Scandium triflate-catalyzed selective ring opening and rearrangement reaction of spiro-epoxyoxindole and carbonyl compounds†
Hui Jiangab,
Haohua Jiec and
Jian Li*c
aState Key Laboratory of Electronic Thin Films and Integrated Devices, University of Electronic Science and Technology of China, No. 4 Second Section Jianshe North Road, Chengdu 610054, P. R. China
bDepartment of Applied Chemistry, University of Electronic Science and Technology of China, No. 4 Second Section Jianshe North Road, Chengdu 610054, P. R. China
cDepartment of Chemistry, Innovative Drug Research Center, Shanghai University, 99 Shangda Road, Shanghai 200444, P. R. China. E-mail: lijian@shu.edu.cn
First published on 17th October 2016
Abstract
Scandium triflate-catalyzed reactions between spiro-epoxyoxindole and carbonyl compounds have been disclosed. The reaction with ketones can be used to synthesize spiro[[1,3]dioxolane-4,3′-indolin] species. Furthermore, an unprecedented rearrangement takes place to yield alkylidene oxindole when aromatic aldehydes are used as carbonyl components.
Isatins are highly valuable building blocks in organic synthesis since they can be easily prepared and work both as electrophiles and nucleophiles.1 It is therefore not surprising that much progress in this research field has been achieved in the past decades.2 In particular, isatin and its derivatives are considered as ideal synthetic precursors for the preparation of the significant synthetic frameworks spirocyclic oxindoles, which are frequently found in large families of natural products and biologically active molecules.3 Of note is the isatin-based methyleneindolinone (also known as alkylidene oxindole), which has enjoyed considerable attention from organic community.4 In this context, the organocatalyzed-asymmetric transformations have been well documented and become a powerful tool, thus providing a vast potential for the construction of various spirooxindole derivatives and corresponding biological evaluation.5 Furthermore, many metal-catalyzed processes as well as other new strategies were also widely investigated in an efficient manner.6 Additionally, much efforts have also been devoted to the development of new reactions and strategies involving isatin-based keteimine,7 and other derivatives.8 In spite of these significant advances, to further explore the synthetic potential of isatin derivatives continues to be desirable.
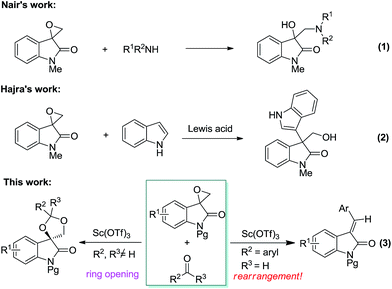
Owing to their easy preparation and versatile reactivity, epoxides have been widely used in a variety of synthetic reactions. As such, the ring opening chemistry of epoxides involving regiospecific C–O9 and C–C10 cleavage has been well established in the past decades. In this light, we become interested in the structural characters and properties of the spiro-epoxyoxindole, which has an epoxy group at 3-position of oxindole. Firstly, these compounds are important antifungal and antitubercular agents.11 Moreover, one of the benzoyl-substituted analogs exhibit biological activities against both melanoma and leukemia.12 Up to now, considerable efforts have been developed to their preparation.13 In contrast, the ring-opening reactions of spiro-epoxyoxindole are less common, and only a few examples have been disclosed. In 2011, Nair disclosed the aminolysis of spiro-epoxyoxindole to synthesize 3-hydroxy-3-aminomethylindolin-2-ones regiospecifically (Scheme 1, eqn (1)).14 In 2012, Zhang reported that spiro-epoxyoxindole underwent selective transformation under photosensitization conditions or Lewis acid catalysis, respectively.15 Furthermore, this structure has also found application in the stereocontrolled synthesis of potent proteasome inhibitor TMC-95A.16 Of late, Hajra and co-workers reported that the reactions between spiro-epoxyoxindoles and indoles afforded structurally complex 3,3′-mixed bisindoles (Scheme 1, eqn (2)).17a This strategy was further applied to gram-scale formal total synthesis of (±)-gliocladin C. Very recently, Friedel–Crafts reaction of spiro-epoxyoxindole and other electron-rich arenes were also described for the selective arylation.17b,c As a consequence, exploring the potential synthetic application of spiro-epoxyoxindole continues to be a valuable target.
