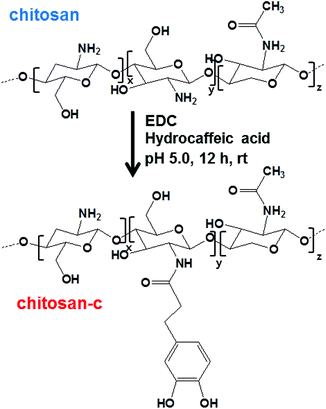
Bio-inspired catechol conjugation converts water-insoluble chitosan into a highly water-soluble, adhesive chitosan derivative for hydrogels and LbL assembly†
Kyuri
Kim‡
a,
Ji Hyun
Ryu‡
a,
Dong Yun
Lee
b and
Haeshin
Lee
*acd
aGraduate School of Nanoscience and Technology (WCU), Korea Advanced Institute of Science and Technology (KAIST), 291 University Rd., Daejeon 305-701, Republic of Korea. E-mail: haeshin@kaist.ac.kr; Fax: +82 42-350-2810; Tel: +82 42-350-2849
bDepartment of Bioengineering, Hanyang University, 17 Haengdang-dong, Seongdong-gu, Seoul, 133-791, Republic of Korea
cDepartment of Chemistry, Korea Advanced Institute of Science and Technology (KAIST), 291 University Rd., Daejeon 305-701, Republic of Korea
dMolecular-Level Interface Research Center, Korea Advanced Institute of Science and Technology (KAIST), 291 University Rd., Daejeon 305-701, Republic of Korea
First published on 2nd May 2013
Abstract
This report describes a simple method to prepare water-soluble chitosan derivative by conjugation of an enediol group, catechol. Chitosan functionalized with a catechol-containing compound, 3,4-dihydroxyhydrocinnamic acid, by a carbodiimide coupling method resulted in chitosan–catechol conjugates. This one-step chemical modification of high-molecular-weight chitosan (approximately 100 kDa) dramatically increased the water solubility of the chitosan derivative to 60 mg mL−1 at pH 7.0. The degree of catechol conjugation was found critical in determining the solubility. The chitosan–catechol conjugates are not only water-soluble but are adhesive, due to the intrinsic adhesive properties of catechol. Also, the water-soluble chitosan derivative allows one to directly form chitosan hydrogel in neutral buffer solutions. The utility of both the water solubility and the adhesive property of the chitosan–catechol was demonstrated by the effective layer-by-layer assembly on substrates. The water-soluble chitosan–catechol is expected to be useful in many areas of surface functionalization, drug delivery, tissue engineering, and tissue adhesives.
Introduction
Despite the variety of beneficial properties of chitosan, such as low toxicity, biodegradability, antitumor activity, enhancement of immunogenicity, and antibacterial activity, the low solubility of chitosan in aqueous solutions at neutral pH has significantly hindered its widespread implementation as a functional biomaterial.1–7 A typical approach used to dissolve chitosan utilizes acidified solutions in which protonated primary amine groups increase the water-solubility of chitosan. However, this approach is limited because the dissolved chitosan begins to precipitate instantaneously as the pH increases.4,8 Therefore, the solubility issue in chitosan limits its widespread application such as hydrogels and layer-by-layer (LbL) assembly. In particular, hydrogel preparation in acid conditions can be toxic to encapsulated entities.Thus, several strategies have been developed to dissolve chitosan at physiological pH. One strategy is to reduce the molecular weight of chitosan to improve aqueous solubility at an alkaline condition because the crystallinity of chitosan decreases as its molecular weight decreases.9,10 However, the decrease in the molecular weight of chitosan changes the activity and properties of chitosan, thereby limiting its widespread implementation.4,9,11
An alternative strategy to improve the aqueous solubility of chitosan is to use chemical modifications. Existing chemistries have focused on the modification of the primary amines of chitosan. Examples of this strategy include the derivation of chitosan with hydrophilic moieties, such as poly(ethylene glycol) (PEG). The excellent hydration properties of PEG make it a widely used polymer to fabricate PEG hydrogels.12,13 PEGylation of chitosan increases its solubility,14–16 but the presence of PEG sterically hinders the active sites of chitosan, thereby obscuring its intrinsic properties.
The glycolation of chitosan is another method used to prepare water-soluble chitosan. The glycolated, water-soluble chitosan contains ethylene glycol units conjugated to the C-6 position of the saccharide ring of chitosan, in which the amine groups remain chemically intact. Additional advantages of glycolation are the enhancement of in vitro and in vivo stabilities and secondary conjugation to the amine groups, which enables multi-functionality to be provided for chitosan.17,18 However, the reactivity of epoxide, which is the precursor of the glycol unit, results in heterogeneous chitosan derivatives. The ethylene oxide favorably reacts with the amine groups rather than with hydroxyl groups, thereby resulting in heterogeneous glycolated chitosan derivatives. A previous 1H-NMR study showed that the glycol chitosan products are heterogeneous.19 Therefore, the number of unreacted amine groups in the glycol chitosan is reduced compared to the proposed structure.
Acetylation, on the other hand, solely targets the primary amine groups of chitosan. The addition of acetyl groups significantly improves solubility because it provides conformational flexibility to the rigid backbone of chitosan. The increase in chain flexibility (i.e., crystallinity reduction) results from the formation of intra- or intermolecular hydrogen bonding by the acetamide.20,21 However, reasonable solubility is achieved by a high degree of chemical modification (approximately 50% of the amine groups).21–23 Thus, such a significant degree of the amine derivatization destroys the unique properties of chitosan, similar to the case of glycol chitosan.
