Open Access Article
Open Access ArticleCrosslinking CO2-switchable polymers for paints and coatings applications†
Raz
Abbasi
a,
Amy
Mitchell
b,
Philip G.
Jessop
 *b and
Michael F.
Cunningham
*b and
Michael F.
Cunningham
 *a
*a
aDepartment of Chemical Engineering, Queen's University, Kingston, ON K7L 3N6, Canada. E-mail: michael.cunningham@queensu.ca
bDepartment of Chemistry, Queen's University, Kingston, ON K7L 3N6, Canada. E-mail: 18avm5@queensu.ca; jessop@queensu.ca
First published on 20th December 2023
Abstract
The emission of volatile organic compounds (VOCs) from solvent-based paints and coatings and its detrimental effect on humans and the environment have encouraged the industry to move towards more environmentally friendly options. Water-based paints and coatings, however, require a complex film formation process involving the coalescence of colloidal polymeric particles in the aqueous medium which adversely affects the critical properties of the film. Despite the advances in water-based formulations, they are still outperformed by solvent-based coatings, particularly in high performance applications. Our group previously demonstrated the potential of CO2-switchable polymers in paints and coatings applications to achieve an aqueous zero-VOC formulation that uses the same film formation process as solvent-based formulations. The normally water-insoluble CO2-switchable polymer can be fully dissolved in carbonated water rather than organic solvents. Subsequently, upon application of the solution to a surface, the polymer switches to a water-insoluble form as the CO2 and water evaporate. This technology offers both environmental friendliness and high performance. In this study, we focus on improving the solvent resistance of the resulting coatings by utilizing a crosslinking reaction. In order to achieve a 1K crosslinking system, the crosslinking reaction should be hindered when CO2 is present to achieve stability and occur during the film formation process as the CO2 evaporates. To accomplish this goal, possible reactions are initially investigated using model compounds that mimic the structure of the polymer and crosslinking agent in terms of their reactivity in the presence and absence of CO2. The promising candidates were then applied in a coating formulation and evaluated in terms of the performance of the crosslinked coatings and the stability of the formulation.
Introduction
Paints and coatings are used in numerous applications and make up the largest single use of organic solvents among different industry sectors, accounting for almost half of all organic solvent usage.1 This widespread usage contributes significantly to the emissions of volatile organic compounds (VOCs) which pose a significant threat to not only human health but also the environment.2,3 For instance, exposure of workers to VOCs leads to inhalation risks, which can result in serious short- and long-term health problems. In addition, these compounds are highly flammable, pose fire hazards, and cause smog formation.2In recent years, there has been a shift from conventional solvent-borne coatings towards more environmentally friendly formulas, such as water-borne, powder, high solid contents, and radiation curable coatings. Water-based technology currently holds the largest architectural and decorative coating market share.4 However, despite the ongoing advances in water-based coatings they are still outperformed by solvent-based coatings in performance factors such as chemical resistance, corrosion, abrasion resistance, and high gloss finish.5 As a result, solvent-based technology is still dominant in most industrial applications that require high performance coatings.
In a solvent-based system, casting of the fully dissolved polymeric solution in an organic solvent on a substrate surface followed by solvent evaporation results in the entanglement of polymer chains into a network. This network forms a film with a high degree of polymer–polymer interpenetration since macromolecules are homogeneously distributed in the solvent.6 This leads to a high performance and high gloss coating. On the other hand, in water-based coatings, also known as latexes, polymeric particles are suspended in the aqueous medium. Film formation begins with water evaporation after application to a substrate. As the coating dries, the colloidal particles come into direct contact, followed by gradual coalescence to form a film.7–9 Softer polymer chains with a low glass-to-rubber transition temperature (Tg) are required to facilitate the coalescence process which has a direct effect on the final properties of the coating.10 The difference in the film formation mechanisms, solvent-based versus water-based, affects the final coating microstructure which in turn influences critical film properties including gloss, sheen, hardness, abrasion resistance, swelling, and durability. For instance, latex coatings generally have rougher microscale surfaces compared to solvent-based coatings which reduce the gloss level.
