Open Access Article
Open Access ArticleTransition metal ion-doped cesium lead halide perovskite nanocrystals: doping strategies and luminescence design
Yunqin
Zhang
a,
Datao
Tu
 *ab,
Luping
Wang
a,
Chenliang
Li
a,
Yuhan
Liu
a and
Xueyuan
Chen
*ab,
Luping
Wang
a,
Chenliang
Li
a,
Yuhan
Liu
a and
Xueyuan
Chen
 *ab
*ab
aCAS Key Laboratory of Design and Assembly of Functional Nanostructures, Fujian Key Laboratory of Nanomaterials, and State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China. E-mail: dttu@fjirsm.ac.cn; xchen@fjirsm.ac.cn
bFujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou, Fujian 350108, China
First published on 23rd September 2023
Abstract
Cesium lead halide perovskite nanocrystals have received considerable attention due to their extraordinary optoelectronic properties including tunable bandgaps over the entire visible spectral region, high photoluminescence quantum yields, and narrow emission band widths. Transition metal ion doping in cesium lead halide perovskite nanocrystals, emerging as an effective method to manipulate the optical properties, is of vital importance for their fundamental research and applications ranging from light-emitting diodes, solar cells, and microlasers to X-ray detection. In this review, we provide an overview of the most recent advances in the design of transition metal ion-doped lead halide perovskite nanocrystals. We briefly introduce several typical strategies for effective doping of transition metal ions in cesium lead halide perovskite nanocrystals. By virtue of transition metal ion doping, we then highlight the manipulation of the optical properties of cesium lead halide perovskite nanocrystals, which includes improving stability, enhancing luminescence efficiency, and tuning emission band and luminescence lifetime. Finally, the challenges and prospects of this active research field are discussed.
Yunqin Zhang earned his B.Sc. degree (2015) in Chemistry from Soochow University. He received his PhD (2021) in Condensed Matter Physics from Fujian Institute of Research on the Structure of Matter (FJIRSM), Chinese Academy of Sciences (CAS). He joined Prof. Xueyuan Chen's group as a postdoctoral associate in 2021. His research focuses on the controlled synthesis and optical properties of inorganic luminescent materials, including lanthanide ions doped nanocrystals, lead halide perovskite nanocrystals and lead-free halide nanocrystals. |
Datao Tu earned his B.Sc. (2006) from the Wuhan University of Technology. He received his PhD (2011) in Materials Physics and Chemistry from FJIRSM, CAS. He joined Prof. Xueyuan Chen's group as a research assistant professor in 2011 and was promoted to a research professor in 2020. His research interest is focused on the chemical synthesis, optical spectroscopy and bioapplications of inorganic nanocrystals. |
Luping Wang earned her B.Sc. degree (2019) in Inorganic Non-Metallic Materials from Shandong University. She is currently a PhD candidate at the University of Chinese Academy of Sciences and studies at FJIRSM, CAS under the supervision of Prof. Xueyuan Chen. Her research focuses on the controlled synthesis and optical properties of inorganic luminescent materials, including lanthanide upconversion nanocrystals and lead-free halide perovskites. |
Chenliang Li earned his B.Sc. degree (2020) in Applied Chemistry from Shandong University. He is currently a PhD candidate at Shanghaitech University and studies at FJIRSM, CAS under the supervision of Prof. Xueyuan Chen. His research focuses on the controlled synthesis and optical properties of inorganic luminescent materials, including lanthanide upconversion nanocrystals and lead-free halide perovskites. |
Yuhan Liu earned her B.Sc. degree (2021) in Polymer materials and engineering from Jining University. She is currently a M.S. candidate at Fuzhou University and studies at FJIRSM, CAS under the supervision of Prof. Xueyuan Chen. Her research focuses on the controlled synthesis and optical properties of lead-free halide perovskites. |
Xueyuan Chen is the editor-in-chief of the Journal of Luminescence. He earned his B.Sc. degree (1993) from the University of Science and Technology of China and his PhD degree (1998) from FJIRSM, CAS. From 2001 to 2005, he was a postdoctoral research associate at the Chemistry Division of Argonne National Laboratory, U.S. Department of Energy, where he studied the photophysics and photochemistry of heavy elements. In 2005, he joined the faculty of FJIRSM, where he is currently a professor and group leader in materials chemistry and physics. His research focuses on the electronic structures, optical properties, and applications of inorganic luminescent materials, such as lanthanide-doped nanoprobes, LED phosphors, and low-dimensional metal-halide perovskites. |
1. Introduction
Since Protesescu reported the synthesis and optical properties of cesium lead halide perovskite nanocrystals (NCs) in 2015, the last few years have witnessed a rapid development of cesium lead halide perovskite NCs in the fields of luminescent materials, light-emitting diodes (LEDs), solar cells, photodetectors, scintillators and ferroelectrics.1–6 Cesium lead halide perovskite NCs are usually represented by CsPbX3 (X = Cl, Br, or I) with a three-dimensional crystal structure or Cs4PbX6 (X = Cl, Br, or I) with a zero-dimensional crystal structure. Although much progress has been made in cesium lead halide perovskites, some baffling problems like the high toxicity of Pb2+ and poor structural stability severely restricted their fundamental research and applications. In this regard, various strategies were proposed, including adjusting their composition, exploring new reaction routes, passivating their surface traps with organic molecules or inorganic salts, and constructing hybrid systems with other materials.7–10 Currently, cation doping especially transition metal ion doping is frequently proposed to tune their composition and optimize their optical properties for versatile applications.11–15Transition metal ions are employed to modulate the photophysical properties of cesium lead halide perovskite NCs by tuning the crystal growth kinetics, changing the crystal structure, or regulating their excited-state dynamics.16–18 Due to the chemical properties and electronic energy level structure, several kinds of transition metal ions are found to be suitable for doping in cesium lead halide perovskite NCs, including Mn2+, Ni2+, Cd2+, Zn2+, Ag+, and Cu+, to name a few.19,20 Among these ions, some cations (e.g., Mn2+, Cd2+) exhibit distinct luminescence, which are utilized as “optically active ions”, while other cations (e.g., Zn2+, Ag+) are usually “non-optically active ions”. When optically active ions are doped into cesium lead halide perovskite NCs, a new emission band may appear. Meanwhile, the exciton emission would be decreased or quenched due to the competition between energy transfer and radiative recombination.21 Generally, since the radii of these transition metal ions are smaller than those of Pb2+ ions, the bandgaps of the cesium lead halide perovskite NCs usually get wider upon doping. Correspondingly, the PL peak may blue shift with widening the bandgap.22 Moreover, transition metal ions may alter the near-band-edge states by eliminating the halide vacancies on the surface. Thus, the PL intensity and stability can be enhanced due to the elimination of these surface trap states.23–27
Cesium lead halide perovskite NCs have shown great promise in versatile applications ranging from LEDs, solar cells, and microlasers to X-ray detection.15,28 Under operating conditions, cesium lead halide perovskite NCs face substantial challenges such as strong light irradiation, applied electric field, unbalanced charge-injection and transport. Doping with transition metal ions is an effective way to improve the efficiency and stability of these NCs to fulfill the requirements of diverse applications.29 For example, in solar cells, transition metal ion doping can modify the optoelectrical properties including charge carrier recombination rate, diffusion length and contact resistance, as well as the open circuit voltage, resulting in improved device performance in terms of power conversion efficiency and operational stability.30,31 For LED applications, transition metal ion-doped CsPbX3 NCs can function as efficient light emitters to fabricate high-performance LEDs with higher luminance and external quantum efficiency than their pure counterparts, by eliminating surface defects and promoting the balance of charge carrier mobilities.32–36 So far, various transition metal ion-doped cesium lead halide perovskite NCs have been explored for multicolor LED applications.14,26,37 For instance, CsPb(Cl/Br)3:Ni2+, CsPbBr3:Mn2+, and CsPbI3:Zn2+ were employed in blue-emitting LEDs, green-emitting LEDs and red-emitting LEDs, respectively.35,38,39
Hitherto, several critical and tutorial reviews have summarized the development of controlled syntheses and regulation of the optical properties of cesium lead halide perovskite NCs.6,40–45 However, there have been few reviews focusing on transition metal ion-doped cesium lead halide perovskite NCs. It is urgent to renew the knowledge about the design of transition metal ion-doped cesium lead halide perovskite NCs because more new understanding or progress has been gained very recently. Rather than being exhaustive, this review aims to highlight the doping strategies and manipulation of the optical properties of transition metal ion-doped cesium lead halide perovskite NCs. This review is organized as follows (Fig. 1). First, the doping strategies of transition metal ions in cesium lead halide perovskite NCs are surveyed, with an emphasis on the hot-injection method, ion exchange method and supersaturated crystallization method. Then, the optical properties manipulation of cesium lead halide perovskite NCs through doping with transition metal ions is systematically discussed, including improving the stability, enhancing the luminescence efficiency, tuning the emission band and tuning the luminescence lifetime. Finally, emerging trends and further efforts are proposed.
 | ||
| Fig. 1 Overview of transition metal ions doped cesium lead halide perovskite NCs from doping strategies to optical properties manipulation. | ||
2. Doping strategies
Because of the fast nucleation and growth process of cesium lead halide perovskite NCs, the controlled doping of transition metal ions remains a challenge.46 Theoretically, transition metal ions can be doped at the A-site (Cs+) or the B-site (Pb2+) of CsPbX3 NCs by in situ synthesis or post-synthesis approaches.47 Nowadays, the hot-injection method and post-synthetic cation exchange method are two commonly used methods for the preparation of high-quality transition metal ion-doped cesium lead halide NCs (Table 1).| Host | Dopant | Synthesis strategy | Reaction temperature (°C) | Feed ratio ([dopant]/[Pb]) | Actual doping content | Size (nm) | Ref. |
|---|---|---|---|---|---|---|---|
| CsPbCl3 | Mn2+ | hot-injection | 200 | 1 | 0.6% | 7.3–8.6 | 111 |
| CsPb(Cl0.6Br0.4)3 | Mn2+ | hot-injection | 250 | 5 | 25% | ∼19 | 13 |
| CsPbCl3 | Mn2+ | cation exchange | 25 | 0.1 | 5.7% | 7.2 | 104 |
| CsPbCl3 | Mn2+ | cation exchange | 25 | 100 | 5.7% | 16.7 | 47 |
| CsPbI3 | Ni2+ | ion exchange | 25 | 0.5 | 1.38% | 15 | 76 |
| CsPbBr3 | Ni2+ | hot-injection | 180 | 2.5 | — | 14-15 | 17 |
| CsPbCl3 | Ni2+ | hot-injection | 210 | 2 | 11.9% | 8.3 | 78 |
| CsPbBr3 | Cd2+ | hot-injection | 220 | 4 | 0.92% | 84 × 16 | 134 |
| CsPbBr3 | Cd2+ | hot-injection | 170 | 2 | 7% | 10.21 | 81 |
| CsPbCl3 | Zn2+ | hot-injection | 210 | 1.5 | 8.6% | 8.4–9.73 | 79 |
| CsPbBr3 | Zn2+ | hot-injection | 90 | 2 | — | 13.1 | 73 |
| CsPbCl3 | Cu2+ | hot-injection | 185 | 1.17 | 7% | 7.0 | 75 |
| CsPb(Cl/Br)3 | Cu+ | anion exchange | 25 | ∼7.3 | — | 10 | 21 |
| CsPb(Cl/Br)3 | Cu2+ | anion exchange | 25 | ∼3.65 | — | 10 | 21 |
| CsPb(Br/I)3 | Ag+ | hot-injection | 25 | 0.04 | 3.5% | 8.1–8.4 | 24 |
| CsPbBrI2 | Fe2+ | hot-injection | 160 | 0.5 | 2.4% | 15.2–18.5 | 25 |
| Cs4PbCl6 | Mn2+ | hot-injection | 180 | 0.25 | 23.6^ | 20 | 92 |
Very recently, other doping strategies including supersaturated crystallization, ligand-assisted ultrasonication, mechanosynthesis, host phase transition method, and modular microfluidics method are proposed.48–51 Supersaturated crystallization and ligand-assisted ultrasonication are both carried out in solution at ambient atmosphere. Supersaturated crystallization utilizes the solubility difference of perovskite solutions in polar solvents and non-polar solvents.52 The ligand-assisted ultrasonication method is a versatile, polar-solvent-free, single-step approach based on the direct ultrasonication of the corresponding precursors in the presence of organic ligands.53 Through mechanosynthesis, powder products can be obtained without using polar solvents, where CsPbX3 NCs are prepared using a planetary ball mill with the addition of ligands.54 The modular microfluidics method involves the injection of two or more component liquids (e.g., droplets) into capillary channels of the automated modular microfluidic platform, where the chemical composition and morphology of the samples can be tailored.51,55
In this section, we will briefly illustrate the doping strategies of the hot-injection method, cation exchange method and supersaturated crystallization method, which were widely employed.
2.1. Hot-injection method
To increase the doping concentration of Mn2+ in cesium lead halide perovskite NCs, the feed ratio of [Mn]/[Pb] precursors and the reaction temperature were investigated. Specifically, when the feed ratio of [Mn]/[Pb] precursors was increased from 1.25 to 10 at a reaction temperature of 170 °C, the Mn2+ doping concentration can be increased from 2% to 27%.60,61 Because Mn2+ doping is a thermodynamically controlled process, a high reaction temperature may facilitate the replacement of Pb2+ with Mn2+ in cesium lead halide perovskite NCs. When the reaction temperature was elevated from 170 °C to 210 °C with the feed [Mn]/[Pb] precursor ratio of 10, the doping concentration of Mn2+ can be further increased from 27% to 46%.62 However, such reaction protocols with a high feed ratio of [Mn]/[Pb] precursors may form byproducts like CsCl or PbCl2, which should be avoided.63,64
In addition, the concentration of Cl− in precursors is also essential for the doping content of transition metal ions in CsPbCl3 NCs.65 For the preparation of Mn2+-doped CsPbCl3 NCs, MnCl2 was found to be more efficient relative to several other manganese salts such as Mn(Ac)2 (Ac: acetate), Mn(acac)2 (acac: acetylacetone), or Mn(oleate)2. The main reason is the similar bond dissociation energy of Mn–Cl (338 kJ mol−1) and Pb–Cl (301 kJ mol−1).57 Despite this, the doping efficiency of Mn2+ is relatively low, where the precursor with a [Mn]/[Pb] ratio of 1.5 may only result in ∼0.2% Mn2+ in the obtained CsPbCl3:Mn2+ NCs.56 To promote the effective doping of Mn2+ in cesium lead halide NCs, a chloride-rich high-temperature reaction was designed, where several chloride chemicals like alkylamine hydrochloride, trimethylchlorosilane, or CuCl2 was used along with Mn2+ salts such as Mn(Ac)2 or MnCl2.13,63,66,67 By employing alkylamine hydrochloride, the doping content of Mn2+ in the obtained CsPbCl3:Mn2+ NCs can be as high as 1.3% with a low [Mn]/[Pb] precursor ratio of 0.05. In another report, trimethylchlorosilane was used to promote the formation of both octahedral structural units [PbCl6]4− and [MnCl6]4− in the solution before Cs+ injection. Correspondingly, the Mn2+ doping content was determined to be 10.3% in the obtained CsPbCl3:Mn2+ NCs with a [Mn]/[Pb] precursor ratio of 1.0.