In the past several years, we have paid much attention to the syntheses of functionalized carbocycles and heterocycles.18,19 Among them, many successful synthetic strategies have been developed using isatin-based derivatives as starting materials. For example, we developed a fast protocol to approach structurally unusual tricyclic spirooxindole using multicomponent reaction strategy.19a Moreover, we disclosed an indium-catalyzed multiple isocyanide insertion reaction with methyleneindolinone.19b Most recently, we disclosed that another cycloaddition reaction between isocyanides and methyleneindolinones to furnish indole-fused polycyclic skeletons with good chemoselectivity.19c As a continuation of our previous research, herein we wish to report that scandium-catalyzed reaction of spiro-epoxyoxindole and ketone can offer a new pathway to spirooxindole structure. Furthermore, an unprecedented rearrangement was observed when aromatic aldehyde was used as carbonyl component (Scheme 1, eqn (3)). To the best of our knowledge, no such examples have been reported yet.
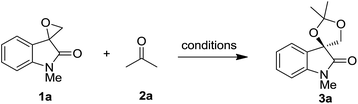
To probe the feasibility of proposed transformation, we selected spiro-epoxyoxindole 1a and acetone 2a as model substrate. As shown in Table 1, in the absence of catalyst, no reaction occurred even the mixture was warmed up to 40 °C (entry 1). Then a variety of Lewis acids were screened to improve the reaction performance. For example, the employment of several metal halides such as CuI and BiCl3 only led to negative results (entries 2 and 3). To our delight, 93% yield of product 3a was isolated with catalytic amount of RuCl3 (entry 4). Encouraged by this result, we subsequently tested several other metal salts to promote this reaction. The experimental result showed that metal triflates including AgOTf and Cu(OTf)2 were all effective to catalyze this transformation (entries 6–8), whereas Sc(OTf)3 exhibited the best performance (entry 9). Upon heating the mixture of 1a and 2a, compound 3a was isolated in 96% yield within half an hour. During our optimization, the influence of reaction temperature was also examined. The experimental results showed that the conversion rate was significantly increased together with shorter reaction time under higher temperature. Finally, when the reaction mixture was warmed up to 60 °C, spiro[[1,3]dioxolane-4,3′-indolin] 3a was produced in 99% within 10 minutes (entry 12).
| Entry | Catalyst | Time (h) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: spiro-epoxyoxindole 1a (1.0 mmol), acetone 2a (5 mL), catalyst (15 mol%), 40 °C.b Yield of product after silica gel chromatography.c In such case, reaction temperature is 60 °C. | |||
| 1 | — | 8.0 | 0 |
| 2 | CuI | 8.0 | 0 |
| 3 | BiCl3 | 8.0 | Trace |
| 4 | RuCl3 | 0.5 | 93 |
| 5 | InCl3 | 0.5 | 91 |
| 6 | AgOTf | 1.5 | 80 |
| 7 | Zn(OTf)2 | 1.0 | 82 |
| 8 | Cu(OTf)2 | 1.0 | 90 |
| 9 | Sc(OTf)3 | 0.5 | 96 |
| 10 | AgOAc | 8.0 | 0 |
| 11 | La(NO3)2 | 8.0 | 0 |
| 12c | Sc(OTf)3 | 10 min | 99 |
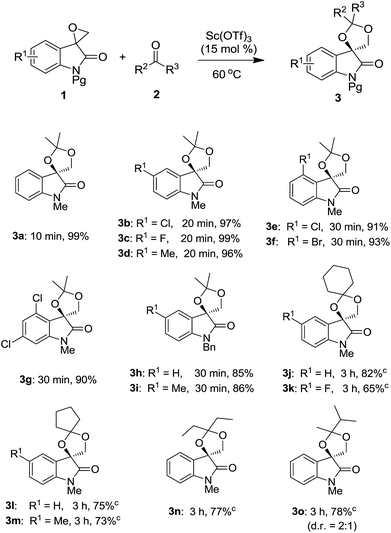
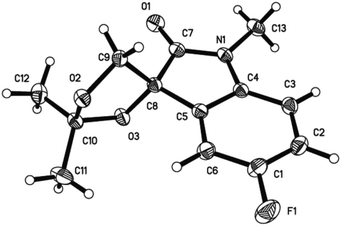