An ideal coating would have the performance advantages of solvent-based coatings and the environmental advantages of water-based coatings. This ideal coating formulation would require polymers that can be dissolved in the aqueous medium before application and can be switched to a water-insoluble form after casting onto a substrate. One of the most promising classes of polymers for this purpose is CO2-responsive polymers.11 CO2 as a trigger for stimuli-responsive materials has attracted considerable attention since it is benign, inexpensive, recyclable, abundant, and does not accumulate in the system.12,13 A polymer with specific functional groups such as amines or amidines becomes protonated (charged) by the addition of CO2 (e.g., in carbonated water) and reverts to the unprotonated (neutral) state upon removal of the trigger (e.g., upon CO2 evaporation). This results in a reversible change in some properties of the polymer including solubility, pH, and osmotic pressure.14,15 As such, CO2-switchable polymers can undergo a reversible hydrophobic–hydrophilic transition upon the addition of CO2 which is of great interest for coating applications. Ho et al. demonstrated the potential of CO2-switchable polymers to serve as a replacement for solvent-based coatings by synthesizing different copolymers containing CO2-switchable monomers including diethylaminoethyl methacrylate (DEAEMA), dimethylaminoethyl methacrylate (DMAEMA), or tert-butylaminoethyl methacrylate (tBAEMA), with well-known non-switchable comonomers in the coating industry such as methyl methacrylate (MMA) and butyl methacrylate (BMA).11
The hydrophobic nature of a CO2-switchable polymer in the neutral state provides water resistance to the final film; however, the ideal coating should offer both water and solvent resistance. For this purpose, crosslinking reactions can be used to enhance the chemical resistance and the mechanical strength by forming a covalently bonded three-dimensional polymer network.16–19 Formulations of polymers and crosslinking agents in coatings can be classified into one-component (1K) and two-component (2K) systems. In 1K systems, the crosslinking agent is always present in the formulation, and the crosslinking reaction does not occur until the time of application. On the other hand, in 2K systems, the crosslinker (known as an activator or hardener) is added to the system just prior to the application.
In this study, we have investigated crosslinking strategies for zero-VOC coatings made from CO2-switchable polymers, specifically targeting 1K crosslinking chemistry. The desired 1K system requires crosslinking chemistry that is inhibited in the presence of CO2 (i.e. while the coating formulation is stored) but reacts rapidly in the absence of CO2 (i.e. after application) at room temperature. Moreover, the crosslinking agent must be soluble in carbonated water and inert to side reactions with CO2.
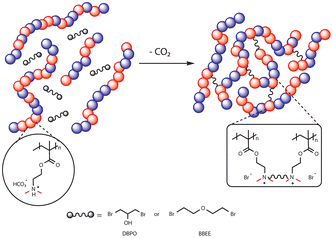
The focus of this study is to achieve crosslinking by alkylation of amines using bromine compounds at room temperature. The low hydrolysis rate of alkyl bromides makes them appropriate crosslinkers for a 1K system by providing a sufficiently long shelf-life.20,21 For this purpose, the crosslinking chemistry of coatings containing CO2-switchable polymers was initially investigated via nuclear magnetic resonance (NMR) spectroscopy using model compounds (small molecule analogues of a polymer chain and crosslinking agent). The crosslinking agents were then applied to a polymeric system to investigate the chemical and water resistance of the crosslinked films as well as the stability of the formulation during storage.
Experimental methods
Materials
Small molecules including 3-bromo-1-propanol (BPO, 97%), 1,3-dibromo-2-propanol (DBPO, 98%), 2-(methylamino)ethanol (MAE, ≥98%), 1,3-bis(tris(hydroxymethyl)methylamino)propane, (BTP, ≥99%), bis(2-bromoethyl)ether (BBEE, ≥95%), 2-dimethylaminoethanol (DMAE, ≥99.5%), 2-diethylaminoethanol (DEAE, ≥99.5%), and glycolic acid (GA) of 99% purity were purchased from MilliporeSigma and used as received. Monomers including diethylaminoethyl methacrylate (DEAEMA, 99%, containing 1500 ppm MEHQ as an inhibitor), dimethylaminoethyl methacrylate (DMAEMA, 98%, containing 700–1000 ppm monomethyl ether hydroquinone as an inhibitor), methyl methacrylate (MMA, 99%, containing ≤30 ppm MEHQ as an inhibitor), and butyl methacrylate (BMA, 99%, containing monomethyl ether hydroquinone as an inhibitor), were purchased from MilliporeSigma and passed through inhibitor remover columns prior to use. Azobisisobutyronitrile (AIBN, 98%) was purchased from MilliporeSigma and recrystallized from its solution in ethanol. Anhydrous dimethylformamide (DMF, 99%) and acetic acid (≥99.7%) were purchased from Thermo Fisher Scientific. Solvents including acetone (≥99.5%), tetrahydrofuran (THF, ≥99.9%), toluene (≥99.5%), methyl ethyl ketone (MEK, ≥99.9%), and methanol (≥99.9%) were purchased from MilliporeSigma and used as received. All the water used in this study was deionized using a Synergy MilliQ system and had a resistivity of 18.2 MΩ cm−1 before exposure to air. Carbon dioxide (grade 3.0) and nitrogen were obtained from Praxair.General procedure for study with small molecules
To gain insight into the reaction between different types of amines (mimicking the pendant group of the polymeric amine) and a bromo compound in the presence and absence of CO2, model compounds were employed. Each selected amine was mixed with 3-bromo-1-propanol (BPO) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio with respect to the number of nitrogen atoms in the amine compound, in deuterium oxide (D2O). The concentration of small molecules in D2O was maintained at 15 wt% for all experiments. The name and structure of the studied model compounds are shown in Table 1, which includes the bulky and non-bulky secondary and tertiary amines with BPO. The solutions were made at room temperature and stirred vigorously. In addition, to simulate the effects of the solution being exposed to CO2, one equivalent of glycolic acid was added to protonate the amine (charged state). Glycolic acid was chosen because it is nonvolatile and its conjugate base, glycolate anion, is a structural mimic of the bicarbonate anion. 1H NMR spectra were recorded at three time intervals (immediately, 1 day, and 1 week after mixing) for both protonated (charged) and unprotonated (neutral) forms. Additionally, mass spectrometry was conducted for each mixture in water after 1 week, to verify the obtained product.
