Crosslinking of diene-modified DNA with bis-maleimides†
Rolf
Tona
and
Robert
Häner
*
Department of Chemistry, University of Bern, CH-3012 Bern, Switzerland. E-mail: robert.haener@ioc.unibe.ch; Tel: +4131 631 4382
First published on 8th March 2005
Abstract
The chemical crosslinking of modified nucleic acids via the Diels–Alder reaction is reported. For this purpose, 1,3-butadiene derived building blocks were incorporated into complementary oligodeoxynucleotides. Treatment of the obtained duplex with difunctional dienophiles results in the clean crosslinking of the two strands. Non-crosslinked adducts arising from a single Diels–Alder reaction of a maleimide to only one strand were not observed, indicating that the first reaction is the rate determining step of the overall process. Based on their thermal denaturation profiles, the crosslinked hybrids behave like two separate, hairpin-like structures, rather than like a single, continuous duplex.
Introduction
The construction of nucleic acid-based molecular structures is a rapidly growing field of supramolecular chemistry.1–5 Due to the simple, yet highly selective, repetitive base pairing pattern, nucleic acids are used for the assembly of nanometer-sized structures.6,7 On the other hand, the geometry of perfectly paired, double stranded DNA or RNA possesses a very well defined geometry. Deviations from this predefined structure towards a more general molecular architecture are possible by a range of manipulations, such as introducing bulged, non-canonical or mismatched nucleotides.8 Alternatively, the structural and functional behaviour of nucleic acids can be influenced by chemical crosslinking. The crosslinking of nucleic acids can be accomplished e.g. through the photo-induced dimerisation of nucleobases9,10 or the use of multifunctional reagents.11 The formation of crosslinks via disulfide bridges at predetermined sites has been applied to the structural and functional study of RNA,12–16 as well as DNA.17 In a series of papers, Glick and coworkers have reported the design, synthesis and analysis of disulfide crosslinked nucleic acids.18–21 More recently, the control of a double helical DNA assembly by the use of disulfide crosslinks22,23 or a difunctional maleimide electrophile24 has been reported by Endo and Majima.A further possibility for the crosslinking of suitably modified nucleic acids consists in the Diels–Alder reaction. Such a method would provide a way of crosslinking, which is orthogonal to the existing procedures. The Diels–Alder reaction has been applied to the derivatisation25,26 and immobilisation27,28 of nucleic acids. Thus, it was shown that oligonucleotides bearing a 5′-linked diene moiety react site-specifically with dienophiles without interference of the many functional groups present in RNA25 and DNA.26,27,29,30 Leuck and coworkers presented a method for the covalent attachment of diene and maleimide functionalized oligonucleotides to a variety of glass surfaces via the Diels–Alder reaction under particularly mild conditions.28 They showed that the method is compatible with the presence of other chemical functionalities. Despite its established chemoselectivity, however, the Diels–Alder reaction has hitherto not found widespread use for the purpose of DNA modification or derivatisation. We recently reported the derivatisation of a diene modified hairpin mimic via the Diels–Alder reaction.31 A variety of functional residues was attached to the modified nucleic acids by reaction with maleimide dienophiles carrying different pendant groups. We have subsequently extended these studies to the investigation of interstrand crosslinking reactions between diene-modified nucleic acids. Here, we wish to report the scope of this method for crosslinking complementary oligonucleotide strands with difunctional maleimide dienophiles.