The introduction of charge groups to chitosan backbones has been yet another method used to increase the solubility of chitosan. The addition of negatively charged conjugates to chitosan results in highly soluble chitosan derivatives. Several zwitterionic chitosan derivatives, such as phosphorylcholine-conjugated chitosan and succinic anhydride-conjugated chitosan, have been demonstrated to improve solubility, due to the charge groups introduced.2,24,25 However, the conjugated moieties may alter the net charge of chitosan derivatives, thereby interfering with its intrinsic functions.
In this study, we report a new strategy that can effectively solubilize high-molecular-weight chitosan. This method involves a simple one-step catechol conjugation to the primary amine groups of chitosan. The catechol-conjugated chitosan (chitosan-c) exhibits excellent water solubility (approximately 60 mg mL−1) at neutral pH, which is approximately four-fold higher than the solubility shown in glycolated chitosan (approximately 17 mg mL−1).26 Furthermore, the high solubility is achieved by a low degree of catechol conjugation (approximately 7.2%). Thus, a low degree of chemical modification is expected to retain the activities of chitosan. Unlike a macromolecular conjugation, such as PEGylation, that can prevent the molecular recognition of chitosan by cells, the small size of catechol can minimize steric hindrance. Most importantly, chitosan-c exhibits adhesive properties, functionalizing solid substrates with ease. The adhesive properties originate from the catechol moiety found in marine mussels.27–29 This report is the first to indicate that catechol conjugation dramatically enhances the water solubility of a water-insoluble biopolymer, which is different from previous catechol conjugation studies with compounds such as hyaluronic acid–catechol,30 heparin–catechol,31 and PEG–catechol,32 in which the polymers are already water-soluble, hydrophilic polymers.
Experimental methods
Materials
Chitosan (Mw 100 kDa, 80% deacetylated) was purchased from Wako Pure Chemical Co. (Japan). Heparin sodium salts (17–19 kDa; Sigma-Aldrich, St. Louis, USA), 3,4-dihydroxy hydrocinnamic acid and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC) were purchased from Sigma-Aldrich. Ethanol was purchased from Merck Chemicals (Darmstadt, Germany). All other chemicals were of analytical grade and were used without further purification.Synthesis of catechol conjugated chitosan (chitosan-c)
Chitosan-c was synthesized as follows. Briefly, chitosan (100 kDa, 3.25 mmol) was hydrated in 1 N HCl (5 mL) solutions, distilled and deionized water (DDW, 45.5 mL) was sequentially added to the solution, and the pH of the solution was adjusted to 5.0 with 5 N NaOH. Then, hydrocaffeic acid (HCA, 1.62, 3.25, or 6.49 mmol) dissolved in a DDW (3 mL) was added to the chitosan solution. To the reaction mixture, EDC (0.65, 1.62, 3.25, 6.49, or 9.47 mmol) dissolved in the 50 mL of DDW and ethanol solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) was then slowly added. The reaction mixture was stirred vigorously at room temperature for 12 h and the pH of the reaction solution was checked every 15 min to be maintained at 5.0. After 12 h, it was dialyzed (molecular weight cutoff: 3500, SpectraPor, USA) in acidified DDW (pH 5.0, HCl) for 2 days, in PBS buffer for 4 h, and in DDW for 4 h. The final product was lyophilized and kept in a moisture-free desiccator. Stock solutions of chitosan (20 mg mL−1) and chitosan-c (20 mg mL−1) were prepared (pH 4.0) for further use. The degree of catechol substitution (DOC) is determined by 1H NMR (Bruker Avance, 400 MHz, D2O).
1, v/v) was then slowly added. The reaction mixture was stirred vigorously at room temperature for 12 h and the pH of the reaction solution was checked every 15 min to be maintained at 5.0. After 12 h, it was dialyzed (molecular weight cutoff: 3500, SpectraPor, USA) in acidified DDW (pH 5.0, HCl) for 2 days, in PBS buffer for 4 h, and in DDW for 4 h. The final product was lyophilized and kept in a moisture-free desiccator. Stock solutions of chitosan (20 mg mL−1) and chitosan-c (20 mg mL−1) were prepared (pH 4.0) for further use. The degree of catechol substitution (DOC) is determined by 1H NMR (Bruker Avance, 400 MHz, D2O).
UV-vis spectrophotometric analysis
Five hundred milliliters of a mixture solution (10 mM tris(hydroxymethyl)aminomethane, sodium acetate, and disodium hydrogen phosphate in DDW) was prepared for a wide pH range buffering. The pH value of the solution was adjusted and allocated to conical tubes with pH values of 2.1, 3.0, 4.0, 5.1, 6.2, 7.2, 7.5, 8.3, 9.0, 10.2, 11.3, 12.0 using 1 N HCl and 1 N NaOH. These pH values are expressed as rounded integers in this report, except for pH 7.5. The stock chitosan and chitosan-c12.7 solutions (20 mg mL−1) were diluted to 2 mg mL−1 by the buffer solutions with pH values from 2.0 to 12.0 for UV-vis measurement. Small changes in the final pH values (<±0.2) were found after dilution. The absorbance of chitosan and chitosan-c12.7 solubilized solutions was measured using a UV-vis spectrophotometer at 600 nm immediately after shaking the samples (UV-1601, Shimadzu) qualitatively from wavelength 200 to 800 nm. Insolubility (i.e. turbidity) of the chitosan and chitosan-c solutions were reported by the scattered value measured at 600 nm.Solubility measurement at varying DOC
A saturating amount of chitosan-c (DOC of 7.2%, 10.8%, 12.7%, and 36%) was each dissolved in 1 mL of pH 5 and pH 7 buffer solutions. The samples were then centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min. Two hundred microliters of the dissolved chitosan-c was carefully pipetted out without disturbing the precipitated chitosan-c concentrated at the bottom of the vials. The pipette samples were stored in the 2.0 mL vials that were pre-weighed. The samples were lyophilized, and weighed. This experiment was repeated three times with all DOC-verified chitosan-c at each pH.