The as-prepared transition metal ion-doped CsPbX3 samples are usually cube-shaped NCs. To tune their morphology, a thermal conversion strategy was explored to synthesize CsPbCl3 nanoplates.68–71 As a typical example, Das Adhikari et al. demonstrated that CsPbCl3:Mn2+ nanoplates with tunable size can be obtained through thermal conversion from Mn2+-contained layered perovskites.50 In their report, butylammonium chloride, PbCl2, MnCl2 and ligands were dissolved at 160 °C in octadecene under heating and then cooled down to 100 °C to form L2MnxPb1−xCl4 (L = n-butylammonium and oleylammonium ions) layered perovskites. Then, a hot solution of Cs-oleates was injected. Upon heating at 230 °C, L2MnxPb1−xCl4 was converted to CsPbCl3:Mn2+ nanoplates (Fig. 3a). Through adjusting the feed [Mn2+]/[Pb2+] precursor ratio from 0 to 1, the size of the final CsPbCl3:Mn2+ nanoplate can be tuned from 580 nm to 20 nm, while the thickness of the platelets remained essentially unchanged (∼5 nm) (Fig. 3b–f).
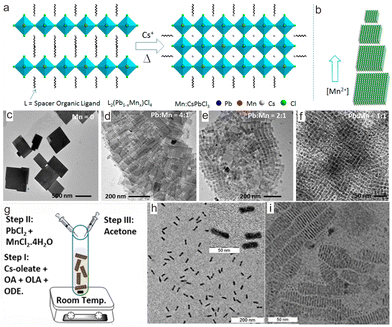 | ||
| Fig. 3 (a) Schematic illustration of the formation of CsPbCl3:Mn2+ nanoplates from layered perovskites L2(Pb1−xMnx)Cl4, wherein L is n-butylammonium and oleylammonium ions. (b) Schematic illustration shows Mn2+ concentration in the reaction mixture with different sizes of CsPbCl3:Mn2+ NCs. Transmission electron microscopy (TEM) images of (c) undoped and (d)–(f) Mn2+-doped CsPbCl3 nanoplates based on different [Pb]:[Mn] precursor ratios. (g) Schematic shows the synthesis of CsPbCl3:Mn2+ nanoplates. OA, OLA, and ODE refer to oleic acid, oleylamine, and 1-octadecene, respectively. (h) TEM image of CsPbCl3:Mn2+ nanoplates lying flat on the TEM grid. Inset shows a magnified view. (i) TEM image of self-assembled CsPbCl3:Mn2+ nanoplates lying perpendicular to the TEM grid. (a)–(f) Reproduced with permission from ref. 68. Copyright 2019, American Chemical Society; (g)–(i) reproduced with permission from ref. 69. Copyright 2020, American Chemical Society. | ||
To circumvent the cumbersome procedures for preparing the layered perovskite of L2MnxPb1−xCl4, a convenient strategy was proposed to directly synthesize Mn2+-doped CsPbCl3 nanoplates by utilizing Cl−-rich precursors to confine the nanocrystal growth at the lattice face 〈001〉.51 To dissolve the metal chloride precursors in solutions for high Cl− content, polar solvents like N,N-dimethylformamide (DMF) or dimethyl sulfoxide (DMSO) were employed, wherein HCl, PbCl2 and MnCl2 were dissolved to prepare Cl−-rich precursors solution. Such a solution was then injected into a toluene solution containing Cs-oleates, followed by adding acetone (Fig. 3g). As a result, CsPbCl3:Mn2+ nanoplates with a thickness of 2.3 nm were obtained, corresponding to four monolayers of CsPbCl3 (Fig. 3h and i). Inspired by this work, CsPbCl3:Mn2+ with hexapod structure was prepared based on oleylamine hydrochloride.52,53
Similar to Mn2+ doping, the hot-injection method was also applied for the doping of other transition metal cations (e.g., Ni2+, Zn2+, Cd2+, Cu2+, Cu+, Ag+, Ti3+, and Fe3+) in cesium lead halide perovskite NCs.14,24,56,72–81 Specifically, copper ion exhibits oxidation numbers of +1 and +2. Generally, Cu2+ can be readily doped in CsPbX3 (X = Cl, Br, I or mixture) NCs.63,82,83 By employing reductive reagents such as trioctylphosphate in the solution, Cu+ can be doped into CsPbCl3 NCs.75
Interestingly, the selection of transition metal precursors may result in different doping sites of transition metal ions in CsPbX3 NCs. In a recent report, two kinds of Zn2+ doping sites were achieved in CsPbI3:Zn2+ NCs. Specifically, it was discovered that Zn2+ dopants may substitute the Pb2+ site in CsPbI3 using ZnI2 precursors, which improved the local ordering of the lattice and reduced the octahedral distortions as confirmed by X-ray absorbance fine structure spectra and X-ray absorption near-edge structure spectra.84 Nevertheless, using non-halide zinc salts (e.g., zinc acetylacetonate) may lead to doping of Zn2+ in the interstitial sites, thus causing lattice expansion as confirmed by the powder X-ray diffraction (XRD) patterns and pair distribution function analyses.36
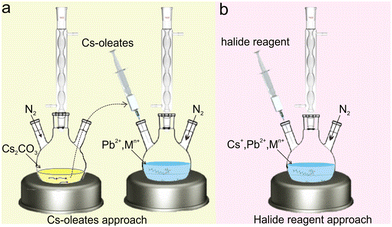
Since the bromide reagents and iodide reagents (e.g., benzoyl bromide, benzoyl iodide) are usually more volatile, corrosive and highly reactive than chloride reagents (e.g., benzoyl chloride), such an approach using halide reagents was mainly applied for the synthesis of transition metal-doped CsPbCl3 NCs. Benefiting from the benzoyl group or phenylphosphoryl of a halide reagent attached to the surface of NCs, the as-prepared transition metal-doped CsPbCl3 NCs often exhibited high photoluminescence quantum yield (PLQY) (>60%) via this approach.85,86 By contrast, the as-synthesized transition metal-doped CsPbCl3 NCs through Cs-oleates approach often suffer from relatively low PLQY (∼5%).87,88 Based on the halide reagent approach, CsPbCl3:Cd2+ and CsPbCl3:Mn2+ NCs with highly efficient emissions were obtained.89–91
By virtue of the flexible regulation of the precursor ratio of halide ions and metal ions, we have synthesized Mn2+-doped rhombohedral phase Cs4PbCl6 NCs (Fig. 4).92 Typically, benzoyl chloride was injected into a hot solution containing CsCO3, Pb(Ac)2 and Mn(Ac)2 with a [benzoyl chloride]/([Pb] + [Mn]) ratio of 6. Benzoyl chloride may release chloride ions very fast at high temperatures. It was discovered that a high [Cl]/[Pb] ratio may be the key to obtaining pure Cs4PbCl6:Mn2+ NCs without the impurities of CsPbCl3. The actual doping concentration of Mn2+ in the as-synthesized NCs was tuned from 0.7 mol% to 23.6 mol% by increasing the precursor ratio of [Pb]/[Mn] in the solution. The successful doping of Mn2+ into the Cs4PbCl6 lattice can be confirmed by the PL spectra and XRD patterns (Fig. 4a and b). As shown in the TEM images, the as-prepared Cs4PbCl6:Mn2+ NCs exhibited a hexagonal shape (Fig. 4c–e), which is markedly different from the well-established CsPbCl3:Mn2+ NCs. Elemental mapping images indicated that Mn2+ ions were distributed uniformly in the obtained NCs (Fig. 4f–k), further confirming the successful doping of Mn2+ in the host lattice.
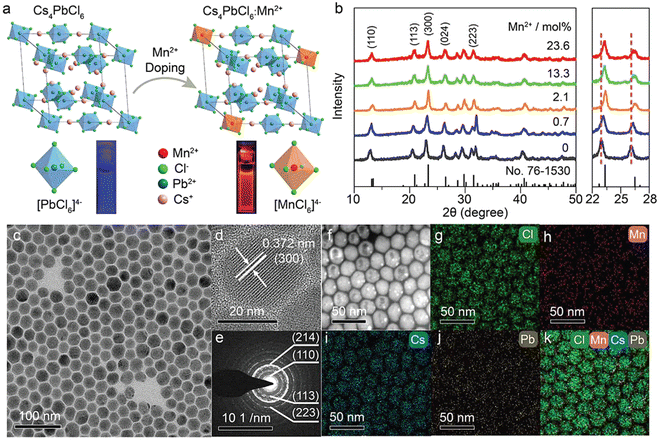 | ||
| Fig. 4 (a) Crystal structure of rhombohedral Cs4PbCl6 and the crystallographic site for Mn2+ dopants. PL photographs of Cs4PbCl6 NCs dispersed in cyclohexane under 304 nm ultraviolet lamp irradiation are presented. (b) XRD patterns of Cs4PbCl6:Mn2+ NCs with different Mn2+ doping concentrations. Bottom lines represent the standard XRD pattern of rhombohedral Cs4PbCl6 (JCPDS No. 76-1530). The enlarged 2θ range (22°–28°) of XRD patterns shows a monotonic shift of the diffraction peaks with increasing the Mn2+ concentration. (c) TEM image, (d) high-resolution TEM image, (e) selected area electron diffraction pattern, (f) scanning transmission electron microscopy (STEM) image, and (g)–(k) corresponding elemental mappings (Cs, Pb, Mn, and Cl) of Cs4PbCl6:Mn2+ NCs. Reproduced with permission from ref. 92. Copyright 2019, Wiley-VCH Verlag GmbH l Co. KGaA, Weinheim. | ||
2.2. Ion exchange method
In the above-mentioned hot-injection method, doping with transition metal ions is carried out with in situ synthesis of the halide perovskite host. As an alternative, doping with transition metal ions can be achieved by a post-synthesis strategy such as ion exchange. The ion (cation or anion) exchange method allows access to a variety of composition and shapes which are not easily attainable through the direct synthesis method.93–97 For transition metal ion-doped cesium lead halide perovskite NCs, ion exchange generally proceeds in non-polar solutions containing transition metal ion salts (e.g., MnBr2, ZnBr2) and cesium lead halide perovskite NCs. It was demonstrated that halide ion exchange was very efficient and fast in the synthesis of cesium lead halide perovskite NCs.98 However, cation exchange in early reports usually led to the decomposition of the host NCs when exchanging Cs+ or Pb2+ with other guest cations (Rb+, Ag+, Cu+, Ba2+, Sn2+, Ge2+, or Bi3+) in CsPbX3 NCs, because the perovskite crystal structure was stabilized primarily by the rigid [PbX6]4− cationic sublattice. As such, the unbalanced rates of extraction of Pb2+ and incorporation of the guest cations resulted in the collapse of the CsPbX3 NCs.99To enable the cation exchange of transition metal ions in CsPbX3 NCs, van der Stam et al. proposed a kinetically controlled method, wherein Pb2+ ions were partially replaced by divalent cations (e.g., Sn2+, Cd2+, and Zn2+) in CsPbBr3 NCs with a doping content of less than 16% (Fig. 5a).99 In their work, an oleylamine solution containing metal halides of MX2 (e.g., ZnBr2) was mixed with a CsPbBr3 NC solution with [M2+]/[NC] ratio varied between ∼8000 and ∼300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. Oleylamine in the solution would aid the formation of halide vacancies on the surface of CsPbX3 NCs. Such halide vacancies can be occupied by MX2, followed by the breaking of the bonds between the surface PbBr2 and the CsPbBr3 NC. Thus, the Pb2+ cations on the surface can be exchanged by the M2+ guest cations. The obtained M2+-doped CsPbBr3 NCs essentially maintained the size and shape as those of the pristine CsPbBr3 NCs (Fig. 5b). By adopting other transition metal halide salts, this method was successfully applied to the doping of Mn2+, Zn2+ and Ni2+ in CsPb(Br/Cl)3 or CsPbI3 NCs.100–102 However, such a cation exchange reaction usually takes a long time (>16 h) because the surface exchange rate and diffusion fluxes for both the outgoing Pb2+ and the incoming M2+ cations are slow.
000. Oleylamine in the solution would aid the formation of halide vacancies on the surface of CsPbX3 NCs. Such halide vacancies can be occupied by MX2, followed by the breaking of the bonds between the surface PbBr2 and the CsPbBr3 NC. Thus, the Pb2+ cations on the surface can be exchanged by the M2+ guest cations. The obtained M2+-doped CsPbBr3 NCs essentially maintained the size and shape as those of the pristine CsPbBr3 NCs (Fig. 5b). By adopting other transition metal halide salts, this method was successfully applied to the doping of Mn2+, Zn2+ and Ni2+ in CsPb(Br/Cl)3 or CsPbI3 NCs.100–102 However, such a cation exchange reaction usually takes a long time (>16 h) because the surface exchange rate and diffusion fluxes for both the outgoing Pb2+ and the incoming M2+ cations are slow.