1 molar ratio with respect to the number of nitrogen atoms in the amine compound, in deuterium oxide (D2O). The concentration of small molecules in D2O was maintained at 15 wt% for all experiments. The name and structure of the studied model compounds are shown in Table 1, which includes the bulky and non-bulky secondary and tertiary amines with BPO. The solutions were made at room temperature and stirred vigorously. In addition, to simulate the effects of the solution being exposed to CO2, one equivalent of glycolic acid was added to protonate the amine (charged state). Glycolic acid was chosen because it is nonvolatile and its conjugate base, glycolate anion, is a structural mimic of the bicarbonate anion. 1H NMR spectra were recorded at three time intervals (immediately, 1 day, and 1 week after mixing) for both protonated (charged) and unprotonated (neutral) forms. Additionally, mass spectrometry was conducted for each mixture in water after 1 week, to verify the obtained product.
1H NMR spectroscopy using a Bruker Avance 400 MHz (at 400.30 MHz) spectrometer was used to monitor the reaction progress with model compounds at 298 K. Small molecules were mixed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio at a concentration of 15 wt% in D2O.
1 molar ratio at a concentration of 15 wt% in D2O.
Mass spectroscopy (MS) was performed using a Velos Pro Orbitrap (ThermoFisher, San Jose, CA) by ESI in positive-ion mode. As a result, the pseudo-molecular ion MH+ (positive ion mode) was observed in the obtained mass spectra. The instrument was controlled using Xcalibur 2.2 SP1 software. Samples were prepared at a concentration of 20 mg mL−1.
Synthesis and characterization of copolymers
CO2-switchable polymers including p(DMAEMA-co-BMA) and p(DEAEMA-co-MMA) were synthesized via free radical polymerization with monomer compositions of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 and 80
50 and 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (mole ratio), respectively. As shown in our previous study, polymers made with these compositions exhibit CO2-switchable behavior.11 In a typical polymerization, anhydrous DMF (250 mL) was added to a 500 mL Schlenk flask and degassed via nitrogen bubbling for 1 h, followed by the addition of 13.8 mL DMAEMA (0.081 mol) and 13 mL BMA (0.082 mol) for p(DMAEMA-co-BMA), and 37.6 mL DEAEMA (0.187 mol) and 5 mL MMA (0.047 mol) for p(DEAEMA-co-MMA). The mixture was degassed with nitrogen for an additional 30 minutes. The AIBN initiator was then added to the solution (0.5 mol% based on the total amount of monomers), and the flask was submerged in an oil bath pre-heated to 75 °C and stirred (400 rpm) for 6 hours under a nitrogen atmosphere. The flask was then cooled to room temperature. The crude reaction mixture was then added dropwise into a beaker with 4 L of water and stirred vigorously. The precipitated polymer was collected by gravity or vacuum filtration and dried under vacuum (65 °C, overnight).
20 (mole ratio), respectively. As shown in our previous study, polymers made with these compositions exhibit CO2-switchable behavior.11 In a typical polymerization, anhydrous DMF (250 mL) was added to a 500 mL Schlenk flask and degassed via nitrogen bubbling for 1 h, followed by the addition of 13.8 mL DMAEMA (0.081 mol) and 13 mL BMA (0.082 mol) for p(DMAEMA-co-BMA), and 37.6 mL DEAEMA (0.187 mol) and 5 mL MMA (0.047 mol) for p(DEAEMA-co-MMA). The mixture was degassed with nitrogen for an additional 30 minutes. The AIBN initiator was then added to the solution (0.5 mol% based on the total amount of monomers), and the flask was submerged in an oil bath pre-heated to 75 °C and stirred (400 rpm) for 6 hours under a nitrogen atmosphere. The flask was then cooled to room temperature. The crude reaction mixture was then added dropwise into a beaker with 4 L of water and stirred vigorously. The precipitated polymer was collected by gravity or vacuum filtration and dried under vacuum (65 °C, overnight).
