrossRef.
- A. Misra and L. Khurana, Int. J. Obes., 2011, 35, 167–187 CrossRef CAS.
- S. Dickinson, E. Colagiuri, P. Faramus Petocz and J. C. Brand-Miller, J Nutr., 2002, 132, 2574–2579 CAS.
- A. T. Merchant, S. S. Anand, L. E. Kelemen, V. Vuksan, R. Jacobs, B. Davis, K. Teo and S. Yusuf, Am. J. Clin. Nutr., 2007, 85, 225–230 CAS.
- Z. Milicevic, I. Raz, S. D. Beattie, B. N. Campaigne, S. Sarwat, E. Gromniak, I. Kowalska, E. Galic, M. Tan and M. Hanefeld, Diabetes Care, 2008, 2, 155–160 CrossRef.
- C. N. C. Juliana, M. W. J. Vasanti, K. Takashi, S. Y. Chittaranjan, K.-H. Yoon and F. B. Hu, JAMA, J. Am. Med. Assoc., 2009, 301, 2129–2140 CrossRef.
- J. H. O'Keefe, N. M. Gheewala and J. O. O'Keefe, J. Am. Coll. Cardiol., 2008, 51, 249–255 CrossRef CAS.
- M. Brownlee and I. Hirsch, JAMA, J. Am. Med. Assoc., 2006, 295, 1707–1708 CrossRef CAS.
- D. Giugliano, A. Ceriello and G. Paolisso, Diabetes Care, 1996, 19, 257–267 CAS.
- M. Brownlee, Nature, 2001, 414, 813–820 CrossRef CAS.
- Editorial, Lancet, 2010, 375, 2193 CrossRef.
- P. N. Mitrou, V. Kipnis, A. C. M. Thiebaut, J. Reedy, A. F. Subar, E. Wirfält, A. Flood, T. Mouw, A. R. Hollenbeck, M. F. Leitzmann and A. Schatzkin, Arch. Intern. Med., 2007, 167, 2461–2468 CrossRef.
- S. Liu, J. E. Manson, I. M. Lee, S. R. Cole, C. H. Henneken, W. C. Willet and J. E. Burning, Am. J. Clin. Nutr., 2000, 72, 922–928 CAS.
- A. Martin, A. Cherubini, C. Andres-Lacueva, M. Paniagua and J. A. Joseph, J. Nutr., Health Aging, 2002, 6, 392–404 CAS.
- S. Wang, J. P. Melnyk, R. Tsao and M. F. Marcone, Food Res. Int., 2011, 44, 14–22 CrossRef CAS.
- P. C. Wooton-Beard, A. Moran and L. Ryan, Food Res. Int., 2011, 44, 217–214 CrossRef.
- K. R. Martin and C. L. Appel, Nutr. Diet. Suppl., 2010, 2, 1–12 CAS.
- J. Serrano, A. Cipak, J. Boada, H. Gonzalo, D. Cacabelos, A. Cassanye, R. Pamplona, N. Zarkovic and M. Portero-Otin, Acta Biochim. Polonica, 2010, 57, 193–198 CAS.
- J. Bouayed and T. Bohn, Oxid. Med. Cell. Longevity, 2010, 3, 228–237 CrossRef.
- S. Diano, Forefront (The American Diabetes Association's Research Magazine), 2009, 6, 26 Search PubMed.
- S. Imai and S. Kajiyama, J. Rehab. Health Sci., 2010, 8, 1–7 Search PubMed.
- E. Alvarez-Parrilla, L. A. L. Rosa, P. Legarreta, L. Saenz, J. Rodrigo-Garcia and G. A. Gonzalez-Agular, Int. J. Food Sci. Nutr., 2010, 61, 369–380 CrossRef CAS.
- A. K. Tiwari, M. Swapna, S. B. Ayesha, A. Zehra, S. B. Agawane and K. Madhusudana, Int. Food Res. J., 2011, 18(3), 915–923 Search PubMed.
- M. M. Bradford, Anal. Biochem., 1976, 72, 248–254 CrossRef CAS.
- Y. Yao, W. Sang, M. Zhou and G. Ren, J. Agric. Food Chem., 2010, 58, 770–774 CrossRef CAS.
- C. Y. Hsieh and S. T. Chang, J. Agric. Food Chem., 2010, 58, 1578–1583 CrossRef CAS.
- M. M. Giusti, R. Rodriguez-Saona and R. E. Wrolstad, J. Agric. Food Chem., 1999, 47, 4631–4637 CrossRef CAS.
- R. B. Walker and J. D. Everette, J. Agric. Food Chem., 2009, 57, 1156–1161 CrossRef CAS.
- T. Yamaguchi, H. Takamura, T. Matoba and J. Terao, Biosci., Biotechnol., Biochem., 1998, 62, 1201–1204 CrossRef CAS.
- P. L. Concklin, S. A. Saracco, S. R. Norris and R. L. Last, Genetics, 2000, 154, 847–856 Search PubMed.
- P. Arumugam, R. Murugan, M. Subathra and A. Ramesh, Med. Chem. Res., 2010, 19, 664–673 CrossRef CAS.
- M. Wettasinghe and F. Shahidi, J. Agric. Food Chem., 1999, 47, 1801–1812 CrossRef CAS.
- B. C. Raju, A. K. Tiwari, J. A. Kumar, A. Z. Ali, S. B. Agawane, G. Saidachary and K. Madhusudhana, Bioorg. Med. Chem., 2010, 18, 358–365 CrossRef CAS.
- R. I. Prior, X. Wu and K. Schaich, J. Agric. Food Chem., 2005, 53, 4290–4302 CrossRef CAS.
- K. Ishige, D. Schubert and Y. Sagara, Free Radical Biol. Med., 2001, 30, 433–446 CrossRef CAS.
- J. M. Awika, L. W. Rooney, X. Wu, R. L. Prior and L. Cisneros-Zevallos, J. Agric. Food Chem., 2003, 51, 6657–6662 CrossRef CAS.
- R. Puildo, L. Bravo and F. Saura-Calixo, J. Agric. Food Chem., 2000, 48, 3396–3402 CrossRef.
- I. Kiefer, P. Prock, C. Lawrence, J. Wise, W. Bieger, P. Bayer, T. Rathmanner, M. Kunze and A. Rieder, J. Am. Coll. Nutr., 2004, 23(3), 205–211 Search PubMed.
- O. I. Auroma, J. Am. Oil Chem. Soc., 1998, 75, 199–212 CrossRef.
- K. Patel and K. Srinivasan, Nahrung, 1997, 41, S, 68–74 CrossRef.
- A. K. Tiwari, Curr. Sci., 2007, 92, 1697–1701 CAS.
- R. C. Khanal, T. J. Rogers, S. E. Wilkes, L. R. Howard and R. L. Prior, Food Funct., 2010, 1, 116–123 CAS.
- I. Emre, D. Turgut-Balik and A. Sahin, Pak. J. Biol. Sci., 2007, 10, 2890–2894 CrossRef CAS.
- I. Emre, D. Turgut-Balik, A. Sahin and M. Kursat, Am.–Eurasian J. Agric. Environ. Sci., 2007, 2, 123–126 Search PubMed.
- E. A. A. A. El-Hady, A. A. A. Haiba, N. R. A. El-Hamid and A. A. Rizkalla, J. Am. Sci., 2010, 6, 434–441 Search PubMed.
Footnote |
| † The authors dedicate this research work to Dr J. Madhusudan Rao, Natural Products Researcher, on the eve of his 60th birthday. |
| This journal is © The Royal Society of Chemistry 2011 |