Results and discussion
The required diene-building blocks 1–3† were synthesised according to the previously published procedure.31 They contain linkers of different lengths, which are connected via ether bonds to the diene-moiety (Scheme 1). Linkers of different lengths were chosen in order to introduce different degrees of flexibility in the modified duplex, which was expected to play an important role in the crosslinking reaction. The different diene-building blocks were integrated into the oligomers Am and Bm (m denotes the number of methylene units in the linkers). The dienes were placed in positions opposite to each other. The unmodified oligonucleotides 4 and 5 were used as reference strands. All oligonucleotides were purified by reverse phase HPLC and characterised by mass spectrometry.†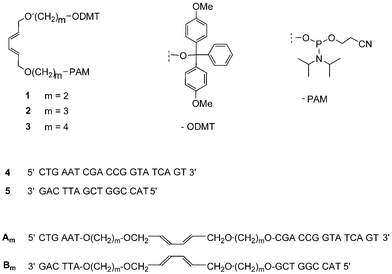 | ||
| Scheme 1 Phosphoramidite building blocks 1–3 used for the synthesis of the diene-modified oligomers Am and Bm. Oligonucleotides 4 and 5 served as controls. | ||
We first investigated the influence of the diene building blocks on the pairing properties of the modified strands by thermal denaturation experiments. All duplexes containing dienes were found to have strongly reduced stabilities. Thus, A2*B2, A3*B3 and A4*B4, containing identical diene-building blocks in the two strands, as well as the duplex A2*B4, having one diene with two-carbon linkers and the other diene with four-carbon linkers had Tm values between 27.5 and 33.5 °C, compared to a Tm of 49.5 °C obtained for the unmodified reference duplex 4*5 (Table 1). The melting curves of the corresponding duplexes are shown in Fig. 1. All curves show sigmoidal shapes with one transition. Destabilizations of these dimensions indicate that the diene-modifications do not support a continuous duplex. The observed melting temperatures correspond quite well with the estimated Tm value of the unmodified longer partial duplex with nine base pairs (approximately 30 °C). The transition of the shorter stem (six base pairs), with an estimated Tm between 15 and 20 °C, was not observed. The modified strands form, therefore, a 9mer duplex. The diene moieties and the further six nucleotides are attached to this duplex stem as random coils.
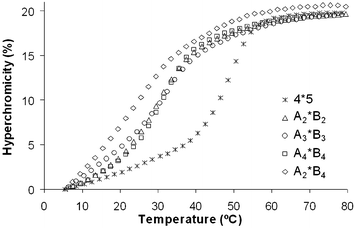 | ||
| Fig. 1 Melting curves of the unmodified (4*5) duplex and the diene-modified (Am*Bm) hybrids. Conditions: 1.0 µM duplex in 10 mM Tris-HCl (pH 4.2) and 100 mM NaCl. | ||
In a next step, the crosslinking reaction between the diene-modified duplexes and dienophiles was investigated. For this purpose, the bis-maleimides 6–8 were prepared according to literature procedures.32,33 The expected crosslinking process is illustrated in Scheme 2.
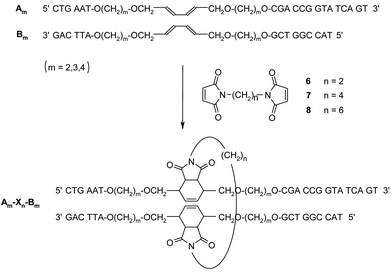 | ||
| Scheme 2 Schematic illustration of the crosslinking reaction between diene-modified duplexes Am*Bm with the bis-maleimides 6–8. Am–Xn–Bm denotes the crosslinked derivatives obtained after the Diels–Alder reaction. | ||
The crosslinking process was monitored by melting curve experiments. We first recorded the melting curve of a solution of duplex A2*B2, in 10 mM Tris-HCl (pH 4.2) and 100 mM NaCl. Straight after adding two equivalents of dimaleimide 8, a second melting curve was recorded, which was congruent with the first one. The solution was then kept at 25 °C and additional melting curves were taken after three days and one week. The different stages of the crosslinking reaction are shown in Fig. 2. The melting curves reveal a gradual transition from a duplex with low stability to the crosslinked product. The curve taken after three days shows the presence of both, non-crosslinked (lower transition) and crosslinked material. After seven days, only the crosslinked duplex could be observed in this qualitative assay.
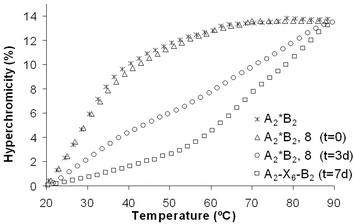 | ||
| Fig. 2 Different stages of the crosslinking experiment of the duplex A2*B2 with the dimaleimide 8 monitored by UV denaturation curves. Reaction times are indicated in days. Conditions: 1.0 µM duplex in 10 mM Tris-HCl (pH 4.2) and 100 mM NaCl. | ||
The reaction mixture was further analysed by ESI-mass spectrometry. After one week at 25 °C, a sample was desalted but not further purified. Fig. 3 shows two peaks corresponding to minor quantities of the two single strands A2 and B2, and the mass of the crosslinked duplex A2–X6–B2. An interesting aspect is the absence of peaks with the mass corresponding to either one of the single strands reacted with the dimaleimide without subsequent crosslinkage. Since the reactions were carried out in the presence of an excess of dimaleimide, these products might well be expected. The absence of any peaks in this mass range, however, indicates that the Diels–Alder reaction of the dimaleimide with one of the two strands is the slow, rate-limiting step while the subsequent step, the crosslinking reaction with the complementary strand, is relatively fast.