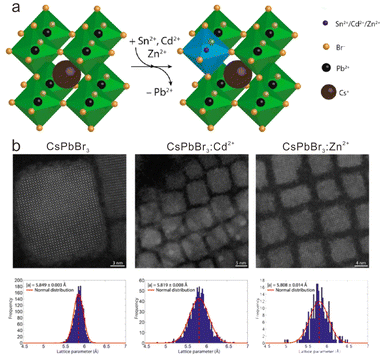 | ||
| Fig. 5 (a) Schematic illustration of partial cation exchange of host Pb2+ with guest M2+ ions (M = Mn, Zn and Cd) in CsPbBr3 NCs. (b) Quantitative high-angle annular dark-field STEM images of CsPbBr3, CsPbBr3:Cd2+ and CsPbBr3:Zn2+. Reproduced with permission from ref. 99. Copyright 2017, American Chemical Society. | ||
It was demonstrated that surface ligands played an important role in the cation exchange reaction.103 As such, several ligands were explored to accelerate the exchange rate of Pb2+ by transition metal dopants through substantial reduction of the activation energy for the formation of B-site vacancies.104,105 Utilizing surface ligand exchange with the formation of Lewis acid/base pair, the halide ions attached with Pb2+ can be replaced with ligand anions like carbonate. Then, Pb2+ can be removed for exchange with transition metal dopants. As a typical example, Yang et al. proposed a surface-ligand-exchange-inspired dynamic ion exchange method to accelerate the ion exchange of Pb2+ with Zn2+ in CsPbBr3 NCs.106 Generally, the surface of CsPbBr3 NCs is Cs-Br terminated or Br-R-NH2+ terminated (R is the bulky organic component). Thus, zinc ethylhexanoate (Zn(Oct)2) was employed to promote the departure of surface R-NH2+ ligands and halide ions, followed by the formation of the surface ion pair Pb-Oct. Such Pb-Oct ion pair was then exchanged by the dopant pair of Zn-Oct on the surface of CsPbBr3 NCs. Such an ion exchange process can be accomplished within serval hours by the ongoing cation diffusion procedure.
Besides the surface ligands, anions were also revealed to promote cation exchange. Compared with cation exchange in cesium lead halide perovskite NCs, anion exchange is much faster. For example, the anion exchange of Br− with Cl− in CsPbBr3 NCs can be readily achieved due to the breaking of the Pb–Br bond and the formation of a Pb–Cl bond. Hence, utilizing the anion exchanged to open up the rigid [PbX6]4− may favor the cation exchange. To exemplify this, Huang et al. dissolved MnCl2 in DMF, which was dropped into a toluene solution containing CsPbBr3 NCs for the synthesis of Mn2+-doped CsPb(Cl/Br)3 NCs.62 They attributed the success of halide-exchange-driven cation exchange to two prerequisites: (i) diffusion of MnCl2 into the CsPbBr3 NC lattice, and (ii) simultaneous existence of halide exchange and cation exchange between MnCl2 and CsPbBr3 NCs. Such a cation exchange process is rapid that can be finished within half an hour.
Initially, the method of halide-exchange-driven cation exchange was mainly applied to CsPbBr3 NCs through direct mixing with dopant salts and ligands. Nevertheless, it was discovered that such a cation exchange may not be achieved in CsPbCl3 NCs by directly mixing with MnCl2 or MnBr2.107 Fortunately, under light irradiation, cation exchange was realized in a dichloromethane (CH2Cl2) solution containing CsPbCl3 NCs and transition metal ion salts. The exchange rates can be controlled by excitation light intensity and lasting time. Such a kind of cation exchange is also called “photo-induced doping”, which benefits from the process called “self-anion exchange” occurring under light irradiation on the surface of CsPbCl3 NCs in CH2Cl2. Thus, it provides a convenient and universal approach for doping the surface of CsPbCl3 NCs with transition metal ions. For example, surface doping of several transition metal ions such as Cu2+, Zn2+, or Cd2+ in CsPbCl3 NCs was realized, based on the slow diffusion rate of these transition metal ions in the CsPbCl3 lattice.21,108,109
2.3. Supersaturated crystallization method
Supersaturated crystallization was also called “ligand-assisted reprecipitation”.110 In principle, it was induced by a change in solubility by mixing Cs+, Pb2+ and transition metal precursors in a “good solvent” (high solubility) into a “poor solvent” (low solubility) with ligands at room temperature. The “good solvents” are generally polar solvents such as DMF, DMSO or acetonitrile, while “bad solvents” are weak-polar or non-polar solvents such as toluene, chlorobenzene, etc.For the preparation of Mn2+-doped CsPb(Br/Cl)3 NCs, a solution containing high concentrations of CsBr, PbBr2, MnCl2 and ligands in DMF was dropped into toluene with ligands such as oleic acid. Thereafter, CsPb(Cl/Br)3:Mn2+ NCs were formed in the mixture solution (Fig. 6a).52 With increasing the ratio of [MnCl2]/[PbBr2] from 0 to 7.5, the Mn2+ concentration in the as-prepared CsPb(Cl/Br)3:Mn2+ NCs can be elevated to 37.7%. However, the length of the as-prepared CsPb(Cl/Br)3:Mn2+ NCs remained at ∼11 nm under different feeding ratios of [MnCl2]/[PbBr2] (Fig. 6b–e). High-resolution TEM image of the as-prepared CsPb(Cl/Br)3:Mn2+ NCs indicated an interplanar distance of 0.56 nm, corresponding to the (100) plane of CsPb(Cl/Br)3 (Fig. 6e). Theoretically, this method does not require heating when the temperature is higher than the melting point of the solvents (the melting points of DMSO and DMF are 19 °C and −61 °C, respectively). Thus, the preparation process is time-saving without cumbersome heating and cooling processes.
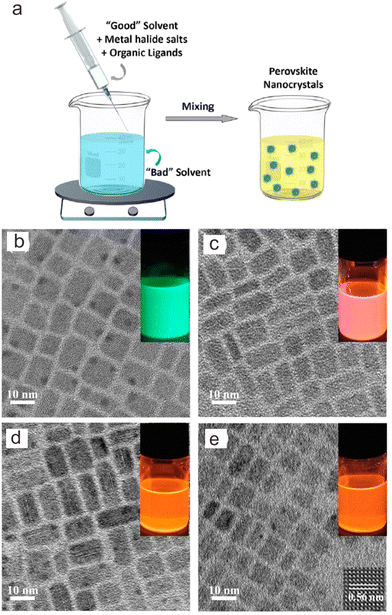 | ||
| Fig. 6 (a) Schematic illustration of supersaturated crystallization method. TEM images of (b) CsPbBr3 NCs and CsPb(Cl/Br)3:Mn2+ NCs with Mn/Pb molar feed ratios of (c) 2.0, (d) 5.0, and (e) 7.5, respectively. Insets show their PL photographs dispersed in cyclohexane under ultraviolet lamp irradiation. Reproduced with permission from ref. 52. Copyright 2017, American Chemical Society. | ||
Moreover, due to the wide choice of solvents and ligands in this approach, the composition and morphologies of transition metal ion-doped cesium lead halide perovskite NCs can be readily manipulated. For instance, CsPbBr3:Mn2+ NCs and magic-sized clusters (MCS) with a size of 300 nm can be prepared based on the ligand of benzoic acid (BA) and benzylamine (BZA).111 Specifically, MnCl2 or MnBr2 was dissolved in DMF containing BA and BAZ, with a [BA]/[Pb] ratio of 30. The DMF solution was then injected rapidly into toluene with vigorous stirring at a [DMF]/[toluene] volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. At a relatively low [BA]/[Pb] concentration ratio of 12, CsPbBr3:Mn2+ NCs with ∼12 nm can be obtained. Nevertheless, the remaining small amount of DMF may be detrimental to CsPbBr3:Mn2+ NCs, because the CsPbBr3:Mn2+ NCs were unstable in DMF. In this regard, Xu et al. developed a DMF-free method to obtain CsPbCl3:Mn2+ NCs. Toluene instead of DMF was used to dissolve CsAc, Pb(Ac)2, Mn(Ac)2 and ligands. After adding HCl into the toluene solution, CsPbCl3 Mn2+ NCs were precipitated.112,113 Similarly, Pan et al. also reported the synthesis of Ni2+-doped CsPb(Cl/Br)3 NCs.114
10. At a relatively low [BA]/[Pb] concentration ratio of 12, CsPbBr3:Mn2+ NCs with ∼12 nm can be obtained. Nevertheless, the remaining small amount of DMF may be detrimental to CsPbBr3:Mn2+ NCs, because the CsPbBr3:Mn2+ NCs were unstable in DMF. In this regard, Xu et al. developed a DMF-free method to obtain CsPbCl3:Mn2+ NCs. Toluene instead of DMF was used to dissolve CsAc, Pb(Ac)2, Mn(Ac)2 and ligands. After adding HCl into the toluene solution, CsPbCl3 Mn2+ NCs were precipitated.112,113 Similarly, Pan et al. also reported the synthesis of Ni2+-doped CsPb(Cl/Br)3 NCs.114
3. Optical properties manipulation
Through the above-mentioned methods, transition metal ion-doped cesium lead halide perovskite NCs can be prepared, where their optical properties can be manipulated via changing the experimental conditions like reaction time, reaction temperature and concentration of the precursors. Specifically, the size, morphology and surface of the NCs may be readily tuned via the hot injection method.115 In the ion exchange method, the composition of the samples obtained is adjustable while maintaining the morphology. As a result, the PL intensity, emission band or PL lifetime of the transition metal ion-doped cesium lead halide perovskite NCs can be manipulated.116 In the supersaturated crystallization method, it is difficult to finely control the morphology due to the rapid precipitation. Consequently, the PL peak of the obtained products may be relatively broad.52In this section, we will survey several typical strategies regarding the manipulation of optical properties of cesium lead halide perovskite NCs by doping them with transition metal ions, in order to improve their stabilities, enhance their luminescence efficiency, and tune their emission band or PL lifetime.
3.1. Improving stability
The applications of cesium lead halide perovskite NCs are severely limited by their poor intrinsic stabilities, which are mainly associated with the low formation energies of perovskite lattices.117 Particularly, cesium lead halide perovskite NCs are prone to deteriorate when in contact with moisture in the air. Moreover, it was reported that heat, oxygen, ultraviolet (UV)-light irradiation, strong sunlight irradiation, and electronic field may accelerate this process.118 These concerns greatly motivate the exploration of transition metal ion-doped cesium lead halide perovskite NCs, aiming to overcome the poor stability of cesium lead halide perovskite NCs.119,120Among the CsPbX3 (X = Cl, Br, or I) NCs, CsPbI3 NCs exhibit the worst stability due to the large ion radius of the I− ion compared with Br− and Cl− ions.119 It was proved that I− ions with a large radius may easily induce lattice distortion as well as phase transformation.121 Furthermore, the crystal structural variation of CsPbI3 NCs may significantly affect their PL properties. In particular, CsPbI3 in cubic-phase (α-CsPbI3, Eg = 1.73 eV) exhibiting excellent optical performance is prone to be transformed to an orthorhombic phase (δ-CsPbI3, Eg = 2.25 eV) with the poor optical performance. Thus, it is critical to maintain the framework of a corner-sharing [PbI6]4− octahedron in α-CsPbI3. Nevertheless, due to the ionic nature of the CsPbI3 NC lattice, the surface halide vacancy would accelerate the breakdown of CsPbI3 NCs. To overcome such an obstacle, several research groups doped small radius ions like Mn2+, Zn2+ or Ni2+ in CsPbI3 NCs to suppress the rotation of [PbI6]4− octahedra by enhancing the energy barrier. Such cation doping induces lattice contractions and the Goldschmidt tolerance factor increments, thus making the CsPbI3 NCs more stable in the cubic phase.27 As a representative example, CsPbI3:Mn2+ still exhibited bright emissions after storage in toluene for more than 30 days, while the undoped CsPbI3 counterparts exhibited weak emissions after several days.58
In addition to colloidal CsPbX3 NCs, the stability of CsPbX3 NCs-based films can also be substantially improved by doping with transition metal ions. When preparing CsPbI3 NCs-based films, the loss of ligands during the washing process may result in iodine vacancy (VI) defects, which would accelerate the phase transition process.35 However, the formation energy of VI defects can be enhanced by transition metal ions doping, enabling the reduction of VI defects and inhibiting phase transition. It was reported that the PLQY of CsPbI3:Zn2+ films maintained 80% of its initial value after 10 days (Fig. 7a and b).80 In contrast, the emission of pure CsPbI3 films disappeared completely after 10 days. As shown in Fig. 7a, the optimal improvement of stability can be achieved with doping of 5 mol% Zn2+ in CsPbI3 NCs.
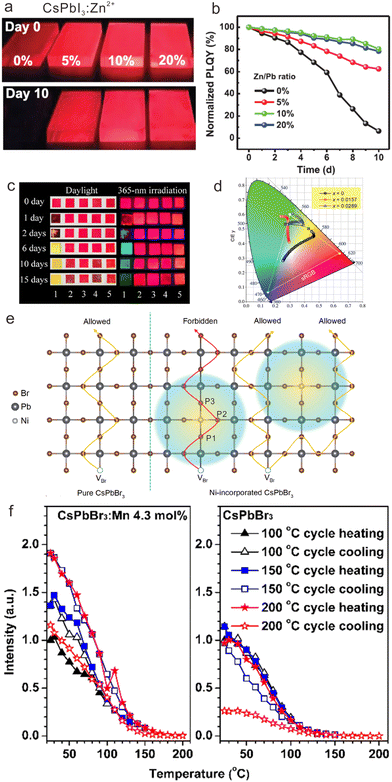 | ||
| Fig. 7 (a) PL photographs and (b) normalized PLQYs for α-phase CsPbI3 and CsPbI3:Zn2+ NCs films as a function of aged days. (c) PL photographs of Cu2+-doped CsPb(Br/I)3 NCs under UV illumination during 15 days. (d) CIE 1931 chromaticity diagram for CsPb1-xNixBr1.5I1.5 with different x values upon different measurement time from 0 to 300 min. (e) Schematic illustration of the repelling effect of Ni2+ on the moving VBr. Compared with pure CsPbBr3, the repelling effect reduces the ionic migration channels and lengthens the migration path. (f) Temperature-dependent PL intensities for excitonic luminescence of CsPbBr3:Mn2+ (4.3 mol%) and pure CsPbBr3 NCs via three heating/cooling cycles at 100, 150, and 200 °C, respectively. (a) and (b) Reproduced with permission from ref. 80. Copyright 2021, Wiley-VCH Verlag GmbH l Co. KGaA, Weinheim; (c) reproduced with permission from ref. 105. Copyright 2019, Elsevier; (d) and (e) reproduced with permission ref. 77. Copyright 2022, Wiley-VCH Verlag GmbH l Co. KGaA, Weinheim; (f) reproduced with permission ref. 39. Copyright 2017, American Chemical Society. | ||
Compared with mono-halide CsPbX3 NCs mentioned above, mixed halide CsPbX3 NCs such as CsPb(Br/Cl)3 and CsPb(Br/I)3 NCs suffer from severe problems of halogen segregation. To solve this issue, Cu2+ ions with a relatively small radius (0.72 Å) were proposed to partly replace Pb2+ ions in the lattice of CsPbBrI2 NCs, in order to enhance the bond strength of Pb-halides and suppress the halogen segregation (Fig. 7c).122 Specifically, the emission color of pristine CsPb(Br/I)3 NC-based films gradually changed from red to green after 2 days, which originated from the halogen segregation of CsPb(Br/I)3 NCs into α-CsPbI3, δ-CsPbI3 and CsPbBr3 NCs. Nevertheless, CsPbBrI2:Cu2+ NCs maintained bright red luminescence for more than 15 days.