000 rpm for 5 min. Two hundred microliters of the dissolved chitosan-c was carefully pipetted out without disturbing the precipitated chitosan-c concentrated at the bottom of the vials. The pipette samples were stored in the 2.0 mL vials that were pre-weighed. The samples were lyophilized, and weighed. This experiment was repeated three times with all DOC-verified chitosan-c at each pH.
Gel permeation chromatography (GPC)
Chitosan, chitosan-c7.2 and chitosan-c12.7 were dissolved in 10 mM sodium acetate buffer (pH 2.0, 1 N HCl) at 1 mg mL−1 and filtered with a sterile filter (0.8 μm, Sartorius stedim). Gel permeation chromatography (1200 Series, Agilent Technologies, Santa Clara, USA) was performed with a flow of 1 mL min−1 for 30 min. The standard curve for hydrodynamic diameter measurement used hyaluronic acids with known molecular weights of 50, 200, and 360 kDa.33Characterization of chitosan-c12.7 hydrogel
50 μL of 1 N NaOH was added to the 1 mL of 20 mg mL−1 chitosan and chitosan-c12.7 solutions and mixed well. The measured pH of the chitosan solution was approximately 12. Elastic modulus was measured at varying frequency using a rheometer (Bohlin, Malvern Instruments, UK). The sol–gel curve of chitosan-c was tested by synthesizing 0.5, 1, 2, 3% chitosan-c12.7 (5, 10, 20, 30 mg mL−1 in PBS buffer solution) hydrogel with the addition of 30, 40, 50, 60, 70 μL of 1 N NaOH.LbL assembly
Twenty nanometers of titanium and 100 nm gold were deposited onto silicon wafers by thermal evaporation, thoroughly washed with DDW, and dried with nitrogen gas. The substrates were then immersed in either chitosan or chitosan-c solubilized in pH 7 buffer solutions, placed on a 3D shaker (TW3, FINEPCR, Seoul, Korea) overnight. Then these substrates were briefly washed with deionized distilled water and immersed in 2 mg mL−1 heparin solution (dissolved in DDW) for 5 min. The substrates were again washed with DDW and immersed in either chitosan or chitosan-c solutions for 5 min. One alternating adsorption process of either (chitosan-c/heparin) or (chitosan/heparin) was counted as one assembly. After the LbL assembly, all the samples were washed with DDW and dried with nitrogen gas.Characterization of thin films
The thickness of LbL assemblies on gold substrates was measured using an ellipsometer (L116S, Gaertner Science Corp., Chicago) and a field emission scanning electron microscope (FE-SEM, S4800, Hitachi, Japan) to measure (chitosan-c/heparin)20 samples. Surface morphology of (chitosan/heparin)2 was examined using a scanning probe microscope (SPM, XE-100, Park Systems, Korea). All images were obtained in non-contact mode at 1.00 Hz. The 3D images were made using XEI program.Results and discussion
The catechols were conjugated to the amine groups of chitosan using EDC chemistry (Scheme 1).34–37 Ethanol was used as a co-solvent to prevent precipitation, and the degree of catechol conjugation (DOC) onto the chitosan backbone was determined by 1H-NMR.32 Briefly, the catechol conjugation was calculated by dividing the integration value of the catechol proton peaks that appeared from 6.5 to 6.7 ppm by the value of the acetyl group protons from 1.9 to 1.93 ppm multiplied by 5 because 20% acetylation was used as provided by the producer (Fig. 1). The chitosan-c with a DOC value of 12.7% is denoted as chitosan-c12.7. Chitosan-c12.7 was water-soluble at pH ranges from 2 to 7 (Fig. 2C). In contrast, the unmodified chitosan precipitated at a pH greater than 2 (Fig. 2A). The difference in water solubility between chitosan and chitosan-c12.7 was further demonstrated by UV-vis spectrophotometry (Fig. 2B and 2D). In general, a turbid solution causes an overall upshift in the visible spectrum from 400 to 600 nm, and the absorbance of a clear solution remains close to the baseline in the visible range. Since chitosan exhibits pH-dependent solubility, it is expected that chitosan causes a baseline upshift as the basicity of an aqueous solution is increased. Indeed, the chitosan solutions (2 mg mL−1) showed baseline up-shifts at pH 4, 6, and 7 (Fig. 2B), indicating poor solubility of chitosan in weak acid and neutral solutions. In contrast, chitosan-c12.7 was soluble in a pH range from 2 to 7. The visible spectra did not show any upshift of baselines, indicating absence of turbidity for the chitosan-c12.7 solutions (Fig. 2D).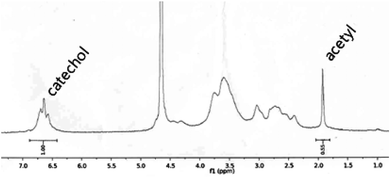 | ||
| Fig. 1 1H-NMR of chitosan-c36.4. | ||
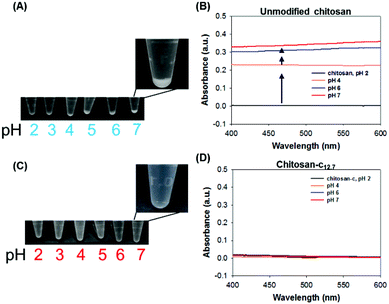 | ||
| Fig. 2 (A) Photographs of the chemically unmodified chitosan solutions at pH values ranging from 2 to 7 and (B) the UV-vis absorbance spectra of the chitosan at pH 2 (green), 4 (orange), 6 (blue), and 7 (red). The arrows indicate the baseline upshifts as pH increases, as an indication of the insolubility of chitosan. (C) Photographs of the chitosan-c solutions at pH ranging from 2 to 7, and (D) the UV-vis absorbance of chitosan-c at pH 2 (green), 4 (orange), 6 (blue), and 7 (red). | ||