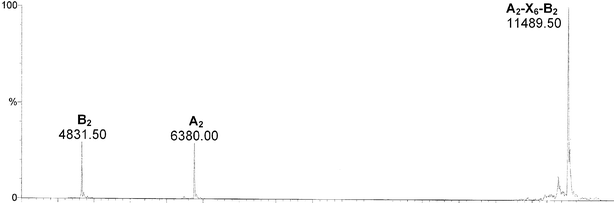 | ||
| Fig. 3 ESI-MS of the crude material obtained from the crosslinking reaction of the duplex A2*B2 with the dimaleimide 8. | ||
After establishing the approximate reaction time required for this type of crosslinking reaction, an extended study involving the diene-modified duplexes An*Bn with the maleimides 6–8 was performed. Each of the different modified duplexes were incubated under the conditions described above with two equivalents of the different dimaleimides. After one week of incubation, the solutions were desalted and analysed by denaturating polyacrylamide gel electrophoresis (PAGE, Fig. 4). In each of the reactions, light bands of unreacted An and Bn (15 and 20 nucleotides excluding the diene building block) can be seen. Furthermore, an additional band, which corresponds to the respective crosslinked products, can be seen in all reactions. In the reaction of A2*B2 with the dimaleimide 7 (lane 3), a further additional band with slightly higher mobility than the crosslinked duplex is observed. Mass spectrometrical analysis of this reaction showed only the masses of the educts and the crosslinked product (data not shown). We have, at present, no firm explanation for the existence of two species of the same mass with different electrophoretic mobility. Additional PAGE experiments showed that all purified crosslinked products exhibited a single band.†
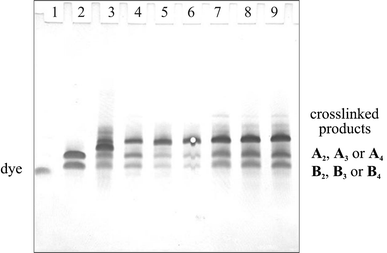 | ||
| Fig. 4 12% Denaturing polyacrylamide gel electrophoresis of the reaction products obtained after treatment of the duplexes An*Bn with 2 equivalents of bis-maleimides 6–8. Lanes: (1) bromophenol blue, (2) A3+B3, (3) A2+B2+7, (4) A3+B3+6, (5) A3+B3+7, (6) A3+B3+8, (7) A4+B4+6, (8) A4+B4+7, (9) A4+B4+8. Bands were visualized using stains-all™ reagent. | ||
The crude materials obtained from the crosslinking reactions were then purified with reverse phase HPLC and further analysed by thermal denaturation expeiments. A strong increase of the melting temperatures was observed after crosslinking of the diene modified duplexes Am*Bm with each of the three bis-maleimides 6–8 (Table 2). Most of the melting curves showed two distinct transitions. Only in the case of A2–X2–B2 and A2–X4–B2 just one transition could be observed. We think, however, that two transitions exist also in these two cases but that they are not resolved (see below). In all cases, where the two transitions can be identified as separate events, the first one occurs at a Tm between 60 and 68 °C and the second one takes place in the range of 78 to 84 °C. As a representative set, the thermal denaturation curves of the crosslinked hybrids formed by reaction of the duplex A4*B4 with the three bis-maleimides 6–8 are shown in Fig. 5.