To shed more light on the mechanism of ionic migration suppression of mixed halide CsPbX3 NCs by transition metal doping, Chen et al. investigated lattice stabilization in CsPb(Br/I)3 NCs by doping with Ni2+ ions.77 For pristine CsPb(Br/I)3 NCs, the PL spectra evolved from one peak (587 nm) to three peaks (610 nm, 521 nm, and 667 nm) after 300 min, and the emission color changed from red to white. With the incorporation of Ni2+ in CsPbBr1.5I1.5, such emission color variability was greatly inhibited as shown in the Commission Internationale de l’Eclairage (CIE) 1931 chromaticity diagram (Fig. 7d), indicative of the effective improvement of photostability. To reveal the mechanism, they illustrated CsPbBr3:Ni2+ as a model. Accordingly, ion migration in CsPbBr3 can be described by a series exchange of Br− with neighboring bromide vacancy (VBr), which were marked as P1, P2 and P3. After Ni2+ doping, the transition states of VBr hopping from P1 to P2 and from P2 to P3 were determined to be 0.318 eV and 0.408 eV, respectively, both of which are higher than the value (0.26 eV) in pure CsPbBr3. Correspondingly, VBr hopping toward Ni2+ ion was forbidden in energy, resulting in a repulsion effect on the migrating VBr and reducing the VBr migration channels. Consequently, the VBr migration length in CsPbBr3 was increased after Ni2+ doping, leading to long-range lattice stabilization (Fig. 7e). They systematically compared Ni2+ with Zn2+ and Bi3+ for the ionic migration suppression effect, which indicated that the coupling between partially filled 3d orbital of Ni2+ and Pb 6s-Br 4p antibonding states is the key to lattice stabilization. Such coupling can passivate the active Pb 6s2 lone-pair electron and enhance the chemical bond strength in surrounding Pb-Br octahedra, thus facilitating long-range lattice stabilization.
Because of the intrinsically low formation energies of perovskite lattices, the thermal stability of cesium lead halide perovskite NCs is another critical issue that needs to be addressed. Doping with transition metal ions may effectively engineer the local structure of cesium lead halide perovskite NCs, which improves their thermal stability by enhancing the formation energies of perovskite lattices. For instance, we doped Mn2+ in CsPbBr3 NCs, which displayed better thermal stability relative to the pristine CsPbBr3 counterparts.39 We compared the temperature-dependent PL spectra of pristine CsPbBr3 and CsPbBr3:Mn2+ NCs by gradually heating them from 25 °C to higher temperatures (100, 150, and 200 °C) and then cooling them to 25 °C. Subsequently, the PL spectra at 25 °C were monitored (Fig. 7f and g). It was found that the room-temperature PL intensities for CsPbBr3:Mn2+ can retain about 120% of their initial intensities undergoing three heating and cooling cycles at 100, 150, and 200 °C, which is superior to their undoped counterparts. Hitherto, the enhancement in thermal stability has also been achieved in Mn2+-doped CsPbCl3 NCs, Cd2+, Co2+ or Zn2+-doped CsPbBr3 NCs, and Ni2+, Zn2+, or Mn2+ doped CsPbI3 NCs, respectively.35,76,109,123,124
Note that the thermal stability of cesium lead halide perovskite NCs was closely associated with defect or trap states. To this regard, Ni2+ doping was proposed, which was demonstrated to reduce the defect or trap states of CsPbCl3 NCs to improve their thermal stability.125 After Ni2+ doping, the thermal activation energy was increased from 58.7 meV to 82.6 meV, which effectively alleviated the thermal quenching of CsPbCl3 NCs. Correspondingly, the thermal quenching temperature was improved from 280 K for CsPbCl3 NCs to 360 K for CsPbCl3:Ni2+ NCs.
3.2. Enhancing luminescence efficiency
The development of cesium lead halide perovskite NCs with excellent luminescence efficiency is a key prerequisite for their practical applications. However, small-sized CsPbX3 NCs often suffer from low PLQYs. Thus, enhancing the luminescence efficiency of cesium lead halide perovskite NCs is an important goal in promoting their fundamental research and applications. Several studies have revealed that doping with transition metal ions may surmount this problem through tuning of near-band edge states or local structures. Hitherto, a series of transition metal ions including Ni2+, Mn2+, Cu2+, and Zn2+ have been widely explored to improve the PLQY of CsPbX3 NCs at different spectral regions, such as CsPbCl3:Ni2+,Pr3+ NCs with near-infrared (NIR) emission, CsPbI3:Zn2+ with deep-red emission, CsPbCl3:Ni2+,Mn2+,Zn2+ NCs with red emission, CsPbBr3:Zn2+ with green emission, CsPbBr1−xClx:Cu2+ NCs and CsPbBr1−xClx:Mn2+ NCs with blue emission, and CsPbCl3:Zn2+ with violet emission.23,35,37,79,83,126–130Compared with CsPbBr3 NCs, the PLQYs of CsPbCl3 and CsPbI3 NCs are relatively low.36 Benefiting from the suppression of defect states by doping with transition metal ions, the PLQY of CsPbI3 NCs can be effectively improved, as revealed by Li et al. through Zn2+ doping.36 In their work, zinc non-halide compounds including zinc acetylacetonate (Zn(acac)2), zinc acetate (ZnAc2) or zinc stearate (ZnSt) were employed as precursors for doping of Zn2+ in CsPbI3 NCs. The as-prepared Zn2+-doped CsPbI3 NCs displayed stronger red emission than the undoped CsPbI3 NCs, owing to the increased electron–hole radiative recombination after Zn2+ doping. Specifically, CsPbI3:Zn2+ prepared from Zn(acac)2 exhibited a PLQY as high as 76%, which was 120% enhancement relative to that of the undoped CsPbI3 counterparts (Fig. 8a and b). On the basis of the absorption spectra, PL decays, and space-charge-limited current measurements for pristine and Zn2+-doped CsPbI3 NCs, it was confirmed the density of the localized defect states near the band edge decreased after Zn2+ doping, thus effectively inhibited nonradiative recombination rate and enhanced the PLQY of CsPbI3 NCs (Fig. 8c and d).
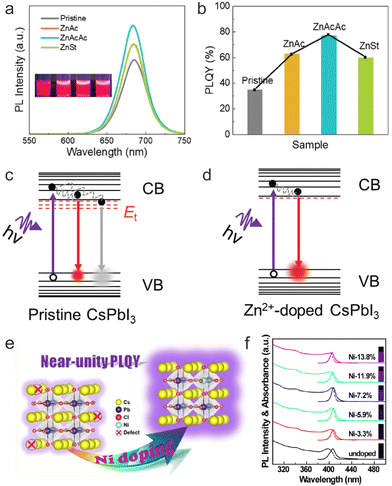 | ||
| Fig. 8 (a) PL spectra of pristine and Zn2+-doped CsPbI3 NCs and their PL photographs under UV lamps. (b) PLQYs of pristine and Zn2+-doped CsPbI3 NCs. Schematic illustration of radiative and nonradiative recombination of CsPbI3 NCs (c) before and (d) after Zn2+ doping. CB and VB refer to conduction band and valence band respectively. (e) Schematic illustration of Ni2+ doping in CsPbCl3 NCs to achieve near-unity PLQY. (f) Absorption and PL spectra of undoped and Ni2+ doped CsPbCl3 NCs. Insets show the photographs of NC solution under UV (365 nm) illumination. (a)–(d) Reproduced with permission from ref. 36. Copyright 2020, Royal Society of Chemistry (United Kingdom); (e) and (f) reproduced with permission from ref. 78. Copyright 2018, American Chemical Society. | ||
Moreover, transition metal dopants may improve the PLQY of cesium lead halide perovskite NCs by tuning the population of band edge states. For example, Ti3+ ions were demonstrated to introduce more band edge states around the conduction band minimum of CsPbCl3, favoring the release of electrons into the conduction band.74 As a result, the PLQY of CsPbCl3 was markedly improved from 0.08% to 48.4%. In another work, Wu et al. proposed that the transition from the T2 energy level of Cu2+ to the conduction band of CsPb(Cl/Br)3 NCs promoted the recombination of excitons via the radiative pathway, thus effectively enhancing the PLQYs of CsPbCl3 NCs from 3% to 51%.82
In addition to near band edge states tuning, the doping with transition metal ions may also engineer the local structure of cesium lead halide perovskite NCs to improve their luminescence efficiency. A typical example is Ni2+ doping in CsPbCl3 NCs, where NiCl2 was employed as a dopant precursor.78 As a result, the PLQY of CsPbCl3 NCs can be increased from 2.4% to 96.5% (Fig. 8e and f). It was confirmed that doping of Ni2+ ions substantially removed the structural defects of VCl, resulting in improved short-range order of the perovskite lattice. Similarly, De et al. reported that Cu+ doping may increase the PLQY of CsPbCl3 NCs from 0.5% to 60%.75 They attributed the significant PLQY enhancement to the rectifying octahedral distortion of the crystal and the passivation of VCl on the surface.
Furthermore, doping with transition metal ions can be utilized to strengthen the quantum confinement effect through engineering the local structure of cesium lead halide perovskite NCs, thus improving their luminescence efficiency. It was demonstrated that Mn2+ doping with a molar concentration of 3 mol% in CsPbCl3 NCs significantly increased their PLQY from 0.5% to 26%, due to the formation of a Ruddlesden–Popper structure in CsPbCl3 NCs.131 In these CsPbCl3:Mn2+ NCs, the excitons were confined to the subdomains separated by the Ruddlesden–Popper structure. Such quantum confinement favored the enhancement of the exciton oscillator strength, which contributed to the prominent exciton resonance in CsPbCl3:Mn2+ NCs, thus enhancing the excitonic emission.
3.3. Tuning emission band
Since the valence band maximum (VBM) of cesium lead halide perovskites is formed by the mixing of halide p-orbitals and metal s-orbitals, the change of composition and crystal structure by doping with transition metal ion may affect their bandgap (Eg) as well as the luminescence emission band.132,133 In addition, several transition metal ion dopants can serve as activators in cesium lead halide perovskite NCs to donate new emission bands.In CsPbX3 NCs, it was reported that replacing Pb2+ (r = 119 pm) with smaller transition metal ions such as Cd2+ (r = 95 pm), or Zn2+ (r = 74 pm) led to blue shifting of the emission band due to the lattice contraction. Specifically, CsPbBr3:Cd2+ and CsPbBr3:Zn2+ NCs exhibited blue emissions, which are 50–60 nm blue-shifted relative to the green-emitting CsPbBr3 NCs.99,134,135 Similarly, the PL peak of CsPbI3 NCs was tuned from 690 nm to 676 nm after doping of Zn2+ ions.35
Recently, we proposed a facile strategy to design efficient UV-emitting cesium lead halide perovskites by engineering the Eg of CsPbCl3 NCs.89 Benefiting from the doping of Cd2+ in CsPbCl3 NCs, we demonstrated that their bandgap can be tuned from the visible region into the UV region with the emission peak at 381 nm (Fig. 9a and b). Cd2+ doping induced lattice contraction as confirmed by XRD patterns. According to theoretical calculations based on density functional theory, the Cd2+ orbital caused little changes in the orbital composition near the Fermi level. Nevertheless, Cd2+ doping increased the bonding interactions between the Cd-5s and Cl-3p states, resulting in the broadening of Eg (Fig. 9c).
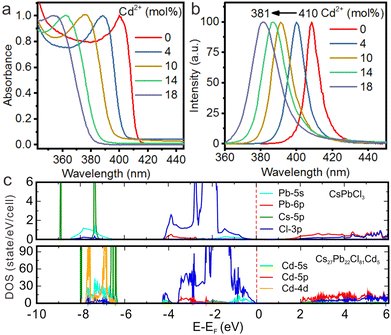 | ||
| Fig. 9 (a) Absorption and (b) PL spectra for the pure CsPbCl3 and CsPbCl3:Cd2+ NCs. (c) Calculated density of states for pure CsPbCl3 and CsPbCl3:Cd2+. Reproduced with permission from ref. 89. Copyright 2021, Wiley-VCH Verlag GmbH l Co. KGaA, Weinheim. | ||
Another common strategy for emission band tuning of cesium lead halide perovskite NCs is the introduction of new activators. Several transition metal ion dopants (e.g., Mn2+, Cd2+) frequently acted as activators in various hosts. Thus, they can donate new emission bands to cesium lead halide perovskite NCs.23,90,91 For example, CsPbCl3 NCs usually exhibit violet emission. It was reported that Cd2+ endowed CsPbCl3 NCs with yellow or red emissions.72 The new emission band peaking at 600 nm was assigned to the transition from 3Eg to the ground state (1A1g) of [CdCl6]4−.
Mn2+ is another frequently utilized activator in various hosts. In CsPbCl3:Mn2+ NCs, we observed a new emission band belonging to 4T1 → 6A1 of Mn2+ (Fig. 10a).39 Through tuning the Mn2+ feeding concentration from 0 to 60 mol%, the PL color of the CsPbCl3:Mn2+ solution can be tuned from purple to yellow due to the change in PL intensity ratio of CsPbCl3 and Mn2+ (Fig. 10b and c). The PL peak of Mn2+ shifted from 570 nm to 625 nm as a result of crystal field variation with increasing Mn2+ concentration. According to the excitation spectra, we confirmed that the strong PL emission of Mn2+ originated from the efficient energy transfer from the exciton of CsPbCl3 to Mn2+ dopants (Fig. 10d). Similarly, energy transfer from exciton to Mn2+ dopants was achieved in CsPbClxBr3−x:Mn2+ NCs.23 With increasing the content of Cl− in CsPbClxBr3−x:Mn2+ NCs, the emission of Mn2+ became stronger due to the increased energy transfer efficiency from the CsPbClxBr3−x host to Mn2+ ions.