At higher pH values, however, both chitosan and chitosan-c precipitated. In the case of chitosan, precipitate formation was consistently observed at pH ranges from 8 to 12, as occurred in acidic solutions (Fig. 3A). The baselines of the spectra were up-shifted, indicating that the chitosan was not dissolved (Fig. 3B). Chitosan-c12.7 precipitated, unlike the acidic and neutral dissolution of chitosan-c12.7 that exhibited transparent solutions. However, the physical properties of the chitosan-c precipitates were different from those observed in the chitosan precipitations. The precipitates of chitosan were solid powders similar to typical polymeric precipitates due to insolubility of the polymer. Thus, the volume of precipitates was small (Fig. 3A). In contrast, the volume of the chitosan-c12.7 precipitates was substantially large, swollen, and sticky (Fig. 3C). The gel-like precipitates which possibly resulted from the chemical crosslinking upon the oxidation of the catechol moieties conjugated to the chitosan were further studied with UV-vis. At pH 8.0, the chitosan-c12.7 showed a broad shoulder after 300 nm up to 600 nm, indicating catecholquinone-initiated oxidative reactions (Fig. 3D, solid red).11,12,38 The catecholquinone oxidation became obvious when the pH value of the solution was increased to 12. A distinct peak at 340 nm in the pH 12 chitosan-c solution indicated the presence of reactions such as catechol-amine reactions (via Schiff base addition or Michael-type addition) and catechol–catechol conjugation (Fig. 3D, blue solid). At pH 12, the chitosan-c12.7 exhibited strong adhesive and cohesive properties, which resulted in hydrogel-like materials that remained strongly attached to the test tube. Therefore, only the solution in the tube was transferred into an UV-vis cuvette for measurement. The measured peak at 340 nm indicates the presence of interesting yet unknown oxidation products, which require a series of further analyses. α,β-Dehydro DOPA shows absorption at 320 nm (Fig. S1†),12 and thus a related compound such as α,β-dehydrocatecholquinone can be a candidate. The UV-vis spectrum of chitosan-c12.7 showed two characteristic peaks at 220 nm and 280 nm (Fig. 3E, right). The peak at 220 nm is the absorbance of the hydroxyl and amine groups in chitosan, and the peak at 280 nm is from the catechol groups. Although the precipitates were detected, a certain degree of the chitosan-c12.7 solubility remained, as proven by the presence of the characteristic chitosan far-UV peak at 220 nm at pH 8 and 12 (Fig. 3E, right). In the case of unmodified chitosan, in contrast, the far-UV peak was absent (Fig. 3E, left, red and blue). Fig. 3F summarizes the comparative solubility of chitosan and chitosan-c. The chemically unmodified chitosan is insoluble in a wide range of pH values from 3 to 12, but the catechol-conjugated chitosan is soluble at acidic and neutral pH and is partially soluble at basic pH.
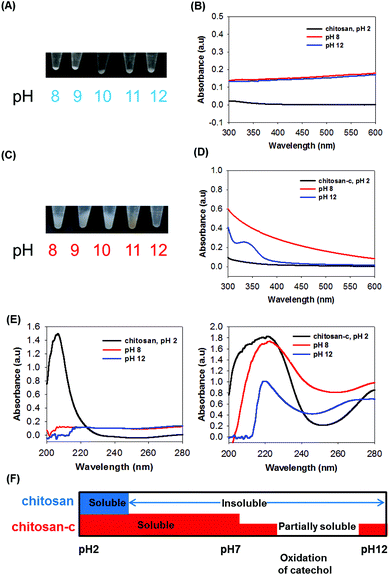 | ||
| Fig. 3 (A) Photographs of the chemically unmodified chitosan solutions at pH values ranging from 2 to 7 and (B) the UV-vis absorbance spectra of the chitosan at pH 2 (green), 4 (orange), 6 (blue), and 7 (red). The arrows indicate the baseline upshifts as pH increases, as an indication of the insolubility of chitosan. (C) Photographs of the chitosan-c solutions at pH ranging from 2 to 7, and (D) the UV-vis absorbance of chitosan-c at pH 2 (green), 4 (orange), 6 (blue), and 7 (red). | ||
The scattering of incident light at 600 nm was used as an indicator of the water solubility of chitosan-c at various concentrations (Fig. 4). We found that 0.5 mg mL−1 chitosan-c12.7 had no turbidity and thus was water-soluble at a wide range of pH values, ranging from 2 to 10 (Fig. 4A, closed circle). However, 0.5 mg mL−1 unmodified chitosan was insoluble at pH above 2.0 (Fig. 4A, open circle). The solubility observed for 0.5 mg mL−1 chitosan-c12.7 is consistent with the existence of a far-UV peak at 220 nm detected at pH 8 and 12 solutions (Fig. 3D). Only when the concentrations of chitosan-c solutions were increased to 1 and 2 mg mL−1, the chitosan-c solutions were turbid at pH greater than 7 (Fig. 4B). The analysis of the UV spectra of chitosan and chitosan-c12.7 showed that chitosan-c12.7 has higher solubility at acidic and neutral pH than chitosan. Thus, we further investigated the maximum solubility of chitosan-c. The solubility of chitosan-c12.7 was substantially high: 34.6 ± 10.1 mg mL−1 at pH 4.9 and 34.3 ± 4.6 mg mL−1 at pH 7.0 (Table 1). We hypothesized that the degree of catechol conjugation has an effect on the solubility of chitosan-c. Chitosan-c variants with DOCs of 1.4% (chitosan-c1.4), 3.0% (chitosan-c3.0), 7.2% (chitosan-c7.2), 10.8% (chitosan-c10.8), 12.7% (chitosan-c12.7), and 36.4% (chitosan-c36.4) were prepared, and their solubilities were measured.