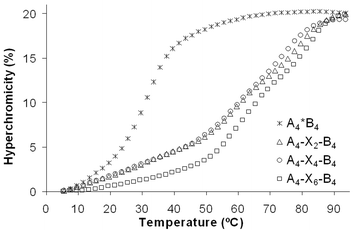 | ||
| Fig. 5 Melting curves of the 1,4-butanediol linked, bis-diene-modified duplex A4*B4 and the crosslinked duplexes obtained after the reaction with the bis-maleimides 6–8. Conditions: 1.0 µM duplex in 10 mM Tris-HCl (pH 4.2) and 100 mM NaCl. | ||
| Duplex | T m/°C | T m of Am–Xn–Bm obtained after reaction with dienophiles 6–8/°C | ||
|---|---|---|---|---|
| 6 (n = 2) | 7 (n = 4) | 8 (n = 6) | ||
| Conditions: oligomer concentration 2.5 µM, 10 mM Tris-HCl, 100 mM NaCl, pH 4.2; temp. gradient: 0.5 °C min−1. | ||||
| 4*5 | 49.5 | — | — | — |
| A2*B2 (m = 2) | 30.5 | 59.5 | 75.5 | 67.5/83.5 |
| A3*B3 (m = 3) | 32.5 | 60.5/78.5 | 60.5/78.5 | 63.5/80.5 |
| A4*B4 (m = 4) | 33.0 | 59.5/80.5 | 59.5/76.5 | 63.5/82.5 |

|
||||
The existence of two transitions indicates that the crosslinked, complementary oligonucleotides do not form a coherent, continuous duplex with a modified building block in the middle of the stem. Rather, they behave like two interconnected hairpin-like structures forming separate secondary structures of different stabilities. Thus, the two Tm's most likely correspond to the denaturation of the shorter and the longer hairpin-like structures. We assume that in the crosslinked duplexes A2–X2–B2 and A2–X4–B2 the analogous two transitions are simply not resolved. Therefore, in these two cases only one apparent transition with an average Tm is observed. The highest Tm's are observed after the crosslinkage with the dienophile 8, which has the longest linker (six methylene units) connecting the two maleimides. The length of the linkers flanking the original diene moieties has less influence.
Based on the data obtained, a model of the crosslinked product A3–X2–B3 was calculated.34Fig. 6 shows the Amber-minimized structure of the two interconnected hairpin-like structures. The scaffold connecting the two duplex arms was optimised separately. It was derived on the basis of two Diels–Alder reactions following the commonly preferred endo-pathway.35 After attaching the two B-form duplexes, the overall structure was again minimised. The bis-hairpin structure is shown in the view perpendicular to the helical axis. The overall hybrid is almost perfectly linear, i.e. the two duplex parts—separated by the Diels–Alder product—share a common helical axis. This is most likely due to the fact that the crosslinking reactions were performed with duplexes carrying identical (and symmetrical) diene-building blocks in the two strands. It is possible that non-linear structures might arise from the use of mixed duplexes (e.g.A2*B4) or duplexes containing unsymmetrical dienes.
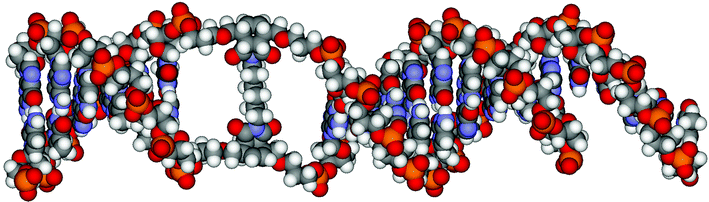 | ||
| Fig. 6 Optimised structures (HyperChem™ 7.0, amber force field) of the crosslinked product A3–X2–B3 consisting of a short stem (6 base pairs, on the left), the region of the crosslink formed by the Diels–Alder reactions and the longer stem to the right (9 base pairs plus a 3′-overhang). | ||
In conclusion, we have shown the synthesis of diene-modified duplexes and their further crosslinking with bis-maleimides. The crosslinking of complementary DNA strands via the Diels–Alder reaction provides a method, which is orthogonal to other existing procedures. The method is simple and robust, since the diene-building blocks are incorporated during automated oligonucleotide synthesis and require no additional deprotection or activation steps. The introduction of diene building blocks occupying opposite positions in complementary strands leads to a significant reduction in hybrid stability. The modified hybrids undergo Diels–Alder reaction with bis-maleimides to give the corresponding crosslinked products. The crosslinking reaction proceeds in a very clean albeit rather slow way. Single adducts of maleimides to one strand were not observed indicating that the first Diels–Alder reaction is rate determining. Based on thermal denaturation experiments, the crosslinked hybrids behave like two separate, hairpin-like structures, rather than like a modified, but coherent duplex.