With the optimized conditions in hand, we turned our attention to establish the scope and limitation of present reaction. A variety of substituted spiro-epoxyoxindoles 1 were used to undergo the optimal conditions and the representative results were summarized in Table 2. All new compounds were characterized by 1H NMR, 13C NMR (see the ESI† for details), and HRMS. Pleasingly, substrates 1 containing electron-deficient and electron-rich substituents on the aromatic ring gave excellent yields in short time (3a–3g). In addition, the structure of compound 3c was unambiguously confirmed by single-crystal X-ray analysis (Fig. 1).20 Furthermore, substrate 1 having substituents at position 4 also worked well to give the desired cycloadducts 3e–3g, which indicated that present reaction was not sensitive to sterical hindrance. The changing of nitrogen protecting group from methyl to benzyl group was subsequently conducted. In such case, a little decreased yield was observed (3h–3i). After a broad spiro-epoxyoxindole scope was established, reactions with substituted ketones 2 were subsequently conducted. Cycloketones such as cyclohexanone and cyclopentanone were firstly examined to produce the desired adducts 3j–3m. Furthermore, ketone 2d having bulky substituent was also proven to be compatible partners to yield 3o in satisfying behavior. Setting a limitation, the reaction did not work for acetophenone and benzophenone probably due to the decreased reactivity of the carbonyl group.
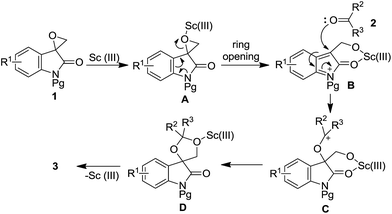
Based on the aforementioned experimental results, a plausible mechanism was proposed to explain this ring opening reaction (Scheme 2). Firstly, the coordination of Sc(III) species to the oxygen atom in spiro-epoxyoxindole 1 leads to the formation of intermediate A, which undergoes the ring opening to generate indole-2-one intermediate B. Then intermediate B is then attacked by the oxygen in ketone 2 followed by cyclization to produce key intermediate D. Finally, the elimination of Sc(III) species from cation D essentially affords product 3.
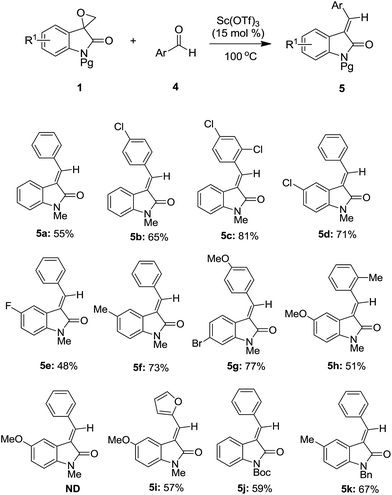
To further demonstrate the versatility of this reaction, benzaldehyde 4a was subsequently examined to react with spiro-epoxyoxindole 1a (Table 3). To our surprise, an interesting rearrangement occurred to yield alkylideneoxindole 5a as the product instead of the expected compound spiro[[1,3]dioxolane-4,3′-indolin] species. Although we were not sure about the exact mechanism for the present unusual result, we sought to briefly investigate the scope and limitation of this rearrangement reaction. As shown in Table 3, substituted aromatic aldehydes 4 were firstly used to react with 1a to produce compounds 5b, 5c. Then, changing the substituent of the aromatic ring of substrate 1 was performed. The experimental outcome indicated that fluoride, chloride, bromide, methyl, and methoxy groups were well tolerated (5d–5i). During this investigation, aromatic aldehydes having methoxy, methyl, and furyl groups were also found to be compatible (5g–5i). Surprisingly, no reaction occurred for unknown reason when benzaldehyde 4a and substrate 1 containing methoxy group was examined. Finally, the replacement of methyl with Bn and Boc as the protecting group of the nitrogen atom was also proven to be compatible (5j–5k). It was also worthy to note that the present strategy demonstrated excellent stereoselectivity and only E-isomers were observed, which was quite unusual.