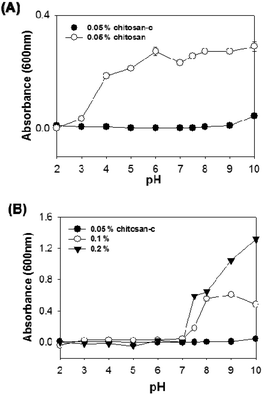 | ||
| Fig. 4 The turbidity of chitosan or chitosan-c dissolved aqueous solutions. (A) The turbidity of 0.5 mg mL−1 chitosan (open circle) and 0.5 mg mL−1 chitosan-c (closed circle) solutions was measured at 600 nm. (B) The effects of chitosan-c concentration on the turbidity of the aqueous solution with varying pH values for 0.5 mg mL−1 (closed circle), 1 mg mL−1 (open circle), and 2 mg mL−1 (closed triangle) chitosan-c. All data were measured with a UV-spectrophotometer in a quartz cuvette (10 mm light path), and each point in the plots represents mean turbidity ± SD, n = 3. | ||
| Chitosan-c1.4 [mg mL−1] | Chitosan-c3.0 [mg mL−1] | Chitosan-c7.2 [mg mL−1] | Chitosan-c10.8 [mg mL−1] | Chitosan-c12.7 [mg mL−1] | Chitosan-c36.4 [mg mL−1] | |
|---|---|---|---|---|---|---|
| pH 4.9 | ≤41.7 ± 4.70 | ≤39.9 ± 5.10 | ≤63.2 ± 10.7 | ≤33.6 ± 9.80 | ≤34.6 ± 10.1 | Turbid (≤6.27 ± 0.55) |
| pH 7.0 | ≤36.2 ± 3.64 | ≤38.5 ± 3.72 | ≤62.0 ± 18.1 | ≤39.9 ± 13.4 | ≤34.3 ± 4.60 | Turbid (≤6.37 ± 1.85) |
The solubility of chitosan-c7.2 was the greatest among the measured values and was 63.2 ± 10.7 mg mL−1 at pH 4.9 and ≤62.0 ± 18.1 mg mL−1 at pH 7. At lower and higher DOC than chitosan-c7.2, solubilities were lower than that of chitosan-c7.2 (Table 1). These solubility values, however, are much higher than fructose-conjugated glycol chitosan, which has a solubility of approximately 17 mg mL−1.26 It is possible that high water solubility of pyrocatechol (approximately 430 mg mL−1) in a neutral aqueous solution plays an important role in enhancing the solubility of chitosan-c conjugates. Also, previous studies have demonstrated that a certain degree of chemical modifications, such as acetylation, breaks intra- and inter-hydrogen bonds of chitosan, resulting in the enhancement of solubility. Similarly, chitosan-c was suspected of structural change. Linear unmodified chitosan polymers stack and form crystalline structures. However, after chemical modifications, this intrinsic crystalline character of the polymers is destroyed, as the rigidity of the polymers decreases and they become amorphous.9 Also, it is important to note that conjugation of catechol groups may have increased the pKa value of the amine groups (pKa = ∼6.5) in the chitosan derivative.39,40 Two hydroxyl groups on the catechol group have pKa values of 9.2 and 13, which are greater than the pKa value of chitosan. This may increase the effective pKa value of the chitosan-c, which might contribute to the increase in solubility of chitosan-c. However, in the case of polypeptides such as p(DOPA-Lys), the solubility of the polymer decreases when DOPA content is elevated.41
The water-soluble chitosan-c reported in this study is a long-chain, high-molecular-weight chitosan derivative. Gel permeation chromatography (GPC) experiments showed that the unmodified chitosan has a broad molecular weight distribution (Fig. 5). The first major peak with a retention time of 12.6 min corresponds to a fairly high molecular weight, weighing approximately 802 kDa, and the second minor peak with a retention time of 14.0 min corresponds to 268 kDa (Table 2). The calibration of the GPC system was performed by using hyaluronic acids. We hypothesized that catechol conjugation may have an effect on the effective molecular weight (i.e., the hydrodynamic volume). In fact, a small alteration occurred in the retention time, changing from 12.6 to 12.76 (Fig. 5B), indicating decreased hydrodynamic volume possibly due to a decrease in rigidity of the backbone of the chitosan derivative or interrupted hydrogen bonding between chitosans. Unlike the chromatogram of chitosan (Fig. 5, red), chitosan-c7.2 (green) and chitosan-c12.7 (black) showed two peaks that were similar in intensity with retention times of 12.76 and 14.0 min, respectively. This indicates that chitosan-c exists in two distinct hydrodynamic volumes although further analysis is needed to explain the presence of peaks 2 and 3. The conjugated catechol-induced interactions such as van der Waals force and/or hydrogen bonds might result in backbone folding. The detection wavelength was set to 210 nm such that the chromatograms were independent of the catechol conjugation.