Experimental
General
All common reagents were purchased from Fluka, Sigma-Aldrich or Acros and were used without further purification. Reactions were monitored by thin layer chromatography (TLC, Machery-Nagel pre-coated TLC plates SIL G-25 UV254) using mixtures of ethylacetate–hexane or dichloromethane–methanol. Compounds were visualized with UV light and/or dipping in permanganate or cerium(IV)-reagent followed by heating. Common work-up consisted of sequential washing of the organic extract with water and brine, followed by drying over anhydrous sodium sulfate and evaporation under reduced pressure. Column chromatography for purification of compounds was performed on silica gel (sds, SILICE 60 A C.C 40–63 µm). For tritylated compounds, TLC-plates and silica gel columns were prepared with solvents containing 0.1% triethylamine. Melting points were recorded using a Büchi 510 apparatus (open capillaries, uncorrected values). 1H (300 Hz), 13C (75 Hz) NMR were recorded on a Bruker AC 300 and fitted with 1D WINNMR and the chemical shifts (δ/ppm) are referred to chloroform-d for 1H and 13C NMR. Mass spectra were recorded on a VG platform mass spectrometer, Micromass Instruments, Manchester, UK.Preparation of the DNA oligomers
The DNA oligomers were assembled on cpg solid supports (Transgenomic, Glasgow, UK) using an ABI 392 or an ABI 394 Nucleic Acid Synthesizer from Applied Biosystems. Standard reagents and solvents for the phosphotriester method were used. Programs for 40 nmol, 0.2 µmol and 1.0 µmol DNA synthesis from Applied Biosystems were used. The coupling time for modified phosphoramidites was extended to 5 min.The DNA oligomers were cleaved from the solid support using 25% aqueous ammonia hydroxide and then deprotected in the same solution at 55 °C for 15 h. After filtration through a 0.45 µm nylon syringe filter, the crude materials were purified with reverse phase HPLC (RP-HPLC) using a LiChroCART 250–4 column from Merck (A: 0.1 M triethyl ammonium acetate in water, B: acetonitrile) at 40 °C. The dried oligonucleotides were desalted with Sep-Pak® Cartridges (Waters) and analysed with ESI-MS and denaturating polyacrylamide gel electrophoresis (PAGE) [Mini-PROTEAN 3 electrophoresis module (Bio-Rad Laboratories), 0.75 mm thick 12% polyacrylamide, 1× TBE (Tris–Borate–EDTA) and 10 M urea, 1× TBE as electrolyte]. Oligonucleotide concentrations were calculated from the absorbance at 260 nm with extinction coefficients calculated according to the program provided at http://paris.chem.yale.edu/extinct.html. For diene-modified and crosslinked oligonucleotides, extinction coefficients of the respective unmodified sequences were used. Pure oligonucleotides were lyophilised and stored at −30 °C.
Crosslinking reactions
A mixture of equal concentrations (approximately 1 µM) of complementary diene-modified oligodeoxyribonucleotides was incubated in 10 mM Tris-HCl (pH 4.2) with 2 equivalents of the bis-maleimide derivatives at room temperature for 7 days. The reaction mixtures were desalted via Sep-Pak® Cartridges (Waters), dried and dissolved in distilled water for the PAGE- and MS-experiments. The crude reaction mixtures were further purified via RP-HPLC [column: LiChroCART® 250–4 (Merck), solvents: A: 0.1 M triethylammonium acetate in H2O (pH 6.5), B: acetonitrile, gradient: 0–35%B in 40 min, retention times: Bm at 13 min (12%B); Am at 17 min (15%B); Am–Xn–Bm at 43 min (35%B)] and desalted via Sep-Pak® Cartridges (Waters) for the UV melting studies.UV melting studies