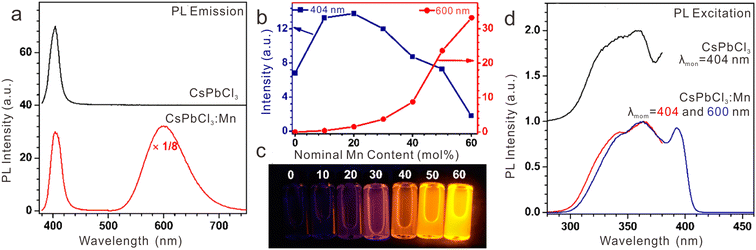 | ||
| Fig. 10 (a) PL emission spectra for pure CsPbCl3 and Mn2+-doped CsPbCl3 NCs upon UV excitation at 362 nm. (b) PL intensities for excitonic or Mn2+-related emissions of CsPbCl3:Mn2+ NCs centered at 404 and 600 nm as a function of the feed doping concentration of Mn2+ ions from 0 to 60 mol%, and (c) their corresponding PL photographs in cyclohexane solution under 362 nm UV lamp irradiation. (d) Comparison of PL excitation spectra for pure CsPbCl3 and Mn2+-doped CsPbCl3 NCs by monitoring the emissions at 404 nm and 600 nm, respectively. Reproduced with permission from ref. 39. Copyright 2017, American Chemical Society. | ||
3.4. Manipulating PL lifetime
The PL lifetimes of excitonic emission for transition metal ion-doped cesium lead halide perovskite NCs are usually several to hundreds of nanoseconds. The PL lifetime (τPL), which is related to radiative (r) and nonradiative (nr) processes, can be depicted as 1/τPL = 1/τr + 1/τnr.89 In most cases, doping transition metal ions into cesium lead halide perovskite NCs may induce more defect states and increase the nonradiative recombination rate (1/τnr). Correspondingly, the PL lifetime of excitonic emission would be shortened. For instance, we found that the nonradiative recombination rate (1/τnr) in CsPbCl3 NCs significantly increased from 0.078 to 0.693 μs−1 after doping of Cd2+.89 As such, the PL lifetime of CsPbCl3 NCs decreased from 5.63 to 1.43 ns after Cd2+ doping. Similarly, it was reported that the PL lifetime of CsPbI3 NCs decreased from 215.2 ns to 85.0 ns by doping Zn2+.35By contrast, it was discovered that several transition metal ions doping may suppress the intrinsic defects of cesium lead halide perovskite NCs, thereby prolonging their PL lifetimes.28,75,135–137 Sun et al. observed that the PL lifetime of CsPbCl3 NCs was markedly increased from 2.58 to 18.39 ns after doping of Ni2+.78 Ni2+ doping substantially eliminated the intrinsic defects of Cl− vacancies in the CsPbCl3 NCs, resulting in increased short-range order of the lattice. Therefore, Ni2+ doped CsPbCl3 NCs exhibited increased defect formation energy and longer PL lifetime than the undoped counterparts.
Furthermore, researchers prolonged the PL lifetime of cesium lead halide perovskite NCs from nanoseconds to microseconds by virtue of the long-lived energy level of transition metal ions. Pradeep et al. proposed a concept of vibrationally assisted delayed fluorescence (VADF) in CsPbClxBr3−x:Mn2+ NCs to harvest delayed fluorescence of Mn2+.138 The excited state electrons of CsPb(Cl/Br)3:Mn2+ NCs can be conserved for several microseconds to milliseconds within the excited-state energy levels of Mn2+. With vibrational assistance, electrons were transferred from the excited-state energy level of Mn2+ to the CsPb(Cl/Br)3 host to obtain the VADF (Fig. 11a). Such energy transfer from Mn2+ to the CsPb(Cl/Br)3 host was significantly affected by vibrational coupling, which was closely related to phonon band structure and temperature (Fig. 11b). To be specific, in CsPb(Cl/Br)3:Mn2+ NCs with small Eg such as CsPbBr3:Mn2+ and CsPb(Cl0.2/Br0.8)3:Mn2+ NCs, substantial gated excitonic emission along with the Mn2+ emission can be observed at 300 K after a delay of 150 μs (Fig. 11c). Nevertheless, it was demonstrated that the energy level of Mn2+ situated deeper in the conductive band of CsPb(Cl/Br)3 with larger Eg (e.g., CsPb(Cl0.6/Br0.4)3:Mn2+ and CsPbCl3:Mn2+NCs), thus making energy transfer assisted by vibrational coupling much harder. Since low temperature may strengthen the coupling, leading to more efficient energy transfer, CsPb(Cl0.6/Br0.4)3:Mn2+ and CsPbCl3:Mn2+NCs with large Eg only exhibited the gated PL of excitons below 170 K (Fig. 11d).
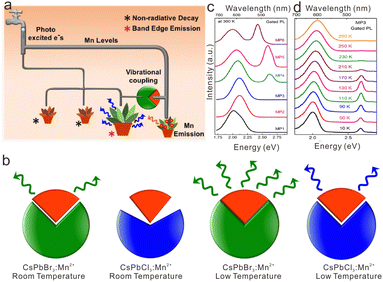 | ||
| Fig. 11 (a) Schematic illustration of vibrationally assisted delayed fluorescence of CsPb(Cl/Br)3:Mn2+ NCs. (b) Schematic illustration of phonon coupling for Mn2+-doped CsPbBr3, and CsPbCl3 perovskite NCs. (c) Room-temperature gated PL emission for CsPb(Cl/Br)3:Mn2+ perovskite NCs (MP1−MP6 stand for the CsPb(Cl/Br)3:Mn2+ with Br content of 0, 20%, 40%, 60%, 80% and 100%). (d) Temperature-dependent gated PL emission for CsPb(Cl/Br)3:Mn2+ NCs. Reproduced with permission from ref. 138. Copyright 2019, American Chemical Society. | ||
4. Conclusion and prospects
During the past decade, doping cesium lead halide perovskite NCs with transition metal ions has been demonstrated to be a robust strategy for engineering their structural, morphological, and optoelectronic properties. With the rapid development of advanced synthesis techniques, significant progress has been made in tackling the problems of controlled synthesis of transition metal ion-doped cesium lead halide perovskite NCs. The unique optical properties of transition metal ion-doped cesium lead halide perovskite NCs bestow great potential for the exploitation of high-performance luminescent materials and novel optoelectronic devices. Despite these achievements, there are still several challenges to be solved to fully exploit the potential of transition metal ion-doped cesium lead halide perovskite NCs, which would undoubtedly accelerate their commercialization.First, due to the ionic crystal nature and the high reactivity of the halide anions in the cesium lead halide perovskite NCs, the nucleation and growth process of cesium lead halide perovskite NCs is fast and uncontrollable. As such, it is essential to precisely regulate the crystallization kinetics of cesium lead halide perovskite NCs, as well as the doping content of transition metal ions. To circumvent the shortcomings of conventional approaches for the fast reaction of preparing cesium lead halide perovskite NCs, novel strategies like vacuum evaporation or light-triggered synthesis may be the smart choice. For example, the ion migration, doping content and composition can be readily controlled in light-triggered synthesis by adjusting either the photon dose or the wavelength of the excitation light.
Second, a comprehensive investigation of the microstructure of transition metal ions in the perovskite hosts is a prerequisite for exploring novel kinds of transition metal ion-doped cesium lead halide perovskite NCs and rationally modulating their optical properties. Especially, low-dimensional cesium lead halide perovskite NCs (e.g., 0D Cs4PbX6 and 2D CsPb2X5) exhibit better environmental stability and higher PLQYs in solid powders than their 3D counterparts. The energy transfer and local electronic structure should be clearly deciphered via fundamental photophysical studies and theoretical calculations, which may contribute to rationally optimizing the luminescent properties of transition metal ion-doped cesium lead halide perovskite NCs.
Last but not least, the studies on cesium lead halide perovskite NCs are mainly restricted to the visible spectral region. Cesium lead halide perovskite NCs with UV/NIR light emission via transition metal ion doping still suffer from weak emissions intensities or poor stabilities, due to the inevitable defects formed during their crystal growth. Thus, it is highly desirable to develop versatile strategies for engineering the local and surface structure of cesium lead halide perovskite NCs to achieve highly efficient UV/NIR-emitting materials for diverse applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key R&D Program of China (No. 2022YFB3503700), the National Natural Science Foundation of China (No. 21975257, 22275188, 22135008, U22A20398), the CAS/SAFEA International Partnership Program for Creative Research Teams, Natural Science Foundation of Fujian Province (No. 2021L3024) and the China Postdoctoral Science Foundation (No. 2021M703219).References
- L. Protesescu, S. Yakunin, M. I. Bodnarchuk, F. Krieg, R. Caputo, C. H. Hendon, R. X. Yang, A. Walsh and M. V. Kovalenko, Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut, Nano Lett., 2015, 15, 3692–3696 CrossRef CAS.
- J. Z. Ye, M. M. Byranvand, C. O. Martnez, R. L. Z. Hoye, M. Saliba and L. Polavarapu, Defect Passivation in Lead-Halide Perovskite Nanocrystals and Thin Films: Toward Efficient LEDs and Solar cells, Angew. Chem., Int. Ed., 2021, 60, 21636–21660 CrossRef CAS PubMed.
- Y. H. Zhang, R. J. Sun, X. Y. Qi, K. F. Fu, Q. S. Chen, Y. C. Ding, L. J. Xu, L. M. Liu, Y. Han, A. V. Malko, X. G. Liu, H. H. Yang, O. M. Bakr, H. Liu and O. F. Mohammed, Metal Halide Perovskite Nanosheet for X-ray High-Resolution Scintillation Imaging Screens, ACS Nano, 2019, 13, 2520–2525 CrossRef CAS PubMed.
- Q. Chen, J. Wu, X. Ou, B. Huang, J. Almutlaq, A. A. Zhumekenov, X. Guan, S. Han, L. Liang, Z. Yi, J. Li, X. Xie, Y. Wang, Y. Li, D. Fan, D. B. L. Teh, A. H. All, O. F. Mohammed, O. M. Bakr, T. Wu, M. Bettinelli, H. Yang, W. Huang and X. Liu, All-inorganic perovskite nanocrystal scintillators, Nature, 2018, 561, 88–93 CrossRef CAS PubMed.
- A. K. Jena, A. Kulkarni and T. Miyasaka, Halide Perovskite Photovoltaics: Background, Status, and Future Prospects, Chem. Rev., 2019, 119, 3036–3103 CrossRef CAS PubMed.
- X. Y. Zhang, L. N. Li, Z. H. Sun and J. H. Luo, Rational chemical doping of metal halide perovskites, Chem. Soc. Rev., 2019, 48, 517–539 RSC.
- D. Yang, X. Li, W. Zhou, S. Zhang and H. Zeng, CsPbBr3 Quantum Dots 2.0: Benzenesulfonic Acid Equivalent Ligand Awakens Complete Purification, Adv. Mater., 2019, 31, 1900767 CrossRef.
- H. Huang, B. Chen, Z. Wang, T. F. Hung, A. S. Susha, H. Zhong and A. L. Rogach, Water resistant CsPbX3 nanocrystals coated with polyhedral oligomeric silsesquioxane and their use as solid state luminophores in all-perovskite white light-emitting devices, Chem. Sci., 2016, 7, 5699–5703 RSC.
- D. Yang, X. Li, Y. Wu, C. Wei, Z. Qin, C. Zhang, Z. Sun, Y. Li, Y. Wang and H. Zeng, Surface Halogen Compensation for Robust Performance Enhancements of CsPbX3 Perovskite Quantum Dots, Adv. Opt. Mater., 2019, 7, 1900276 CrossRef.
- J. J. Wei, W. Zheng, P. Huang, Z. L. Gong, Y. Liu, S. Lu, Z. Li and X. Y. Chen, Direct photoinduced synthesis of lead halide perovskite nanocrystals and nanocomposites, Nano Today, 2021, 39, 101179 CrossRef CAS.
- J. S. Yao, J. Ge, B. N. Han, K. H. Wang, H. B. Yao, H. L. Yu, J. H. Li, B. S. Zhu, J. Z. Song, C. Chen, Q. Zhang, H. B. Zeng, Y. Luo and S. H. Yu, Ce3+-Doping to Modulate Photoluminescence Kinetics for Efficient CsPbBr3 Nanocrystals Based Light-Emitting Diodes, J. Am. Chem. Soc., 2018, 140, 3626–3634 CrossRef CAS PubMed.
- H. Huang, R. Li, S. Jin, Z. Li, P. Huang, J. Hong, S. Du, W. Zheng, X. Chen and D. Chen, Ytterbium-Doped CsPbCl3 Quantum Cutters for Near-Infrared Light-Emitting Diodes, ACS Appl. Mater. Interfaces, 2021, 13, 34561–34571 CrossRef CAS.
- D. Q. Chen, S. Zhou, F. F. Tian, H. T. Ke, N. Z. Jiang, S. J. Wang, Y. Z. Peng and Y. Liu, Halogen-Hot-Injection Synthesis of Mn-Doped CsPb(Cl/Br)3 Nanocrystals with Blue/Orange Dual-Color Luminescence and High Photoluminescence Quantum Yield, Adv. Opt. Mater., 2019, 7, 1901082 CrossRef CAS.
- M. L. Liu, N. Z. Jiang, H. Huang, J. D. Lin, F. Huang, Y. P. Zheng and D. Q. Chen, Ni2+-doped CsPbI3 perovskite nanocrystals with near-unity photoluminescence quantum yield and superior structure stability for red light-emitting devices, Chem. Eng. J., 2021, 413, 127547 CrossRef CAS.
- D. X. Yang and D. X. Huo, Cation doping and strain engineering of CsPbBr3-based perovskite light emitting diodes, J. Mater. Chem. C, 2020, 8, 6640–6653 RSC.
- N. Pradhan, Mn-Doped Semiconductor Nanocrystals: 25 Years and Beyond, J. Phys. Chem. Lett., 2019, 10, 2574–2577 CrossRef CAS PubMed.
- H. Kim, S. R. Bae, T. H. Lee, H. Lee, H. Kang, S. Park, H. W. Jang and S. Y. Kim, Enhanced Optical Properties and Stability of CsPbBr3 Nanocrystals Through Nickel Doping, Adv. Funct. Mater., 2021, 31, 2102770 CrossRef CAS.
- B. Su, G. Zhou, J. Huang, E. Song, A. Nag and Z. Xia, Mn2+-Doped Metal Halide Perovskites: Structure, Photoluminescence, and Application, Laser Photon. Rev., 2020, 15, 2000334 CrossRef.