Conclusions
In conclusion, we have disclosed the Sc(OTf)3-catalyzed selective reaction of spiro-epoxyoxindole and carbonyl compound. The reaction with ketone provided a rapid access for the construction of spiro[[1,3]dioxolane-4,3′-indolin] species. Remarkably, an unprecedented rearrangement took place to yield alkylidene oxindole as the product when aromatic aldehyde was used as reaction component. This method is also distinguished by its broad substrate scope and convenient experimental set-up. Further extension of present strategy to build up its versatility is currently underway in our laboratory.Acknowledgements
We thank the Fundamental Research Funds for the Central Universities (ZYGX2015J026) and the National Natural Science Foundation of China (no. 21002061, 21272148) for financial support.Notes and references
-
(a) G. S. Singh and Z. Y. Desta, Chem. Rev., 2012, 112, 6104 CrossRef CAS PubMed
; (b) F. Zhou, Y.-L. Liu and J. Zhou, Adv. Synth. Catal., 2010, 352, 1381 CrossRef CAS
.
-
(a) W. Dai, X.-L. Jiang, Q. Wu, F. Shi and S.-J. Tu, J. Org. Chem., 2015, 80, 5737 CrossRef CAS PubMed
; (b) C.-S. Wang, R.-Y. Zhu, J. Zheng, F. Shi and S.-J. Tu, J. Org. Chem., 2015, 80, 512 CrossRef CAS PubMed
; (c) Y.-L. Liu, X. Wang, Y.-L. Zhao, F. Zhu, X.-P. Zeng, L. Chen, C.-H. Wang, X.-L. Zhao and J. Zhou, Angew. Chem., Int. Ed., 2013, 52, 13735 CrossRef CAS PubMed
; (d) R.-G. Shi, X.-H. Wang, R. Liu and C.-G. Yan, Chem. Commun., 2016, 52, 6280 RSC
; (e) Y. Murata, M. Takahashi, F. Yagishita, M. Sakamoto, T. Sengoku and H. Yoda, Org. Lett., 2013, 15, 6182 CrossRef CAS PubMed
.
-
(a) C. Marti and E. M. Carreira, Eur. J. Org. Chem., 2003, 2209 CrossRef CAS
; (b) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748 CrossRef CAS PubMed
; (c) S. Rana, E. C. Blowers, C. Tebbe, J. I. Contreras, P. Radhakrishnan, S. Kizhake, T. Zhou, R. N. Rajule, J. L. Arnst, A. R. Munkarah, R. Rattan and A. Natarajan, J. Med. Chem., 2016, 59, 5121 CrossRef CAS PubMed
; (d) R. M. Williams, J. Cao and H. Tsujishima, Angew. Chem., Int. Ed., 2000, 39, 2540 CrossRef CAS
; (e) P. S. Baran and J. M. Richter, J. Am. Chem. Soc., 2005, 127, 15394 CrossRef CAS PubMed
; (f) A. Jossang, P. Jossang, H. A. Hadi, T. Sevenet and B. Bodo, J. Org. Chem., 1991, 56, 6527 CrossRef CAS
.
-
(a) X.-H. Chen, Q. Wei, S.-W. Luo, H. Xiao and L.-Z. Gong, J. Am. Chem. Soc., 2009, 131, 13819 CrossRef CAS PubMed
; (b) Y. Wang, M.-S. Tu, L. Yin, M. Sun and F. Shi, J. Org. Chem., 2015, 80, 3223 CrossRef CAS PubMed
; (c) A. Singh, A. L. Loomer and G. P. Roth, Org. Lett., 2012, 14, 5266 CrossRef CAS PubMed
; (d) F. Zhong, X. Han, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2011, 50, 7837 CrossRef CAS PubMed
; (e) Y. Han, Y.-J. Sheng and C.-G. Yan, Org. Lett., 2014, 16, 2654 CrossRef CAS PubMed
; (f) J. Sun, Y. Sun, H. Gong, Y.-J. Xie and C.-G. Yan, Org. Lett., 2012, 14, 5172 CrossRef CAS PubMed
; (g) J. J. Badillo, C. J. A. Ribeiro, M. M. Olmstead and A. K. Franz, Org. Lett., 2014, 16, 6270 CrossRef CAS PubMed
; (h) Y.-L. Lu, J. Sun, Y.-J. Xie and C.-G. Yan, RSC Adv., 2016, 6, 23390 RSC
.