UV melting curves were recorded on a Cary 3E UV/VIS Spectrophotometer (Varian) equipped with a Cary Temperature Controller, a Sample Transport Accessory and a Multi Cell Block. Aqueous solutions of oligomer (2.5 µM), Tris-HCl (10 mM, pH 4.2) and NaCl (100 mM) were used. The strands were allowed to anneal by heating at 85 °C for 1–2 min, followed by slow cooling to room temperature. The samples were heated at a rate of 0.5 °C min−1 and the absorbance at 260 nm was plotted every 1 min. The percent hyperchromicity at 260 nm was plotted as a function of temperature. Melting temperatures were determined from the maximum of the first derivative of the melting curve (A260 against temperature); each Tm is the average of three independent experiments; experimental error: ±0.5 °C.General method for the preparation of dimaleimides 6–832,33
The diamine in absolute CH2Cl2 (1 ml mmol−1) was added to maleic acid anhydride (2.2 equiv.) in absolute CH2Cl2 (1 ml mmol−1) and heated to 50 °C for 1 h. After evaporation of the solvent, acetic anhydride (20 ml mmol−1) and sodium acetate (1 g mmol−1) were added. The suspension was heated to 80 °C for 3 h; after filtration, the solvent was evaporated under reduced pressure.N,N′-Ethylenedimaleimide (6)
Synthesized according to the general method from ethane-1,2-diamine and maleic acid anhydride. The crude material was purified by silica chromatography (EtOAc–Hex, 1 ∶ 1). Yield: 15%, white solid. 1H-NMR (CDCl3) δ: 3.73(s, 4 H), 6.68 (s, 4 H). 13C-NMR (CDCl3) δ: 36.50, 134.17, 170.48. A sample was recrystallised from EtOAc; mp: 196 °C; ESI-MS (pos.): 220 (11%, C10H8N2O4, calc; 220.18).N,N′-Tetramethylenedimaleimide (7)
Synthesized according to the general method from butane-1,4-diamine and maleic acid anhydride. The crude material was purified by silica chromatography (EtOAc–Hex, 1 ∶ 1). Yield: 51%, white solid. 1H-NMR (CDCl3) δ: 1.53–1.58 (m, 4 H), 3.47–3.54 (m, 4 H), 6.66 (s, 4 H). 13C-NMR (CDCl3) δ: 25.76, 37.17, 134.10, 170.74. A sample was recrystallised from EtOAc; mp: 206 °C; ESI-MS (pos.): 248 (15%; C12H12N2O4, calc. 248.24).N,N′-Hexamethylenedimaleimide (8)
Synthesized according to the general method from hexane-1,6-diamine and maleic acid anhydride. The crude material was purified by silica chromatography (EtOAc–Hex, 1 ∶ 1). Yield: 60%, white solid. 1H NMR (CDCl3) δ: 1.20–1.29 (m, 4 H), 1.50–1.59 (m, 4 H), 3.47 (t, 4 H, J = 7.3 Hz), 6065 (s, 4 H). 13C NMR (CDCl3) δ: 26.15, 28.32, 37.67, 134.03, 170.84. A sample was recrystallised from EtOAc; mp: 144 °C; ESI-MS (pos.): 267 (18%; C14H16N2O4, calc. 276.29).Financial support of this project by the Swiss National Foundation (grant 31-63380.00) is gratefully acknowledged.
References
- C. A. Mirkin, Inorg. Chem., 2000, 39, 2258–2272 CrossRef CAS.
- C. M. Niemeyer, Angew. Chem. Int. Ed. Engl., 2001, 40, 4128–4158 CrossRef CAS.
- N. C. Seeman, Nature, 2003, 421, 427–431 CrossRef.
- A. I. H. Murchie and D. M. J. Lilley, Methods Enzymol., 1992, 211, 158–180 CrossRef CAS.
- Y. G. Li, Y. D. Tseng, S. Y. Kwon, L. D'Espaux, J. S. Bunch, P. L. Mceuen and D. Luo, Nat. Mater., 2004, 3, 38–42 CrossRef CAS.
- J. Wengel, Org. Biomol. Chem., 2004, 2, 277–280 RSC.
- R. Bashir, Superlattices Microstruct., 2001, 29, 1–16 CrossRef CAS.
- T. Hermann and D. J. Patel, J. Mol. Biol., 1999, 294, 829–849 CrossRef CAS.
- L. S. Behlen, J. R. Sampson and O. C. Uhlenbeck, Nucleic Acids Res., 1992, 20, 4055–4059 CrossRef CAS.
- D. E. Bergstrom and N. J. Leonard, Biochemistry, 1972, 11, 1–& CrossRef CAS.