The molecular weight and dispersity (Đ) of copolymers were determined by size exclusion chromatography (SEC) using a Waters 2695 separation module equipped with a set of Waters Styragel columns, connected to a Waters 2414 differential refractometer at 40 °C. THF was used as the eluent with a flow rate of 0.3 mL min−1 and the injection volume was set to 30 μL. Polymers were dissolved in THF and filtered through a 0.2 μm syringe filter prior to the injection. The average molar masses (Mn and Mw) and dispersity were derived according to a calibration curve based on the low dispersity pMMA standards ranging from 535 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 020
020![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 (InfinityLab EasiVial, Agilent Technologies).
000 g mol−1 (InfinityLab EasiVial, Agilent Technologies).
Additionally, the composition of the purified copolymers was determined using 1H NMR spectroscopy with a Bruker Avance 500 MHz at 298 K (CDCl3 used as solvent).
Differential scanning calorimetry (DSC) was performed using a TA Instruments Q100 DSC analyzer to determine the copolymer's Tg. Nitrogen was used in DSC experiments with a flow rate of 50 mL min−1. Three cycles were performed starting at −20 °C and heating to 200 °C, followed by an isothermal hold then cooling back to −20 °C and heating back to 200 °C. All heating and cooling rates were 10 °C min−1.
Evaluation of coating formulation stability and coat formation
To dissolve the copolymers in carbonated water, Milli-Q water was added to a 100 mL round bottom flask equipped with a gas dispersion tube (connected to a CO2 tank via a gas regulator) and a vent needle. CO2 was sparged through the water via the gas dispersion tube for 30 min to obtain carbonated water with a pH of approximately 4. The polymer was then crushed and gradually added to carbonated water. The concentration of the polymer in carbonated water was 20 wt% for all experiments. The flask was left under a CO2 atmosphere overnight to achieve a clear solution of dissolved polymer in carbonated water. Afterward, the crosslinking agent was added in different molar ratios (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 2
10, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and 1
10, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 for the crosslinking agent
30 for the crosslinking agent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) amine repeating unit) to the given solution, stirred, and left for 24 h. The resulting mixture was then cast onto a flat silicon mold and allowed to cure at room temperature for 1 week to obtain the crosslinked films.
amine repeating unit) to the given solution, stirred, and left for 24 h. The resulting mixture was then cast onto a flat silicon mold and allowed to cure at room temperature for 1 week to obtain the crosslinked films.
Additionally, the stability of the coating formulations (with the polymers in their charged state) over time in the presence of the crosslinking agent was studied using an Agilent 1260 Infinity II SEC system equipped with a set of PSS NOVEMA Max Lux columns, connected to an Agilent GPC/SEC MDS triple detection unit. A solution of 0.02 wt% LiBr and 0.3 M formic acid in HPLC grade water was used as the eluent. The flow rate and injection volume were set to 1 mL min−1 and 100 μL, respectively. The temperature of the columns and RI detector was set to 40 °C. The coating's mixture containing the polymer and the crosslinking agent dissolved in carbonated water was allowed to age for 1 month. Subsequently, a solution of GA was added to the mixture prior to analysis (to prevent any further crosslinking reaction during the analysis). The samples were filtered using a 0.2 μm filter prior to the injection. The RI detector was calibrated using a set of low dispersity poly(2-vinylpyridine) standards ranging from 1110 to 446![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 (PSS Polymer Standard Service GmbH).
000 g mol−1 (PSS Polymer Standard Service GmbH).
The viscosity of the coating formulation was measured using an Anton Paar MCR 702 rotational rheometer, with a cup-bob geometry employed. The temperature was maintained at 20 °C during the test.
To obtain gel fractions, the crosslinked films were weighed, placed into a filtration cellulose thimble and Soxhlet extracted using 250 mL of refluxing THF for 7 days. The films were then dried under a vacuum for at least 48 h. The gel fractions of the dried films were calculated using eqn (1).
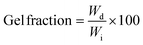 | (1) |
To evaluate the solvent and water resistance of the crosslinked films and their behavior in contact with different solvents, the swelling index was measured by immersing 0.2 g of a crosslinked film in an excess of different solvents and shaking for 24 h at room temperature. The swollen films were then taken out from the solvent, quickly filtered, and weighed.22 The swelling ratio is calculated using eqn (2).