- Y. M. Chen, Y. S. Liu and M. C. Hong, Cation-doping matters in caesium lead halide perovskite nanocrystals: from physicochemical fundamentals to optoelectronic applications, Nanoscale, 2020, 12, 12228–12248 RSC.
- Y. H. Kye, C. J. Yu, C. H. Kim, Y. S. Kim and U. G. Jong, Influence of Metal-Ion Replacement on the Phase Stabilization of Cubic All-Inorganic Cesium Lead Halide Perovskites: an Ab Initio Thermodynamic Formalism for Solution-Processed Cation Doping, J. Phys. Chem. C, 2021, 125, 13195–13211 CrossRef CAS.
- Y. C. Chen, H. L. Chou, J. C. Lin, Y. C. Lee, C. W. Pao, J. L. Chen, C. C. Chang, R. Y. Chi, T. R. Kuo, C. W. Lu and D. Y. Wang, Enhanced Luminescence and Stability of Cesium Lead Halide Perovskite CsPbX3 Nanocrystals by Cu2+-Assisted Anion Exchange Reactions, J. Phys. Chem. C, 2019, 123, 2353–2360 CrossRef CAS.
- Y. J. Guo, J. Su, L. Wang, Z. H. Lin, Y. Hao and J. J. Chang, Improved Doping and Optoelectronic Properties of Zn-Doped CsPbBr3 Perovskite through Mn Codoping Approach, J. Phys. Chem. Lett., 2021, 12, 3393–3400 CrossRef CAS PubMed.
- Y. Z. Zheng, X. Yuan, J. Yang, Q. Y. Li, X. X. Yang, Y. Fan, H. B. Li, H. L. Liu and J. L. Zhao, Cu doping-enhanced emission efficiency of Mn2+ in cesium lead halide perovskite nanocrystals for efficient white light-emitting diodes, J. Lumin., 2020, 227, 117586 CrossRef CAS.
- D. Ghosh, M. Y. Ali, A. Ghosh, A. Mandal and S. Bhattacharyya, Heterovalent Substitution in Mixed Halide Perovskite Quantum Dots for Improved and Stable Photovoltaic Performance, J. Phys. Chem. C, 2021, 125, 5485–5493 CrossRef CAS.
- Y. B. Chen, H. W. Yang, J. Song and B. Zhang, An effective Fe(II) doping strategy for stable and highly photoluminescent CsPbBrI2 nanocrystals, J. Mater. Chem. C, 2022, 10, 5147–5154 RSC.
- M. Lu, X. Y. Zhang, X. Bai, H. Wu, X. Y. Shen, Y. Zhang, W. Zhang, W. T. Zheng, H. W. Song, W. W. Yu and A. L. Rogach, Spontaneous Silver Doping and Surface Passivation of CsPbl3 Perovskite Active Layer Enable Light-Emitting Devices with an External Quantum Efficiency of 11.2%, ACS Energy Lett., 2018, 3, 1571–1577 CrossRef CAS.
- A. Swarnkar, W. J. Mir and A. Nag, Can B-Site Doping or Alloying Improve Thermal- and Phase-Stability of All-Inorganic CsPbX3 (X = CI, Br, I) Perovskites?, ACS Energy Lett., 2018, 3, 286–289 CrossRef CAS.
- A. Dey, J. Z. Ye, A. De, E. Debroye, S. K. Ha, E. Bladt, A. S. Kshirsagar, Z. Y. Wang, J. Yin, Y. Wang, L. N. Quan, F. Yan, M. Y. Gao, X. M. Li, J. Shamsi, T. Debnath, M. H. Cao, M. A. Scheel, S. Kumar, J. A. Steele, M. Gerhard, L. Chouhan, K. Xu, X. G. Wu, Y. X. Li, Y. N. Zhang, A. Dutta, C. Han, I. Vincon, A. L. Rogach, A. Nag, A. Samanta, B. A. Korgel, C. J. Shih, D. R. Gamelin, D. H. Son, H. B. Zeng, H. Z. Zhong, H. D. Sun, H. V. Demir, I. G. Scheblykin, I. Mora-Sero, J. K. Stolarczyk, J. Z. Zhang, J. Feldmann, J. Hofkens, J. M. Luther, J. Perez-Prieto, L. Li, L. Manna, M. I. Bodnarchuk, M. V. Kovalenko, M. B. J. Roeffaers, N. Pradhan, O. F. Mohammed, O. M. Bakr, P. D. Yang, P. Muller-Buschbaum, P. V. Kamat, Q. L. Bao, Q. Zhang, R. Krahne, R. E. Galian, S. D. Stranks, S. Bals, V. Biju, W. A. Tisdale, Y. Yan, R. L. Z. Hoye and L. Polavarapu, State of the Art and Prospects for Halide Perovskite Nanocrystals, ACS Nano, 2021, 15, 10775–10981 CrossRef CAS PubMed.
- A. R. Kirmani, J. M. Luther, M. Abolhasani and A. Amassian, Colloidal Quantum Dot Photovoltaics: Current Progress and Path to Gigawatt Scale Enabled by Smart Manufacturing, ACS Energy Lett., 2020, 5, 3069–3100 CrossRef CAS.
- J. Huang, Y. Yuan, Y. Shao and Y. Yan, Understanding the physical properties of hybrid perovskites for photovoltaic applications, Nat. Rev. Mater., 2017, 2, 17042 CrossRef CAS.
- C. H. Lu, G. V. Biesold, Y. J. Liu, Z. T. Kang and Z. Q. Lin, Doping and ion substitution in colloidal metal halide perovskite nanocrystals, Chem. Soc. Rev., 2020, 49, 4953–5007 RSC.
- Y. R. He, J. X. Yan, L. Xu, B. M. Zhang, Q. Cheng, Y. Cao, J. Zhang, C. Tao, Y. Q. Wei, K. C. Wen, Z. Y. Kuang, G. M. Chow, Z. X. Shen, Q. M. Peng, W. Huang and J. P. Wang, Perovskite Light-Emitting Diodes with Near Unit Internal Quantum Efficiency at Low Temperatures, Adv. Mater., 2021, 33, 2006302 CrossRef CAS.
- B. S. B. Karunathilaka, U. Balijapalli, C. A. M. Senevirathne, S. Yoshida, Y. Esaki, K. Goushi, T. Matsushima, A. S. D. Sandanayaka and C. Adachi, Suppression of external quantum efficiency rolloff in organic light emitting diodes by scavenging triplet excitons, Nat. Commun., 2020, 11, 4926 CrossRef CAS PubMed.
- J. Pan, L. N. Quan, Y. Zhao, W. Peng, B. Murali, S. P. Sarmah, M. Yuan, L. Sinatra, N. M. Alyami, J. Liu, E. Yassitepe, Z. Yang, O. Voznyy, R. Comin, M. N. Hedhili, O. F. Mohammed, Z. H. Lu, D. H. Kim, E. H. Sargent and O. M. Bakr, Highly Efficient Perovskite-Quantum-Dot Light-Emitting Diodes by Surface Engineering, Adv. Mater., 2016, 28, 8718–8725 CrossRef CAS PubMed.
- X. Y. Shen, Y. Zhang, S. V. Kershaw, T. S. Li, C. C. Wang, X. Y. Zhang, W. Y. Wang, D. G. Li, Y. H. Wang, M. Lu, L. J. Zhang, C. Sun, D. Zhao, G. S. Qin, X. Bai, W. W. Yu and A. L. Rogach, Zn-Alloyed CsPbI3 Nanocrystals for Highly Efficient Perovskite Light-Emitting Devices, Nano Lett., 2019, 19, 1552–1559 CrossRef CAS.
- J. H. Li, J. W. Chen, L. M. Xu, S. N. Liu, S. Lan, X. S. Li and J. Z. Song, A zinc non-halide dopant strategy enables efficient perovskite CsPbI3 quantum dot-based light-emitting diodes, Mater. Chem. Front., 2020, 4, 1444–1453 RSC.
- S. C. Hou, M. K. Gangishetty, Q. M. Quan and D. N. Congreve, Efficient Blue and White Perovskite Light-Emitting Diodes via Manganese Doping, Joule, 2018, 2, 2421–2433 CrossRef CAS.
- B.-B. Zhang, S. Yuan, J.-P. Ma, Y. Zhou, J. Hou, X. Chen, W. Zheng, H. Shen, X.-C. Wang, B. Sun, O. M. Bakr, L.-S. Liao and H.-T. Sun, General Mild Reaction Creates Highly Luminescent Organic-Ligand-Lacking Halide Perovskite Nanocrystals for Efficient Light-Emitting Diodes, J. Am. Chem. Soc., 2019, 141, 15423–15432 CrossRef CAS PubMed.
- S. Zou, Y. Liu, J. Li, C. Liu, R. Feng, F. Jiang, Y. Li, J. Song, H. Zeng, M. Hong and X. Chen, Stabilizing Cesium Lead Halide Perovskite Lattice through Mn (II)-Substitution for Air-Stable Light-Emitting Diodes, J. Am. Chem. Soc., 2017, 139, 11443–11450 CrossRef CAS.
- X. Zhu, L. Ge, Y. Wang, M. Li, R. Q. Zhang, M. C. Xu, Z. H. Zhao, W. Z. Lv and R. F. Chen, Recent Advances in Enhancing and Enriching the Optical Properties of Cl-Based CsPbX3 Nanocrystals, Adv. Opt. Mater., 2021, 9, 2100058 CrossRef CAS.
- Y. Zhou, J. Chen, O. M. Bakr and H. T. Sun, Metal-Doped Lead Halide Perovskites: Synthesis, Properties, and Optoelectronic Applications, Chem. Mater., 2018, 30, 6589–6613 CrossRef CAS.
- M. V. Kovalenko, L. Protesescu and M. I. Bodnarchuk, Properties and potential optoelectronic applications of lead halide perovskite nanocrystals, Science, 2017, 358, 745–750 CrossRef CAS.
- Z. Liu, X. Qin, Q. H. Chen, T. C. Jiang, Q. S. Chen and X. G. Liu, Metal-Halide Perovskite Nanocrystal Superlattice: Self-Assembly and Optical Fingerprints, Adv. Mater., 2023, 35, e2209279 CrossRef PubMed.
- J. Xu, S. Yu, X. Shang and X. Chen, Temperature Dependence of Bandgap in Lead-Halide Perovskites with Corner-Sharing Octahedra, Adv. Photon. Res., 2023, 4, 2200193 CrossRef CAS.
- H. H. Yao, J. Zhao, Z. Z. Li, Z. P. Ci and Z. W. Jin, Research and progress of black metastable phase CsPbI3 solar cells, Mater. Chem. Front., 2021, 5, 1221–1235 RSC.
- Q. J. Gao, J. H. Qi, K. Chen, M. H. Xia, Y. Hu, A. Y. Mei and H. W. Han, Halide Perovskite Crystallization Processes and Methods in Nanocrystals, Single Crystals, and Thin Films, Adv. Mater., 2022, 34, 2200720 CrossRef CAS.
- J. Ren, X. Zhou and Y. Wang, Water triggered interfacial synthesis of highly luminescent CsPbX3:Mn2+ quantum dots from nonluminescent quantum dots, Nano Res., 2020, 13, 3387–3395 CrossRef CAS.
- Y. Tong, E. Bladt, M. F. Aygüler, A. Manzi, K. Z. Milowska, V. A. Hintermayr, P. Docampo, S. Bals, A. S. Urban, L. Polavarapu and J. Feldmann, Highly Luminescent Cesium Lead Halide Perovskite Nanocrystals with Tunable Composition and Thickness by Ultrasonication, Angew. Chem., Int. Ed., 2016, 55, 13887–13892 CrossRef CAS PubMed.
- D. J. Kubicki, D. Prochowicz, A. Hofstetter, B. J. Walder and L. Emsley, Cd-113 Solid-State NMR at 21.1 T Reveals the Local Structure and Passivation Mechanism of Cadmium in Hybrid and All-Inorganic Halide Perovskites, ACS Energy Lett., 2020, 5, 2964–2971 CrossRef CAS.
- T. Guner, B. Akbali, M. Ozcan, G. Topcu, M. M. Demir and H. Sahin, Monitoring the Doping and Diffusion Characteristics of Mn Dopants in Cesium Lead Halide Perovskites, J. Phys. Chem. C, 2018, 122, 11543–11549 CrossRef CAS.
- F. Bateni, R. W. Epps, K. Abdel-latif, R. Dargis, S. Han, A. A. Volk, M. Ramezani, T. Cai, O. Chen and M. Abolhasani, Ultrafast cation doping of perovskite quantum dots in flow, Matter, 2021, 4, 2429–2447 CrossRef CAS.
- J. R. Zhu, X. L. Yang, Y. H. Zhu, Y. W. Wang, J. Cai, J. H. Shen, L. Y. Sun and C. Z. Li, Room-Temperature Synthesis of Mn-Doped Cesium Lead Halide Quantum Dots with High Mn Substitution Ratio, J. Phys. Chem. Lett., 2017, 8, 4167–4171 CrossRef CAS.
- C. Otero-Martinez, J. Z. Ye, J. Sung, I. Pastoriza-Santos, J. Perez-Juste, Z. G. Xia, A. Rao, R. L. Z. Hoye and L. Polavarapu, Colloidal Metal-Halide Perovskite Nanoplatelets: Thickness-Controlled Synthesis, Properties, and Application in Light-Emitting Diodes, Adv. Mater., 2022, 34, 2107105 CrossRef CAS PubMed.
- Z. Y. Zhu, Q. Q. Yang, L. F. Gao, L. Zhang, A. Y. Shi, C. L. Sun, Q. Wang and H. L. Zhang, Solvent-Free Mechanosynthesis of Composition-Tunable Cesium Lead Halide Perovskite Quantum Dots, J. Phys. Chem. Lett., 2017, 8, 1610–1614 CrossRef CAS.
- K. Abdel-Latif, R. W. Epps, C. B. Kerr, C. M. Papa, F. N. Castellano and M. Abolhasani, Facile Room-Temperature Anion Exchange Reactions of Inorganic Perovskite Quantum Dots Enabled by a Modular Microfluidic Platform, Adv. Funct. Mater., 2019, 29, 1900712 CrossRef.
- D. Parobek, B. J. Roman, Y. Dong, H. Jin, E. Lee, M. Sheldon and D. H. Son, Exciton-to-Dopant Energy Transfer in Mn-Doped Cesium Lead Halide Perovskite Nanocrystals, Nano Lett., 2016, 16, 7376–7380 CrossRef CAS.