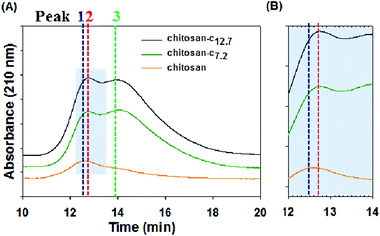 | ||
| Fig. 5 (A) Gel permeation chromatograms of chitosan (orange), chitosan-c7.2 (green), and chitosan-c12.7 detected at 210 nm. The blue dotted line corresponds to the peak of chitosan at a retention time of 12.6 min, the red line corresponds to the peak of chitosan-c7.2 at a retention time of 12.76, and the green line corresponds to the peak of chitosan-c12.7 at a retention time of 14.0 min. (B) The shaded region in panel A from 12 to 14 min was enlarged to precisely distinguish the peaks of chitosan (orange) and chitosan-c (green and black). All three samples had concentrations of 2 mg mL−1. | ||
| Peak number | Hydrodynamic diameter (kDa) |
|---|---|
| Peak 1 (12.60 min) | 802 |
| Peak 2 (12.76 min) | 707 |
| Peak 3 (14.00 min) | 268 |
In addition to solubility enhancement, conjugated catechol groups can also be used as crosslinkers for hydrogel formation and surface functionalizing molecules in LbL assembly. Without any prior treatment, the excellent water solubility of chitosan-c12.7 allows one to form chitosan hydrogel directly from PBS pH 7.4 with ease. Chemically unmodified chitosan is insoluble in the near neutral PBS solution, resulting in precipitation, although the chitosan-c rapidly formed hydrogels upon treatment of a base such as sodium hydroxide (NaOH) (Fig. 6A) in which the catechols form intermolecular catechol–amine and/or catechol–catechol adducts.34,42 The brown color of the chitosan-c hydrogel is due to the aforementioned catechol-mediated crosslinkings. Thus, the conjugated catechol provides solubility enhancement of the chitosan in a wide range of pH values as well as chemical reactivity to form hydrogel. We found that the 3% solution (30 mg mL−1) of chitosan-c12.7 became hydrogels independent of the base concentration. However, 1% chitosan-c12.7 solution (10 mg mL−1) did not become hydrogels in all tested concentrations of the base solution. In the case of 2% solution (20 mg mL−1), hydrogels were formed only at high concentration of the base (>50 μL mL−1). Low concentrations of base showed partial gelation (triangles). The elastic modulus of one of these conditions, the 2% w/v chitosan-c12.7 hydrogel (20 mg mL−1) mixed with 50 μL of 5 N NaOH, demonstrated that the chitosan-c12.7 hydrogel is soft (approximately 2500 Pa) (Fig. 6C).
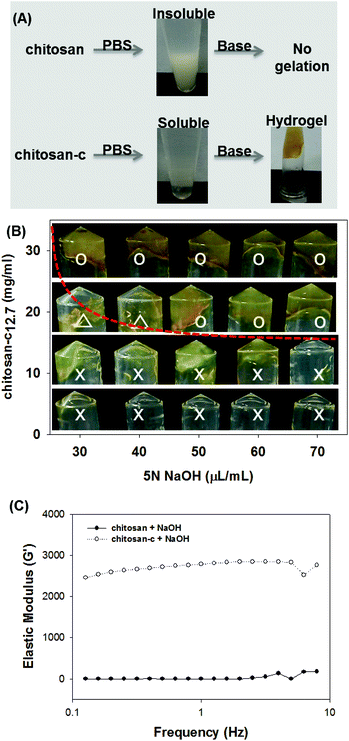 | ||
| Fig. 6 (A) Direct hydrogel formation of chitosan-c in PBS. Chitosan is insoluble in PBS preventing hydrogel formation (upper). Chitosan-c is water-soluble in PBS, allowing facile formation of chitosan hydrogels upon base addition (lower). (B) Sol–gel pseudo-diagram of chitosan-c12.7. (C) Effect of chitosan-c12.7 (20 mg ml−1) and 50 μL of 5 N NaOH in chitosan-c12.7 hydrogel formation. | ||
Catechol conjugation provides an additional utility to chitosan, which is enhancement of adhesive properties. Catechol-containing polymers, such as poly(ethylene glycol)-catechol,32,43,44 heparin–catechol,31 hyaluronic acid–catechol,44 chitosan–catechol,34 poly(ethylenimine)-catechol,45 poly(dopamine),27,28,46 poly(norepinephrine),47 and others, have exhibited improved surface adhesion. Thus, we hypothesized that water-soluble chitosan-c can be effectively used as the first polymeric layer in layer-by-layer (LbL) assembly. In fact, despite its beneficial properties, such as biocompatibility and anti-bacterial activity, the use of chitosan in the LbL assembly process has been problematic due to the poor solubility of chitosan. The first layer as a chemical primer is critical in the overall stability of LbL films on substrates.45 We chose heparin as an anionic counterpart polymer, due to its negative charge. As expected, (chitosan/heparin)2 LbL assembly performed using chitosan and heparin at a chitosan-insoluble condition (pH 7.0) resulted in barely functionalized Au substrates (Fig. 7A, bottom). In contrast, LbL assembly performed under the same conditions using water-soluble chitosan-c instead of chemically unmodified chitosan showed successful surface functionalization (Fig. 7A, top). As the number of the LbL assembly cycle increased, the film thickness showed an exponential increase when the water-soluble chitosan-c was used. However, such an increase was not observed when chemically unmodified chitosan was utilized (Fig. 7B). Previous reports showed that the growth of film thickness becomes exponential as the molecular weight of an anionic polymer decreases, due to enhanced diffusion.48 The low molecular weight of heparin we used in this study might play a similar role in the exponential growth of the film thickness. The scanning electron microscope (SEM) section image of the assembled film of (chitosan-c/heparin)20 showed a thick polymeric film with a thickness of approximately 1 μm (Fig. 7C).