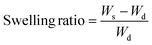 | (2) |
The double rub test was conducted on the crosslinked and uncrosslinked films according to ASTM D5402 – 19.23 The cotton swab was dipped into different solvents listed in Table 2 and rubbed on the coated films on the steel plates. After every 25 rubs, the cotton swabs were charged with the solvent.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mole ratio) crosslinked with the BBEE crosslinker
50 mole ratio) crosslinked with the BBEE crosslinker
| Solvent | Swelling ratio (BBEE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAEMA repeating unit) DMAEMA repeating unit) |
||
|---|---|---|---|
| Uncrosslinked | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
|
| D = film dissolved. | |||
| Acetone | D | 0.15 | 0.41 |
| THF | D | 0.19 | 0.46 |
| Toluene | D | 0.16 | 0.56 |
| MEK | D | 0.23 | 0.45 |
| Methanol | D | 0.17 | 0.51 |
| 1 M acetic acid | D | 0.92 | 2.45 |
| Water | 0.23 | 0.16 | 0.24 |
| Carbonated water | D | 0.25 | 0.32 |
Results and discussion
To establish an effective 1K crosslinking system suitable for paints and coatings prepared using CO2 switchable technology, it is crucial for the polymers to remain relatively free of crosslinking while the formulation is stored (e.g., in the can) but undergo extensive crosslinking while they are forming a coating on the substrate. To achieve this objective, the progression of the reaction between the pendant group of polymeric amines and the dibromide crosslinking agent was monitored over time under two conditions: when the amine is protonated (with glycolic acid) and when it is in its neutral state. To simulate the polymer structure and investigate these processes, model compounds were employed that resemble the structure of the polymers and the crosslinker. The reaction progress and resulting products were analyzed using 1H NMR and mass spectroscopy. In the subsequent section, the reactions between different amines and a bromo alcohol (BPO), as outlined in Table 1, will be discussed.Reaction between tertiary amines and BPO
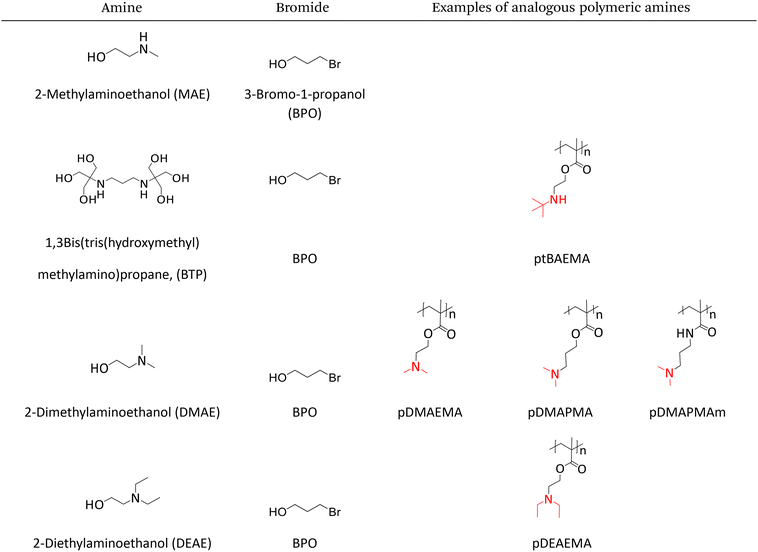
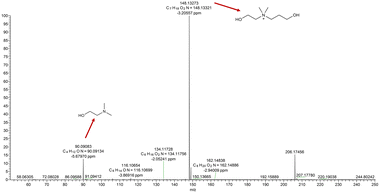
The Menshutkin reaction between unprotonated DMAE (non-bulky tertiary amine) and BPO resulted in the expected quaternary ammonium salt.24 The key indication is the appearance of a new singlet peak at 3.04 ppm associated with the methyl groups attached to the nitrogen in the product (Fig. 1).25 This peak is present in the spectrum recorded immediately after mixing and increases in area and height significantly over 1 week, after which all of the dominant peaks in the 1H NMR spectrum are associated with the product (Fig. S1–S3† present the integration values and 2D-NMR spectra of the product). The formation of the product (m/z: 148.13) was also confirmed by mass spectroscopy (Fig. 2).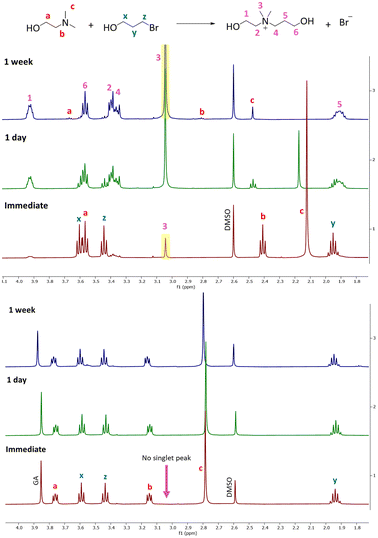 | ||
| Fig. 1 Overlaid 1H NMR spectra of DMAE and BPO, when the amine is in the neutral state (above) and protonated state (below). | ||
In this reaction, chemical shifts associated with the remaining DMAE shifted down-field over time with the signals associated with the methylene and methyl groups attached to the nitrogen showing a significant change in the position. This phenomenon is attributed to the change in pH and concentration over the course of the reaction. As the reaction proceeds the concentration of DMAE (starting material) decreases, which leads to lower pH as well as higher percent protonation of the remaining DMAE, causing the down-field shift in the NMR spectrum.26
When protonated DMAE (protonated with glycolic acid) was exposed to BPO, no expected quaternary salt formed. The spectra shown in Fig. 1 do not exhibit any significant peak associated with the quaternary salt product as indicated by the singlet at 3.04 pm over 1 week. In summary, the NMR analysis reveals that this mixture undergoes a rapid reaction in the neutral state and an extremely slow reaction when the amines are protonated which aligns with the desired behaviors for a 1K crosslinking system.