-
(a) D. Cheng, Y. Ishihara, B. Tan and C. F. Barbas III, ACS Catal., 2014, 4, 743 CrossRef CAS
; (b) W. Sun, G. Zhu, C. Wu, G. Li, L. Hong and R. Wang, Angew. Chem., Int. Ed., 2013, 52, 8633 CrossRef CAS PubMed
; (c) L.-T. Shen, W.-Q. Jia and S. Ye, Angew. Chem., Int. Ed., 2013, 52, 585 CrossRef CAS PubMed
; (d) G. Bencivenni, L.-Y. Wu, A. Mazzanti, B. Giannichi, F. Pesciaioli, M.-P. Song, G. Bartoli and P. Melchiorre, Angew. Chem., Int. Ed., 2009, 48, 7200 CrossRef CAS PubMed
; (e) A. Quintavalla, F. Lanza, E. Montroni, M. Lombardo and C. Trombini, J. Org. Chem., 2013, 78, 12049 CrossRef CAS PubMed
.
-
(a) B. M. Trost, N. Cramer and S. M. Silverman, J. Am. Chem. Soc., 2007, 129, 12396 CrossRef CAS PubMed
; (b) B. M. Trost, N. Cramer and H. Bernsmann, J. Am. Chem. Soc., 2007, 129, 3086 CrossRef CAS PubMed
; (c) Y.-H. Jiang and C.-G. Yan, RSC Adv., 2016, 6, 42173 RSC
.
-
(a) Y.-M. Wang, H.-H. Zhang, C. Li, T. Fan and F. Shi, Chem. Commun., 2016, 52, 1804 RSC
; (b) S. Hajra, S. M. Aziz, B. Jana, P. Mahish and D. Das, Org. Lett., 2016, 18, 532 CrossRef CAS PubMed
; (c) M. Montesinos-Magraner, C. Vila, R. Cantón, G. Blay, I. Fernández, M. C. Muñoz and J. R. Pedro, Angew. Chem., Int. Ed., 2015, 54, 6320 CrossRef CAS PubMed
; (d) T. Arai, K. Tsuchiya and E. Matsumura, Org. Lett., 2015, 17, 2416 CrossRef CAS PubMed
; (e) X.-P. Yin, X.-P. Zeng, Y.-L. Liu, F.-M. Liao, J.-S. Yu, F. Zhou and J. Zhou, Angew. Chem., Int. Ed., 2014, 53, 13740 CrossRef CAS PubMed
; (f) H. Gao, J. Sun and C.-G. Yan, J. Org. Chem., 2014, 79, 4131 CrossRef CAS PubMed
.
-
(a) P. B. Alper, C. Meyers, A. Lerchner, D. R. Siegel and E. M. Carreira, Angew. Chem., Int. Ed., 1999, 38, 3186 CrossRef CAS
; (b) C. Marti and E. M. Carreira, J. Am. Chem. Soc., 2005, 127, 11505 CrossRef CAS PubMed
; (c) S. Kato, T. Yoshino, M. Shibasaki, M. Kanai and S. Matsunaga, Angew. Chem., Int. Ed., 2012, 51, 7007 CrossRef CAS PubMed
; (d) X. Li, W. Tan, Y.-X. Gong and F. Shi, J. Org. Chem., 2015, 80, 1841 CrossRef CAS PubMed
; (e) B. Viswambharan, K. Selvakumar, S. Madhavan and P. Shanmugam, Org. Lett., 2010, 12, 2108 CrossRef CAS PubMed
.
-
(a) M. L. Jamieson, P. A. Hume, D. P. Furkert and M. A. Brimble, Org. Lett., 2016, 18, 468 CrossRef CAS PubMed
; (b) K. W. Armbrust, M. G. Beaver and T. F. Jamison, J. Am. Chem. Soc., 2015, 137, 6941 CrossRef CAS PubMed
; (c) L. Yao, Q. Zhu, L. Wei, Z.-F. Wang and C.-J. Wang, Angew. Chem., Int. Ed., 2016, 55, 5829 CrossRef CAS PubMed
; (d) Y. Liu, R. C. Klet, J. T. Hupp and O. Farha, Chem. Commun., 2016, 52, 7806 RSC
; (e) J. Zhang, S. Wu, J. Wu and Z. Li, ACS Catal., 2015, 5, 51 CrossRef CAS
.