- W. Liu, Q. Lin, H. Li, K. Wu, I. Robel, J. M. Pietryga and V. I. Klimov, Mn2+-Doped Lead Halide Perovskite Nanocrystals with Dual-Color Emission Controlled by Halide Content, J. Am. Chem. Soc., 2016, 138, 14954–14961 CrossRef CAS PubMed.
- Q. A. Akkerman, D. Meggiolaro, Z. Dang, F. De Angelis and L. Manna, Fluorescent Alloy CsPbxMn1–xI3 Perovskite Nanocrystals with High Structural and Optical Stability, ACS Energy Lett., 2017, 2, 2183–2186 CrossRef CAS PubMed.
- P. Arunkumar, H. B. Cho, K. H. Gil, S. Unithrattil, Y. H. Kim and W. Bin Im, Probing Molecule-Like Isolated Octahedra via-Phase Stabilization of Zero-Dimensional Cesium Lead Halide Nanocrystals, Nat. Commun., 2018, 9, 4691 CrossRef.
- H. Liu, Z. Wu, J. Shao, D. Yao, H. Gao, Y. Liu, W. Yu, H. Zhang and B. Yang, CsPbxMn1-xCl3 Perovskite Quantum Dots with High Mn Substitution Ratio, ACS Nano, 2017, 11, 2239–2247 CrossRef CAS PubMed.
- J. Meng, Z. Y. Lan, M. Abdellah, B. Yang, S. Mossin, M. L. Liang, M. Naumova, Q. Shi, S. L. G. Alvarez, Y. Liu, W. H. Lin, I. E. Castelli, S. E. Canton, T. Pullerits and K. B. Zheng, Modulating Charge-Carrier Dynamics in Mn-Doped All-Inorganic Halide Perovskite Quantum Dots through the Doping-Induced Deep Trap States, J. Phys. Chem. Lett., 2020, 11, 3705–3711 CrossRef CAS.
- G. G. Huang, C. L. Wang, S. H. Xu, S. F. Zong, J. Lu, Z. Y. Wang, C. G. Lu and Y. P. Cui, Postsynthetic Doping of MnCl2 Molecules into Preformed CsPbBr3 Perovskite Nanocrystals via a Halide Exchange-Driven Cation Exchange, Adv. Mater., 2017, 29, 1700095 CrossRef.
- S. Das Adhikari, R. K. Behera, S. Bera and N. Pradhan, Presence of Metal Chloride for Minimizing the Halide Deficiency and Maximizing the Doping Efficiency in Mn(II)-Doped CsPbCl3 Nanocrystals, J. Phys. Chem. Lett., 2019, 10, 1530–1536 CrossRef CAS PubMed.
- Y. C. Li, C. Y. Wang, G. C. Hu, W. Meng, S. Q. Sui and Z. T. Deng, Promoting the doping efficiency and photoluminescence quantum yield of Mn-doped perovskite nanocrystals via two-step hot-injection, Chem. Commun., 2022, 58, 941–944 RSC.
- J. P. Ma, Y. M. Chen, L. M. Zhang, S. Q. Guo, J. D. Liu, H. Li, B. J. Ye, Z. Y. Li, Y. Zhou, B. B. Zhang, O. M. Bakr, J. Y. Zhang and H. T. Sun, Insights into the local structure of dopants, doping efficiency, and luminescence properties of lanthanide-doped CsPbCl3 perovskite nanocrystals, J. Mater. Chem. C, 2019, 7, 3037–3048 RSC.
- S. Das Adhikari, S. K. Dutta, A. Dutta, A. K. Guria and N. Pradhan, Chemically Tailoring the Dopant Emission in Manganese-Doped CsPbCl3 Perovskite Nanocrystals, Angew. Chem., Int. Ed., 2017, 56, 8746–8750 CrossRef CAS PubMed.
- S. Zhou, Y. W. Zhu, J. S. Zhong, F. F. Tian, H. Huang, J. K. Chen and D. Q. Chen, Chlorine-additive-promoted incorporation of Mn2+ dopants into CsPbCl3 perovskite nanocrystals, Nanoscale, 2019, 11, 12465–12470 RSC.
- S. Das Adhikari, A. Dutta, S. K. Dutta and N. Pradhan, Layered Perovskites L-2(Pb1-XMnX)C14 to Mn-Doped CsPbCl3 Perovskite Platelets, ACS Energy Lett., 2018, 3, 1247–1253 CrossRef CAS.
- W. J. Mir, M. Jagadeeswararao, S. Das and A. Nag, Colloidal Mn-Doped Cesium Lead Halide Perovskite Nanoplatelets, ACS Energy Lett., 2017, 2, 537–543 CrossRef CAS.
- S. K. Dutta and N. Pradhan, Coupled Halide-Deficient and Halide-Rich Reaction System for Doping in Perovskite Armed Nanostructures, J. Phys. Chem. Lett., 2019, 10, 6788–6793 CrossRef CAS PubMed.
- N. Pradhan, Alkylammonium Halides for Facet Reconstruction and Shape Modulation in Lead Halide Perovskite Nanocrystals, Acc. Chem. Res., 2021, 54, 1200–1208 CrossRef CAS PubMed.
- H. M. Li, W. Dong, X. Y. Shen, C. D. Ge, Y. L. Song, Z. S. Wang, A. R. Wang, Z. Q. Yang, M. W. Hao, Y. Zhang, W. T. Zheng, X. Y. Zhang and Q. F. Dong, Enhancing the Efficiency and Stability of CsPbI3 Nanocrystal-Based Light-Emitting Diodes through Ligand Engineering with Octylamine, J. Phys. Chem. C, 2022, 126, 1085–1093 CrossRef CAS.
- S. Zhang, L. F. Yuan, H. L. Liu, G. F. Zhou, W. G. Ding, Z. P. Qin, X. G. Li and S. R. Wang, Tunable White Light-Emitting Devices Based on Unilaminar High-Efficiency Zn2+-Doped Blue CsPbBr3 Quantum Dots, J. Phys. Chem. Lett., 2021, 12, 8507–8512 CrossRef CAS PubMed.
- Y. Li, Q. S. Liu, X. J. Liu, J. Feng, L. J. He, H. W. Li, C. Y. Li and H. J. Zhang, Simultaneous Enhancement of Photoluminescence and Stability of CsPbCl3 Perovskite Enabled by Titanium Ion Dopant, J. Phys. Chem. Lett., 2021, 12, 10746–10752 CrossRef CAS PubMed.
- A. De, S. Das, N. Mondal and A. Samanta, Highly Luminescent Violet- and Blue-Emitting Stable Perovskite Nanocrystals, ACS Mater. Lett., 2019, 1, 116–122 CrossRef CAS.
- R. K. Behera, A. Dutta, D. Ghosh, S. Bera, S. Bhattacharyya and N. Pradhan, Doping the Smallest Shannon Radii Transition Metal Ion Ni(II) for Stabilizing alpha-CsPbI3 Perovskite Nanocrystals, J. Phys. Chem. Lett., 2019, 10, 7916–7921 CrossRef CAS.
- X. Chen, Z. G. Sun, B. Cai, X. M. Li, S. H. Zhang, D. Fu, Y. S. Zou, Z. Y. Fan and H. B. Zeng, Substantial Improvement of Operating Stability by Strengthening Metal-Halogen Bonds in Halide Perovskites, Adv. Funct. Mater., 2022, 32, 2112129 CrossRef CAS.
- Z. Yong, S. Guo, J. Ma, J. Zhang, Z. Li, Y. Chen, B. Zhang, Y. Zhou, J. Shu, J. Gu, L. Zheng, O. M. Bakr and H. Sun, Doping-Enhanced Short-Range Order of Perovskite Nanocrystals for Near-Unity Violet Luminescence Quantum Yield, J. Am. Chem. Soc., 2018, 140, 9942–9951 CrossRef CAS PubMed.
- V. Naresh and N. Lee, Zn(II)-Doped Cesium Lead Halide Perovskite Nanocrystals with High Quantum Yield and Wide Color Tunability for Color-Conversion Light-Emitting Displays, ACS Appl. Nano Mater., 2020, 3, 7621–7632 CrossRef CAS.
- L. Zhang, C. Kang, G. Zhang, Z. Pan, Z. Huang, S. Xu, H. Rao, H. Liu, S. Wu, X. Wu, X. Li, Z. Zhu, X. Zhong and A. K. Y. Jen, All-Inorganic CsPbI3 Quantum Dot Solar Cells with Efficiency over 16% by Defect Control, Adv. Funct. Mater., 2021, 31, 2005930 CrossRef CAS.
- J. Li, Z. Guo, S. Xiao, Y. Tu, T. He and W. Zhang, Enhanced Performance of Two-Photon Excited Amplified Spontaneous Emission by Cd-Alloyed CsPbBr3 Nanocrystals, Inorg. Chem., 2022, 61, 4735–4742 CrossRef CAS PubMed.
- R. Wu, Z. Bai, J. Jiang, H. Yao and S. Qin, Research on the photoluminescence properties of Cu2+-doped perovskite CsPbCl3 quantum dots, RSC Adv., 2021, 11, 8430–8436 RSC.
- C. Bi, S. Wang, Q. Li, S. V. Kershaw, J. Tian and A. L. Rogach, Thermally Stable Copper(II)-Doped Cesium Lead Halide Perovskite Quantum Dots with Strong Blue Emission, J. Phys. Chem. Lett., 2019, 10, 943–952 CrossRef CAS PubMed.
- C. H. Bi, X. J. Sun, X. Huang, S. X. Wang, J. F. Yuan, J. X. Wang, T. Pullerits and J. J. Tian, Stable CsPb1-xZnxI3 Colloidal Quantum Dots with Ultralow Density of Trap States for High-Performance Solar Cells, Chem. Mater., 2020, 32, 6105–6113 CrossRef CAS.
- M. Imran, V. Caligiuri, M. Wang, L. Goldoni, M. Prato, R. Krahne, L. De Trizio and L. Manna, Benzoyl Halides as Alternative Precursors for the Colloidal Synthesis of Lead-Based Halide Perovskite Nanocrystals, J. Am. Chem. Soc., 2018, 140, 2656–2664 CrossRef CAS PubMed.
- C. Y. Zhang, Q. Wan, L. K. Ono, Y. Q. Liu, W. L. Zheng, Q. G. Zhang, M. M. Liu, L. Kong, L. Li and Y. B. Qi, Narrow-Band Violet-Light-Emitting Diodes Based on Stable Cesium Lead Chloride Perovskite Nanocrystals, ACS Energy Lett., 2021, 6, 3545–3554 CrossRef CAS.
- D. P. Nenon, K. Pressler, J. Kang, B. A. Koscher, J. H. Olshansky, W. T. Osowiecki, M. A. Koc, L.-W. Wang and A. P. Alivisatos, Design Principles for Trap-Free CsPbX3 Nanocrystals: Enumerating and Eliminating Surface Halide Vacancies with Softer Lewis Bases, J. Am. Chem. Soc., 2018, 140, 17760–17772 CrossRef CAS.
- N. Pradhan, Tips and Twists in Making High Photoluminescence Quantum Yield Perovskite Nanocrystals, ACS Energy Lett., 2019, 4, 1634–1638 CrossRef CAS.
- Y. Zhang, X. Cheng, D. Tu, Z. Gong, R. Li, Y. Yang, W. Zheng, J. Xu, S. Deng and X. Chen, Engineering the Bandgap and Surface Structure of CsPbCl3 Nanocrystals to Achieve Efficient Ultraviolet Luminescence, Angew. Chem., Int. Ed., 2021, 60, 9693–9698 CrossRef CAS PubMed.
- T. Cai, H. Yang, K. Hills-Kimball, J.-P. Song, H. Zhu, E. Hofman, W. Zheng, B. M. Rubenstein and O. Chen, Synthesis of All-Inorganic Cd-Doped CsPbCl3 Perovskite Nanocrystals with Dual-Wavelength Emission, J. Phys. Chem. Lett., 2018, 9, 7079–7084 CrossRef CAS.
- T. Cai, J. Y. Wang, W. H. Li, K. Hills-Kimball, H. J. Yang, Y. Nagaoka, Y. C. Yuan, R. Zia and O. Chen, Mn2+/Yb3+ Codoped CsPbCl3 Perovskite Nanocrystals with Triple-Wavelength Emission for Luminescent Solar Concentrators, Adv. Sci., 2020, 7, 2001317 CrossRef CAS.
- W. Zhang, J. Wei, Z. Gong, P. Huang, J. Xu, R. Li, S. Yu, X. Cheng, W. Zheng and X. Chen, Unveiling the Excited-State Dynamics of Mn2+ in 0D Cs4PbCl6 Perovskite Nanocrystals, Adv. Sci., 2020, 7, 2002210 CrossRef CAS.
- B. J. Beberwyck, Y. Surendranath and A. P. Alivisatos, Cation Exchange: A Versatile Tool for Nanomaterials Synthesis, J. Phys. Chem. C, 2013, 117, 19759–19770 CrossRef CAS.
- S. Gupta, S. V. Kershaw and A. L. Rogach, 25th Anniversary Article: Ion Exchange in Colloidal Nanocrystals, Adv. Mater., 2013, 25, 6923–6943 CrossRef CAS PubMed.
- W. van der Stam, E. Bladt, F. T. Rabouw, S. Bals and C. D. Donega, Near-Infrared Emitting CuInSe2/CuInS2 Dot Core/Rod Shell Heteronanorods by Sequential Cation Exchange, ACS Nano, 2015, 9, 11430–11438 CrossRef CAS PubMed.
- H. B. Li, R. Brescia, R. Krahne, G. Bertoni, M. J. P. Alcocer, C. D’Andrea, F. Scotognella, F. Tassone, M. Zanella, M. De Giorgi and L. Manna, Blue-UV-Emitting ZnSe(Dot)/ZnS(Rod) Core/Shell Nanocrystals Prepared from CdSe/CdS Nanocrystals by Sequential Cation Exchange, ACS Nano, 2012, 6, 1637–1647 CrossRef CAS PubMed.
- L. De Trizio and L. Manna, Forging Colloidal Nanostructures via Cation Exchange Reactions, Chem. Rev., 2016, 116, 10852–10887 CrossRef CAS PubMed.
- G. Nedelcu, L. Protesescu, S. Yakunin, M. I. Bodnarchuk, M. J. Grotevent and M. V. Kovalenko, Fast Anion-Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I), Nano Lett., 2015, 15, 5635–5640 CrossRef CAS PubMed.