DEAE underwent only a very slow reaction with BPO at room temperature in the neutral state. The signal associated with the methylene protons in the ethyl groups of the product appeared after 1 day and developed further over 1 week (Fig. S4†). However, the conversion of the product is only about 23% after 1 week (based upon the integration of the triplet peaks at 1.20 ppm for the product, and 0.93 ppm for the starting material). Compared with DMAE, the steric hindrance of the two ethyl groups attached to the nitrogen slows the reaction significantly. Steric hindrance and solvent polarity are known to have an impact on the quaternization rate in the Menshutkin reaction.27,28 This reaction requires elevated temperatures to promote the formation of the product.29 It is therefore not appropriate for use in a paint formulation.
The reaction between secondary amines and BPO
The reaction between MAE (non-bulky secondary amine) and BPO can lead to a mono addition product via nucleophilic aliphatic substitution (Fig. S5†).30 In the spectrum recorded immediately after mixing the two compounds, the dominant peaks are associated with the starting materials. As the reaction proceeds, the peaks belonging to the product grow in comparison with the starting materials (after 1 day). This trend continues to the 1 week spectrum when all chemical shifts associated with the expected amino alcohol product (3-amino-2-(((2-hydroxyethyl)(methyl)amino)methyl)propan-1-ol) are present in the spectra along with a trace of residual starting materials (labeled in Fig. S5†). In this mixture, the chemical shifts of the protons on BPO stay at the same position over the course of the reaction. In contrast, the peaks of the MAE (starting material) and the amino alcohol product shift down-field with protons on the methylene and methyl groups attached to the nitrogen displaying significant movement. As mentioned previously, this is attributed to the change in pH and concentration over the course of the reaction. In addition, the HBr byproduct also affects the system by further protonating MAE which leads to a reduction of the electron density around the nitrogen atom due to the presence of the positively charged hydrogen ion.The mass-to-charge ratio (m/z) of 134.11 observed in the mass spectrum matches the expected m/z of the product (Fig. S7†). The MAE and BPO mixture, therefore, resulted in a rapid reaction at room temperature when the amine was in a neutral state, the desired outcome for a 1K crosslinking system.
Performing the same MAE–BPO reaction but in the presence of an equivalent of acid led to a low but non-negligible product yield after 1 week. The product conversion was 18% after 1 day and did not further increase over 1 week, indicating that the reaction did not significantly proceed after the first day. The cause of this limited product formation may have been the incomplete protonation of the MAE with glycolic acid. In this case, a small amount of unprotonated MAE may have reacted with BPO to form a product in 1 day. Since there is no more unprotonated amine in the medium, it prevents the reaction from progressing further. In summary, the reaction between the non-bulky secondary amine MAE and bromo compound BPO resulted in fast product formation at room temperature if the amine is neutral but a low yield of the product when the amine is protonated, although it is believed the protonation was incomplete.
The reaction of the bulky secondary amine (BTP) and BPO in the absence of added acid yielded no detectable product after 1 week (Fig. S6†), likely due to the steric hindrance of the BTP preventing the reaction. Mass spectroscopy showed a significant peak with the exact mass of BTP (m/z: 283.2) starting material and a peak belonging to m/z of the possible mono-addition product (Fig. S8†). However, it seems that the conversion was not large enough for the product to be detected by NMR spectroscopy.