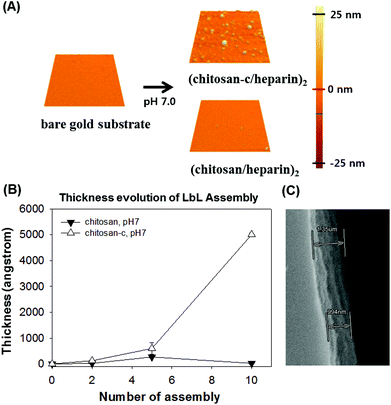 | ||
| Fig. 7 (A) SPM images of the LbL assembly on bare gold substrate (top left). An SPM image of the Au substrate functionalized by the use of water-soluble chitosan-c, (chitosan-c/heparin)2 (top right). An SPM image of the Au substrate modified by the use of chemically unmodified chitosan, (chitosan/heparin)2, which remained clean. All images are 5 μm × 5 μm in size. (B) The analysis of the LbL film thickness by an ellipsometer. Changes in the assembled film thickness as a function of the number of layers assembled of heparin and chitosan (closed triangle) or chitosan-c (open triangle) at pH 7.0. (C) A SEM image of the (chitosan-c/heparin)20 film. | ||
Conclusion
In this study, we described a simple method to prepare a water-soluble chitosan derivative, known as chitosan-c, via catechol conjugation. The resulting chitosan-c was highly water-soluble in an aqueous solution at pH values of up to 7.0 (approximately 60 mg mL−1). In an alkaline solution (pH 7.5–12), chitosan-c was partially water-soluble (0.5 mg mL−1). The molecular weight of the chitosan used in this study was high (approximately 800 kDa), indicating that this conjugation technique is useful for the solubilization of high molecular weight chitosan. In addition, the conjugation of catechol provides adhesive properties, which may be useful for application in surface modification, drug delivery, tissue engineering, and tissue adhesives.Acknowledgements
This study was supported by grants from the WCU program (R31-10071), Molecular-level Interface Research Center (2011-0001319), and Future Fundamental Technology Development Program (2010-0028765) funded by the Ministry of Education, Science and Technology, Republic of Korea.References
- T. Kean and M. Thanou, Adv. Drug Delivery Rev., 2010, 62, 3–11 CrossRef CAS.
- C. Dufes, J. M. Muller, W. Couet, J. C. Olivier, I. F. Uchegbu and A. G. Schatzlein, Pharm. Res., 2004, 21, 101–107 CAS.
- D. A. Zaharoff, C. J. Rogers, K. W. Hance, J. Schlom and J. W. Greiner, Vaccine, 2007, 25, 2085–2094 Search PubMed.
- G. Bajaj, W. G. Van Alstine and Y. Yeo, PLoS ONE, 2012, 7, e3089–e30899 Search PubMed.
- P. Li, Y. F. Poon, W. Li, H. Y. Zhu, S. H. Yeap, Y. Cao, X. Qi, C. Zhou, M. Lamrani, R. W. Beuerman, E. T. Kang, Y. Mu, C. M. Li, M. W. Chang, S. S. Jan Leong and M. B. Chan-Park, Nat. Mater., 2011, 10, 149–156 CrossRef CAS.
- S. H. Yang, Y. S. Lee, F. H. Lin, J. M. Yang and K. S. Chen, J. Biomed. Mater. Res., B: Appl. Biomater., 2007, 83, 304–313 Search PubMed.
- E. I. Rabea, M. E. Badawy, C. V. Stevens, G. Smagghe and W. Steurbaut, Biomacromolecules, 2003, 4, 1457–1465 CrossRef CAS.
- A. C. Williams, I. A. Sogias and V. V. Khutoryanskiy, Macromol. Chem. Phys., 2010, 211, 426–433 CrossRef CAS.
- S. J. Lu, X. F. Song, D. Y. Cao, Y. P. Chen and K. D. Yao, J. Appl. Polym. Sci., 2004, 91, 3497–3503 Search PubMed.
- C. K. S. Pillai, W. Paul and C. P. Sharma, Prog. Polym. Sci., 2009, 34, 641–678 CrossRef CAS.
- H. K. No, N. Y. Park, S. H. Lee and S. P. Meyers, Int. J. Food Microbiol., 2001, 74, 65–72 Search PubMed.
- B. P. Lee, J. L. Dalsin and P. B. Messersmith, Biomacromolecules, 2002, 3, 1038–1047 CrossRef CAS.
- C. C. Lin and K. S. Anseth, Pharm. Res., 2009, 26, 631–643 CrossRef CAS.
- Y. L. Hsieh and J. Du, Cellulose, 2007, 14, 543–552 Search PubMed.
- J. W. Nah, Y. I. Jeong, D. G. Kim and M. K. Jang, Carbohydr. Res., 2008, 343, 282–289 Search PubMed.
- S. R. Mao, X. T. Shuai, F. Unger, M. Wittmar, X. L. Xie and T. Kissel, Biomaterials, 2005, 26, 6343–6356 CrossRef CAS.