-
(a) J. Zhang, Z. Chen, H.-H. Wu and J. Zhang, Chem. Commun., 2012, 48, 1817 RSC
; (b) Z. Chen, Z. Tian, J. Zhang, J. Ma and J. Zhang, Chem.–Eur. J., 2012, 18, 8591 CrossRef CAS PubMed
; (c) G.-W. Wang, H.-T. Yang, P. Wu, C.-B. Miao and Y. Xu, J. Org. Chem., 2006, 71, 4346 CrossRef CAS PubMed
; (d) T. Wang and J. Zhang, Chem.–Eur. J., 2011, 17, 86 CrossRef CAS PubMed
.
- A. Dandia, R. Singh, M. Saha and A. Shivpuri, Pharmazie, 2002, 57, 602 CAS
.
- K. A. Marx, P. O'Neil, P. Hoffman and M. L. Ujwal, J. Chem. Inf. Comput. Sci., 2003, 43, 1652 CrossRef CAS PubMed
.
-
(a) Y. Kuang, Y. Lu, Y. Tang, X. Liu, L. Lin and X. Feng, Org. Lett., 2014, 16, 4244 CrossRef CAS PubMed
; (b) V. Schulz, M. Davoust, J.-F. Lohier, J. S. de Oliveria Santos, P. Metzner and J.-F. Brière, Org. Lett., 2007, 9, 1745 CrossRef CAS PubMed
; (c) M. Chouhan, A. Pal, R. Sharma and V. A. Nair, Tetrahedron Lett., 2013, 54, 7119 CrossRef CAS
.
- M. Chouhan, K. R. Senwar, R. Sharma, V. Grover and V. A. Nair, Green Chem., 2011, 13, 2553 RSC
.
- L. Wang, Y. Su, X. Xu and W. Zhang, Eur. J. Org. Chem., 2012, 6606 CAS
.
- M. Inoue, H. Furuyama, H. Sakazaki and M. Hirama, Org. Lett., 2001, 3, 2863 CrossRef CAS PubMed
.
-
(a) S. Hajra, S. Maity and R. Maity, Org. Lett., 2015, 17, 3430 CrossRef CAS PubMed
; (b) S. Hajra, S. Maity and S. Roy, Adv. Synth. Catal., 2016, 358, 2300 CrossRef CAS
; (c) M. Luo, R. Yuan, X. Liu, L. Yu and W. Wei, Chem.–Eur. J., 2016, 22, 9797 CrossRef CAS PubMed
.
-
(a) C. L. Li, H. M. Deng, C. J. Li, X. S. Jia and J. Li, Org. Lett., 2015, 17, 5718 CrossRef CAS PubMed
; (b) S. L. Jia, S. K. Su, C. J. Li, X. S. Jia and J. Li, Org. Lett., 2014, 16, 5604 CrossRef CAS PubMed
; (c) G. S. Cheng, X. He, L. M. Tian, J. W. Chen, C. J. Li, X. S. Jia and J. Li, J. Org. Chem., 2015, 80, 11100 CrossRef CAS PubMed
; (d) S. B. Xu, S. K. Su, H. T. Zhang, L. M. Tian, P. C. Liang, J. W. Chen, Y. Zhang, C. J. Li, X. S. Jia and J. Li, Synthesis, 2015, 47, 2414 CrossRef CAS
.
-
(a) S. K. Su, C. J. Li, X. S. Jia and J. Li, Chem.–Eur. J., 2014, 20, 5096 Search PubMed
; (b) Y. M. Tian, L. M. Tian, X. He, C. J. Li, X. S. Jia and J. Li, Org. Lett., 2015, 17, 4874 CrossRef CAS PubMed
; (c) Y. M. Tian, L. M. Tian, C. J. Li, X. S. Jia and J. Li, Org. Lett., 2016, 18, 840 CrossRef CAS PubMed
; (d) J. Li, S. K. Su, M. Y. Huang, B. Y. Song, C. J. Li and X. S. Jia, Chem. Commun., 2013, 49, 10694 RSC
; (e) J. Li, Y. J. Liu, C. J. Li and X. S. Jia, Chem.–Eur. J., 2011, 17, 7409 CrossRef CAS PubMed
.
- CCDC 1469608 for compound 3c contains the supplementary crystallographic data for this paper.†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1469608. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra21264f |
| This journal is © The Royal Society of Chemistry 2016 |