Based on the reactions studied using model compounds, DMAE and MAE with BPO demonstrated the most promising results for use in 1K crosslinking reactions in CO2-switchable coating formulations. The tertiary amine DMAE is still preferred since the secondary amine MAE is sterically unhindered and forms carbamates in the presence of CO2 which would lead to a mixture of carbamates and bicarbonates in carbonated water. Since carbamates are thermodynamically stable, they typically require heating to drive off CO2 and revert them back to their neutral state. In contrast, tertiary amines exclusively form bicarbonates, which are more easily converted back to the neutral form.26,31
Crosslinking study in the polymeric system
The names and structures of CO2-switchable polymers containing non-bulky tertiary amines, which the NMR study suggested could undergo a crosslinking reaction in the presence of a dibromide crosslinking agent at room temperature, are provided in Table 1. pDMAEMA, chosen for this study, has a pKaH (the pKa of the protonated form) in the range of 7.0–7.5 and forms a cationic polyelectrolyte when its amine groups become protonated at lower pH values.32 As shown in our previous study, the copolymerization of DMAEMA with a more hydrophobic monomer like BMA in a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 monomer composition (mole ratio) results in a hydrophobic CO2-switchable copolymer which forms a homogeneous solution in carbonated water, whereas the copolymer is insoluble in regular (noncarbonated) water.11 p(DMAEMA-co-BMA), synthesized via free radical polymerization, was selected for further crosslinking study (Scheme 1). The 1H NMR spectrum of the purified copolymer is provided in Fig. S11.† Moreover, the properties of the synthesized copolymer are shown in Table S1.† The coating formulation used in the crosslinking study contains 20 wt% polymer for all experiments. With a relatively low viscosity of 27 mPa s (Fig. S13†), this allows for additional modifications, including the potential incorporation of additives.
50 monomer composition (mole ratio) results in a hydrophobic CO2-switchable copolymer which forms a homogeneous solution in carbonated water, whereas the copolymer is insoluble in regular (noncarbonated) water.11 p(DMAEMA-co-BMA), synthesized via free radical polymerization, was selected for further crosslinking study (Scheme 1). The 1H NMR spectrum of the purified copolymer is provided in Fig. S11.† Moreover, the properties of the synthesized copolymer are shown in Table S1.† The coating formulation used in the crosslinking study contains 20 wt% polymer for all experiments. With a relatively low viscosity of 27 mPa s (Fig. S13†), this allows for additional modifications, including the potential incorporation of additives.
In the following sections, the influence of the dibromide crosslinking agent structure, the degree of crosslinking, the stability of the coating formula, and the behavior of the crosslinked coatings in different solvents will be discussed.
1,3-Dibromo-1-propanol (DBPO) was initially chosen as a dibromide water-soluble crosslinker. DBPO at 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 2
10, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and 1
10, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 molar ratios (crosslinking agent
30 molar ratios (crosslinking agent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAEMA repeating unit) was added to a 20 wt% solution of the p(DMAEMA-co-BMA) copolymer in carbonated water. The mixture was then cast on a silicon mold to form a film. The dried film was then allowed to sit for 1 week at room temperature. To determine the gel fraction of the dried films after crosslinking, Soxhlet extraction was conducted for 1 week in refluxing THF. The crosslinked films revealed 97, 97, 96, and 83% gel fractions, respectively (Fig. 3). Furthermore, to determine if there was any effect of the solvent type on the degree of crosslinking, a crosslinked sample was prepared using a solution of p(DMAEMA-co-BMA) copolymer in THF with the DBPO crosslinker added at a molar ratio of 5
DMAEMA repeating unit) was added to a 20 wt% solution of the p(DMAEMA-co-BMA) copolymer in carbonated water. The mixture was then cast on a silicon mold to form a film. The dried film was then allowed to sit for 1 week at room temperature. To determine the gel fraction of the dried films after crosslinking, Soxhlet extraction was conducted for 1 week in refluxing THF. The crosslinked films revealed 97, 97, 96, and 83% gel fractions, respectively (Fig. 3). Furthermore, to determine if there was any effect of the solvent type on the degree of crosslinking, a crosslinked sample was prepared using a solution of p(DMAEMA-co-BMA) copolymer in THF with the DBPO crosslinker added at a molar ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (DBPO
10 (DBPO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAEMA repeating unit). The process of making the crosslinked film was conducted under the same conditions as the polymer dissolved in carbonated water. The gel fraction was approximately identical for both experiments (96% and 97%) showing that both final films were highly crosslinked.