- S. Gogev, K. de Fays, M. F. Versali, S. Gautier and E. Thiry, Vaccine, 2004, 22(15–16), 1946–1953 Search PubMed.
- J. H. Park, S. Kwon, M. Lee, H. Chung, J. H. Kim, Y. S. Kim, R. W. Park, I. S. Kim, S. B. Seo, I. C. Kwon and S. Y. Jeong, Biomaterials, 2006, 27, 119–126 CrossRef CAS.
- D. K. Knight, S. N. Shapka and B. G. Amsden, J. Biomed. Mater. Res. A, 2007, 83, 787–798 Search PubMed.
- J. Li, Y. Du and H. Liang, J. Appl. Polym. Sci., 2006, 102, 1098–1105 Search PubMed.
- K. Kurita, M. Kamiya and S. I. Nishimura, Carbohydr. Polym., 1991, 16, 83–89 Search PubMed.
- N. M. Alves and J. F. Mano, Int. J. Biol. Macromol., 2008, 43, 401–414 CrossRef CAS.
- B. M. Bishop, E. A. Papanastasiou, Q. Y. Hua, A. Sandouk, U. H. Son, A. J. Christenson and M. L. Van Hoek, APMIS, 2009, 117, 492–499 CrossRef CAS.
- R. Zeng, H. Fu and Y. F. Zhao, Macromol. Rapid Commun., 2006, 27, 548–552 Search PubMed.
- P. S. Xu, G. Bajaj, T. Shugg, W. G. Van Alstine and Y. Yeo, Biomacromolecules, 2010, 11, 2352–2358 Search PubMed.
- Y. C. Chung, C. L. Kuo and C. C. Chen, Bioresour. Technol., 2005, 96, 1473–1482 Search PubMed.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS.
- S. Hong, K. Y. Kim, H. J. Wook, S. Y. Park, K. D. Lee, D. Y. Lee and H. Lee, Nanomedicine, 2011, 6, 793–801 CrossRef CAS.
- J. H. Waite and M. L. Tanzer, Science, 1981, 212, 1038–1040 CrossRef CAS.
- H. Lee, K. Lee, I. K. Kim and T. G. Park, Adv. Funct. Mater., 2009, 19, 1884–1890 CrossRef CAS.
- I. You, S. M. Kang, Y. Byun and H. Lee, Bioconjugate Chem., 2011, 22, 1264–1269 Search PubMed.
- H. Lee, K. D. Lee, K. B. Pyo, S. Y. Park and H. Lee, Langmuir, 2010, 26, 3790–3793 CrossRef CAS.
- M. Lavertu, Z. Xia, A. N. Serreqi, M. Berrada, A. Rodrigues, D. Wang, M. D. Buschmann and A. Gupta, J. Pharm. Biomed. Anal., 2003, 32, 1149–1158 CrossRef CAS.
- J. H. Ryu, Y. Lee, W. H. Kong, T. G. Kim, T. G. Park and H. Lee, Biomacromolecules, 2011, 12, 2653–2659 CrossRef CAS.
- A. O. Aytekin, S. Morimura and K. Kida, J. Biosci. Bioeng., 2011, 111, 212–216 Search PubMed.
- J. Ren, Q. Li, F. Dong, Y. Feng and Z. Guo, Int. J. Biol. Macromol., 2013, 53, 77–81 Search PubMed.
- C. Nunes, E. Maricato, A. Cunha, A. Nunes, J. A. da Silva and M. A. Coimbra, Carbohydr. Polym., 2013, 91, 236–243 Search PubMed.
- H. Xu, J. Nishida, W. Ma, H. Wu, M. Kobayashi, H. Otsuka and A. Takahara, Macro Lett., 2012, 1, 457–460 Search PubMed.
- J. W. Park, K. H. Choi and K. K. Park, Bull. Korean Chem. Soc., 1983, 4, 68–72.
- A. Domard, Int. J. Biol. Macromol., 1987, 9, 98–104 CrossRef CAS.
- E. P. Howlka and T. J. Deming, Macromol. Biosci., 2010, 10, 496–502 CAS.
- S. Ryu, Y. Lee, J. W. Hwang, S. Hong, C. Kim, T. G. Park, H. Lee and S. H. Hong, Adv. Mater., 2011, 23, 1971–1975 CrossRef CAS.
- K. Chawla, S. Lee, B. P. Lee, J. L. Dalsin, P. B. Messersmith and N. D. Spencer, J. Biomed. Mater. Res. A., 2009, 90, 742–749 Search PubMed.
- Y. H. Lee, H. Lee, Y. B. Kim, J. Y. Kim, T. Hyeon, H. Park, P. B. Messersmith and T. G. Park, Adv. Mater., 2008, 20, 4154–4157 CAS.
- H. Lee, Y. Lee, A. R. Statz, J. Rho, T. G. Park and P. B. Messersmith, Adv. Mater., 2008, 20, 1619–1623 CrossRef CAS.
- S. M. Kang, I. You, W. K. Cho, H. K. Shon, T. G. Lee, I. S. Choi, J. M. Karp and H. Lee, Angew. Chem., Int. Ed., 2010, 49, 9401–9404 CrossRef CAS.
- S. M. Kang, J. Rho, I. S. Choi, P. B. Messersmith and H. Lee, J. Am. Chem. Soc., 2009, 131, 13224–13225 CrossRef CAS.
- B. Sun, C. M. Jewell, N. J. Fredin and D. M. Lynn, Langmuir, 2007, 23, 8452–8459 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3bm00004d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2013 |