- S. R. Rajski and R. M. Williams, Chem. Rev., 1998, 98, 2723–2795 CrossRef CAS.
- M. Y. X. Ma, L. S. Reid, S. C. Climie, W. C. Lin, R. Kuperman, M. Sumnersmith and R. W. Barnett, Biochemistry, 1993, 32, 1751–1758 CrossRef CAS.
- M. Y. X. Ma, K. Mccallum, S. C. Climie, R. Kuperman, W. C. Lin, M. Sumnersmith and R. W. Barnett, Nucleic Acids Res., 1993, 21, 2585–2589 CrossRef CAS.
- D. J. Fu, F. Benseler and L. W. Mclaughlin, J. Am. Chem. Soc., 1994, 116, 4591–4598 CrossRef CAS.
- C. R. Allerson and G. L. Verdine, Chem. Biol., 1995, 2, 667–675 CrossRef CAS.
- S. T. Sigurdsson, T. Tuschl and F. Eckstein, RNA, 1995, 1, 575–583 CAS.
- J. Milton, B. A. Connolly, T. T. Nikiforov and R. Cosstick, Chem. Commun., 1993, 779–780 RSC.
- G. D. Glick, Biopolymers, 1998, 48, 83–96 CrossRef CAS.
- R. J. Cain, E. R. Zuiderweg and G. D. Glick, Nucleic Acids Res., 1995, 23, 2153–2160 CrossRef CAS.
- R. J. Cain and G. D. Glick, Nucleic Acids Res., 1997, 25, 836–842 CrossRef CAS.
- J. T. Goodwin, S. E. Osborne, E. J. Scholle and G. D. Glick, J. Am. Chem. Soc., 1996, 118, 5207–5215 CrossRef CAS.
- M. Endo and T. Majima, Angew. Chem. Int. Ed. Engl., 2003, 42, 5744–5747 CrossRef CAS.
- M. Endo and T. Majima, J. Am. Chem. Soc., 2003, 125, 13654–13655 CrossRef CAS.
- M. Endo and T. Majima, Chem. Commun., 2004, 1308–1309 RSC.
- B. Seelig and A. Jaschke, Tetrahedron Lett., 1997, 38, 7729–7732 CrossRef CAS.
- K. W. Hill, J. Taunton-Rigby, J. D. Carter, E. Kropp, K. Vagle, W. Pieken, D. P. C. Mcgee, G. M. Husar, M. Leuck, D. J. Anziano and D. P. Sebesta, J. Org. Chem., 2001, 66, 5352–5358 CrossRef CAS.
- G. M. Husar, D. J. Anziano, M. Leuck and D. P. Sebesta, Nucleosides Nucleotides Nucleic Acids, 2001, 20, 559–566 CrossRef CAS.
- H. A. Latham-Timmons, A. Wolter, J. S. Roach, R. Giare and M. Leuck, Nucleosides Nucleotides Nucleic Acids, 2003, 22, 1495–1497 CrossRef CAS.
- D. Graham, A. Grondin, C. McHugh, L. Fruk and W. E. Smith, Tetrahedron Lett., 2002, 43, 4785–4788 CrossRef CAS.
- L. Fruk, A. Grondin, W. E. Smith and D. Graham, Chem. Commun., 2002, 2100–2101 RSC.
- R. Tona and R. Häner, Chem. Commun., 2004, 1908–1909 RSC.
- P. Kovacic and R. W. Hein, J. Am. Chem. Soc., 1959, 81, 1187–1190 CrossRef CAS.
- J. C. Cheronis, E. T. Whalley, K. T. Nguyen, S. R. Eubanks, L. G. Allen, M. J. Duggan, S. D. Loy, K. A. Bonham and J. K. Blodgett, J. Med. Chem., 1992, 35, 1563–1572 CrossRef CAS.
- HyperChem(TM), Hypercube, Inc., 1115 NW 4th Street, Gainesville, Florida 32601, USA.
- I. Fleming, Frontier Orbitals and Organic Chemical Reactions, John Wiley & Sons, 1980 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: experimental methods, NMR data, mass spectra of the oligonucleotides and results from analyses of the cross-linked products. See http://www.rsc.org/suppdata/mb/b4/b418502a/ |
| This journal is © The Royal Society of Chemistry 2005 |