- W. van der Stam, J. J. Geuchies, T. Altantzis, K. H. W. van den Bos, J. D. Meeldijk, S. Van Aert, S. Bals, D. Vanmaekelbergh and C. D. M. Donega, Highly Emissive Divalent-Ion-Doped Colloidal CsPb1-xMxBr3 Perovskite Nanocrystals through Cation Exchange, J. Am. Chem. Soc., 2017, 139, 4087–4097 CrossRef CAS PubMed.
- F. Li, Z. Xia, C. Pan, Y. Gong, L. Gu, Q. Liu and J. Z. Zhang, High Br- Content CsPb(ClyBr1-y)3 Perovskite Nanocrystals with Strong Mn2+ Emission through Diverse Cation/Anion Exchange Engineering, ACS Appl. Mater. Interfaces, 2018, 10, 11739–11746 CrossRef CAS.
- A. Shapiro, M. W. Heindl, F. Horani, M. H. Dahan, J. Tang, Y. Amouyal and E. Lifshitz, Significance of Ni Doping in CsPbX3 Nanocrystals via Postsynthesis Cation-Anion Coexchange, J. Phys. Chem. C, 2019, 123, 24979–24987 CrossRef.
- W. J. Mir, A. Swarnkar and A. Nag, Postsynthesis Mn-doping in CsPbI3 Nanocrystals to Stabilize the Black Perovskite Phase, Nanoscale, 2019, 11, 4278–4286 RSC.
- C. Otero-Martinez, M. Imran, N. J. Schrenker, J. Z. Ye, K. Y. Ji, A. Rao, S. D. Stranks, R. L. Z. Hoye, S. Bals, L. Manna, J. Perez-Juste and L. Polavarapu, Fast A-Site Cation Cross-Exchange at Room Temperature: Single-to Double- and Triple-Cation Halide Perovskite Nanocrystals, Angew. Chem., Int. Ed., 2022, 61, e202205617 CrossRef CAS.
- K. M. Hills-Kimball, M. J. Perez, Y. Nagaoka, T. Cai, H. J. Yang, A. H. Davis, W. W. Zheng and O. Chen, Ligand Engineering for Mn2+ Doping Control in CsPbCl3 Perovskite Nanocrystals via a Quasi-Solid-Solid Cation Exchange Reaction, Chem. Mater., 2020, 32, 2489–2500 CrossRef CAS.
- L. Z. Wu, Y. Wang, M. Kurashvili, A. Dey, M. H. Cao, M. Doblinger, Q. Zhang, J. Feldmann, H. Huang and T. Debnath, Interfacial Manganese-Doping in CsPbBr3 Nanoplatelets by Employing a Molecular Shuttle, Angew. Chem., Int. Ed., 2022, 61, e202115852 CrossRef CAS PubMed.
- Z. Yang, M. Wei, O. Voznyy, P. Todorovic, M. Liu, R. Quintero-Bermudez, P. Chen, J. Z. Fan, A. H. Proppe, L. N. Quan, G. Walters, H. Tan, J.-W. Chang, U. S. Jeng, S. O. Kelley and E. H. Sargent, Anchored Ligands Facilitate Efficient B-Site Doping in Metal Halide Perovskites, J. Am. Chem. Soc., 2019, 141, 8296–8305 CrossRef CAS PubMed.
- T. Qiao, D. Parobek, Y. Dong, E. Ha and D. H. Son, Photoinduced Mn doping in cesium lead halide perovskite nanocrystals, Nanoscale, 2019, 11, 5247–5253 RSC.
- F. Li, Y. Liu, H. Wang, Q. Zhan, Q. Liu and Z. Xia, Postsynthetic Surface Trap Removal of CsPbX3 (X = CI, Br, or I) Quantum Dots via a ZnX2/Hexane Solution toward an Enhanced Luminescence Quantum Yield, Chem. Mater., 2018, 30, 8546–8554 CrossRef CAS.
- C. Xie, Y. Zhao, W. Shi and P. Yang, Postsynthetic Surface-Treatment of CsPbX3 (X = Cl, Br, or I) Nanocrystals via CdX2 Precursor Solution toward High Photoluminescence Quantum Yield, Langmuir, 2021, 37, 1183–1193 CrossRef CAS PubMed.
- X. Li, Y. Wu, S. Zhang, B. Cai, Y. Gu, J. Song and H. Zeng, CsPbX3 Quantum Dots for Lighting and Displays: Room-Temperature Synthesis, Photoluminescence Superiorities, Underlying Origins and White Light-Emitting Diodes, Adv. Funct. Mater., 2016, 26, 2435–2445 CrossRef CAS.
- K. Xu, E. T. Vickers, B. B. Luo, A. C. Allen, E. F. Chen, G. Roseman, Q. H. Wang, D. S. Kliger, G. L. Millhauser, W. J. Yang, X. M. Li and J. Z. Zhang, First Synthesis of Mn-Doped Cesium Lead Bromide Perovskite Magic Sized Clusters at Room Temperature, J. Phys. Chem. Lett., 2020, 11, 1162–1169 CrossRef CAS PubMed.
- K. Y. Xu, C. C. Lin, X. B. Xie and A. Meijerink, Efficient and Stable Luminescence from Mn2+ in Core and Core-Isocrystalline Shell CsPbCl3 Perovskite Nanocrystals, Chem. Mater., 2017, 29, 4265–4272 CrossRef CAS PubMed.
- Q. Shan, J. Song, Y. Zou, J. Li, L. Xu, J. Xue, Y. Dong, B. Han, J. Chen and H. Zeng, High Performance Metal Halide Perovskite Light-Emitting Diode: From Material Design to Device Optimization, Small, 2017, 13, 1701770 CrossRef.
- G. C. Pan, X. Bai, W. Xu, X. Chen, Y. Zhai, J. Y. Zhu, H. Shao, N. Ding, L. Xu, B. Dong, Y. L. Mao and H. W. Song, Bright Blue Light Emission of Ni2+ Ion-Doped CsPbClxBr3-x Perovskite Quantum Dots Enabling Efficient Light-Emitting Devices, ACS Appl. Mater. Interfaces, 2020, 12, 14195–14202 CrossRef CAS PubMed.
- Y. K. Wang, X. Y. Liu, Q. Q. He, G. Y. Chen, D. D. Xu, X. D. Chen, W. B. Zhao, J. C. Bao, X. X. Xu, J. L. Liu and X. Wang, Reversible Transformation between CsPbBr3 Perovskite Nanowires and Nanorods with Polarized Optoelectronic Properties, Adv. Funct. Mater., 2021, 31, 2011251 CrossRef CAS.
- S. Ye, M. J. Zhao, J. Song and J. L. Qu, Controllable emission bands and morphologies of high-quality CsPbX3 perovsKite nanocrystals prepared in octane, Nano Res., 2018, 11, 4654–4663 CrossRef CAS.
- A. Q. Liu, G. Y. Guan, X. M. Chai, N. N. Feng, M. Lu, X. Bai and Y. Zhang, Metal Halide Perovskites toward Electrically Pumped Lasers, Laser Photon. Rev., 2022, 16, 2200189 CrossRef CAS.
- C. Otero-Martinez, N. Fiuza-Maneiro and L. Polavarapu, Enhancing the Intrinsic and Extrinsic Stability of Halide Perovskite Nanocrystals for Efficient and Durable Optoelectronics, ACS Appl. Mater. Interfaces, 2022, 14, 34291–34302 CrossRef CAS PubMed.
- A. Matuhina, G. K. Grandhi, M. N. Liu, J. H. Smatt, N. S. M. Viswanath, H. Ali-Loytty, K. Lahtonen and P. Vivo, Octahedral distortion driven by CsPbI3 nanocrystal reaction temperature - the effects on phase stability and beyond, Nanoscale, 2021, 13, 14186–14196 RSC.
- Q. A. Akkerman, G. Raino, M. V. Kovalenko and L. Manna, Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals, Nat. Mater., 2018, 17, 394–405 CrossRef CAS.
- Y. Wang, G. Chen, D. Ouyang, X. He, C. Li, R. Ma, W.-J. Yin and W. C. H. Choy, High Phase Stability in CsPbI3 Enabled by Pb-I Octahedra Anchors for Efficient Inorganic Perovskite Photovoltaics, Adv. Mater., 2020, 32, 2000186 CrossRef CAS.
- J. B. Zhang, L. W. Zhang, P. Cai, X. G. Xue, M. K. Wang, J. Zhang and G. L. Tu, Enhancing stability of red perovskite nanocrystals through copper substitution for efficient light-emitting diodes, Nano Energy, 2019, 62, 434–441 CrossRef CAS.
- J. Y. Woo, Y. Kim, J. Bae, T. G. Kim, J. W. Kim, D. C. Lee and S. Jeong, Highly Stable Cesium Lead Halide Perovskite Nanocrystals through in Situ Lead Halide Inorganic Passivation, Chem. Mater., 2017, 29, 7088–7092 CrossRef CAS.
- H. Yang, W. Yin, W. Dong, L. Gao, C.-H. Tan, W. Li, X. Zhang and J. Zhang, Enhancing the light-emitting performance and stability in CsPbBr3 perovskite quantum dots via simultaneous doping and surface passivation, J. Mater. Chem. C, 2020, 8, 14439–14445 RSC.
- X. Chen, K. Xing, Z. Cao, X. Yuan and J. Zhao, Improving Thermal Stability of Photoluminescence in Violet-emitting CsPbCl3 Perovskite Nanocrystals Using Ni Doping, Chin. J. Lumin., 2019, 40, 1220–1227 CrossRef CAS.
- M. Xie, J. Guo, X. Zhang, C. Bi, L. Zhang, Z. Chu, W. Zheng, J. You and J. Tian, High-Efficiency Pure-Red Perovskite Quantum-Dot Light-Emitting Diodes, Nano Lett., 2022, 22, 8266–8273 CrossRef CAS PubMed.
- J. K. Lyu, B. Dong, G. C. Pan, L. H. Sun, X. Bai, S. T. Hu, B. Shen, B. S. Zhou, L. Wang, W. Xu, D. L. Zhou, L. Xu and H. W. Song, Ni2+ and Pr3+ Co-doped CsPbCl3 perovskite quantum dots with efficient infrared emission at 1300 nm, Nanoscale, 2021, 13, 16598–16607 RSC.
- K. Xing, X. Yuan, Y. Wang, J. Li, Y. J. Wang, Y. Fan, L. Yuan, K. Li, Z. J. Wu, H. B. Li and J. L. Zhao, Improved Doping and Emission Efficiencies of Mn-Doped CsPbCl3 Perovskite Nanocrystals via Nickel Chloride, J. Phys. Chem. Lett., 2019, 10, 4177–4184 CrossRef CAS PubMed.
- P. J. Song, B. Qiao, D. D. Song, J. Y. Cao, Z. H. Shen, Z. Xu, S. L. Zhao, S. Wageh and A. Al-Ghamdi, Modifying the Crystal Field of CsPbCl3:Mn2+ Nanocrystals by Co-doping to Enhance Its Red Emission by a Hundredfold, ACS Appl. Mater. Interfaces, 2020, 12, 30711–30719 CrossRef CAS PubMed.
- J. K. Zhang, Y. Z. Zheng, G. T. Liu, Y. Z. Ma, L. Gong, R. Q. Guan, X. Y. Cui, J. J. Yan, J. L. Zhao and J. H. Yang, Pressure-Engineered Optical and Charge Transport Properties of Mn2+/Cu2+ Codoped CsPbCl3 Perovskite Nanocrystals via Structural Progression, ACS Appl. Mater. Interfaces, 2020, 12, 48225–48236 CrossRef CAS PubMed.
- S. Paul, E. Bladt, A. F. Richter, M. Doblinger, Y. Tong, H. Huang, A. Dey, S. Bals, T. Debnath, L. Polavarapu and J. Feldmann, Manganese-Doping-Induced Quantum Confinement within Host Perovskite Nanocrystals through Ruddlesden-Popper Defects, Angew. Chem., Int. Ed., 2020, 59, 6794–6799 CrossRef CAS PubMed.
- Z. Song, J. Zhao and Q. Liu, Luminescent perovskites: recent advances in theory and experiments, Inorg. Chem. Front., 2019, 6, 2969–3011 RSC.
- L. Chouhan, S. Ghimire, C. Subrahmanyam, T. Miyasaka and V. Biju, Synthesis, optoelectronic properties and applications of halide perovskites, Chem. Soc. Rev., 2020, 49, 2869–2885 RSC.
- J. Guo, Y. Fu, M. Lu, X. Zhang, S. V. Kershaw, J. Zhang, S. Luo, Y. Li, W. W. Yu, A. L. Rogach, L. Zhang and X. Bai, Cd-Rich Alloyed CsPb1-xCdxBr3 Perovskite Nanorods with Tunable Blue Emission and Fermi Levels Fabricated through Crystal Phase Engineering, Adv. Sci., 2020, 7, 2000930 CrossRef CAS PubMed.
- M. Imran, J. Ramade, F. Di Stasio, M. De Franco, J. Buha, S. Van Aert, L. Goldoni, S. Lauciello, M. Prato, I. Infante, S. Bals and L. Manna, Alloy CsCdxPb1-xBr3 Perovskite Nanocrystals: The Role of Surface Passivation in Preserving Composition and Blue Emission, Chem. Mater., 2020, 32, 10641–10652 CrossRef CAS.
- C. Zhang, B. Wang, Q. Wan, L. Kong, W. Zheng, Z. Li and L. Li, Critical role of metal ions in surface engineering toward brightly luminescent and stable cesium lead bromide perovskite quantum dots, Nanoscale, 2019, 11, 2602–2607 RSC.
- G. H. Ahmed, Y. Liu, I. Bravic, X. Ng, I. Heckelmann, P. Narayanan, M. S. Fernandez, B. Monserrat, D. N. Congreve and S. Feldmann, Luminescence Enhancement Due to Symmetry Breaking in Doped Halide Perovskite Nanocrystals, J. Am. Chem. Soc., 2022, 144, 15862–15870 CrossRef CAS PubMed.
- K. R. Pradeep, D. Acharya, P. Jain, K. Gahlot, A. Yadav, A. Camellini, M. Zavelani-Rossi, G. Cerullo, C. Narayana, S. Narasimhan and R. Viswanatha, Harvesting Delayed Fluorescence in Perovskite Nanocrystals Using Spin-Forbidden Mn d States, ACS Energy Lett., 2020, 5, 353–359 CrossRef.
| This journal is © the Partner Organisations 2024 |