DMAEMA repeating unit). The process of making the crosslinked film was conducted under the same conditions as the polymer dissolved in carbonated water. The gel fraction was approximately identical for both experiments (96% and 97%) showing that both final films were highly crosslinked.
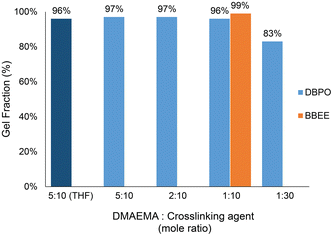 | ||
Fig. 3 The gel fractions of p(DMAEMA-co-BMA) (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mole ratio) crosslinked with DBPO and BBEE. Gel fractions were determined by Soxhlet extraction in refluxing THF for 1 week. 50 mole ratio) crosslinked with DBPO and BBEE. Gel fractions were determined by Soxhlet extraction in refluxing THF for 1 week. | ||
In addition, the crosslinking of pDEAEMA was studied under the same conditions. The pKaH value of the homopolymer of DEAEMA is in the range of 6.9–7.5.32 In addition, it is quite hydrophobic in its neutral form which provides water resistance for the final coating. However, according to the NMR studies with model compounds, unprotonated DEAE (the small-molecule analogue for pDEAEMA) did not result in a high reaction yield over the course of 1 week. In order to determine whether the reaction with an analogous polymeric amine would also be slow, DEAEMA was copolymerized with MMA in a 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 MMA
80 MMA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEAEMA mole ratio to give a Tg that is appropriate for coatings, as done in our previous study.11 The 1H NMR spectrum of the copolymer after purification is illustrated in Fig. S12.† The crosslinking degree of p(DEAEMA-co-MMA) was evaluated in the presence of a DBPO crosslinking agent at room temperature with a 5
DEAEMA mole ratio to give a Tg that is appropriate for coatings, as done in our previous study.11 The 1H NMR spectrum of the copolymer after purification is illustrated in Fig. S12.† The crosslinking degree of p(DEAEMA-co-MMA) was evaluated in the presence of a DBPO crosslinking agent at room temperature with a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 molar ratio of crosslinking agent
10 molar ratio of crosslinking agent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEAEMA repeating unit. The gel fraction of the dried film was 16%, which is in approximate agreement with the NMR small molecule study that resulted in a 23% product conversion after 1 week.
DEAEMA repeating unit. The gel fraction of the dried film was 16%, which is in approximate agreement with the NMR small molecule study that resulted in a 23% product conversion after 1 week.
The stability of the coating formula
The dibromo alcohol crosslinking agent (DBPO) was mixed with DMAE in D2O followed by sparging CO2. The reaction was then monitored via NMR analysis and compared with the same mixture in which the amine functional group is protonated using GA, to evaluate the reactivity of the solution when CO2 is present in the formula. The results suggested that DBPO reacts with CO2 leading to the formation of a cyclic carbonate by cycloaddition of carbon dioxide to DBPO (Fig. S9 and S10†). The synthesis of cyclic carbonates using alkylene halohydrins as one of the reagents with CO2 has been shown in different studies.33–36This side reaction appears to have a low product yield; however, it could affect the stability and shelf-life of the system. This is due to a possible decrease in the efficiency of the crosslinking agent in the long term as a portion of the crosslinker could convert to cyclic carbonates and become unavailable to participate in the crosslinking reaction. Consequently, DBPO is more suitable for a 2K system or possibly a 1K system with a limited shelf-life.
To achieve a self-crosslinking 1K formula, the formation of the cyclic carbonate side product can be avoided by selecting dibromo ether (BBEE) as a crosslinker that does not contain hydroxyl groups. To assess the extent of a potential side reaction using BBEE, an NMR study was conducted. No detectable reaction was found when the amine (DMAE) was protonated in the presence of CO2. Moreover, it is anticipated that the alkyl dibromide compounds will possess a satisfactory half-life suitable for a 1K crosslinking agent system because hydrolysis of structurally similar alkylbromides has a half-life on the order of a year or more.20 A crosslinked film of p(DMAEMA-co-BMA) (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 molar ratio of monomer composition) using the BBEE crosslinker was then obtained under the same conditions as the previous tests. The Soxhlet extraction revealed a 99% gel fraction with the addition of BBEE in a molar ratio of 1
50 molar ratio of monomer composition) using the BBEE crosslinker was then obtained under the same conditions as the previous tests. The Soxhlet extraction revealed a 99% gel fraction with the addition of BBEE in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (crosslinking agent
10 (crosslinking agent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAEMA repeating unit). Therefore, using the dibromo ether crosslinker BBEE eliminates the issue of the cyclic carbonate side product formation from the reaction with CO2 and results in a high degree of crosslinking.
DMAEMA repeating unit). Therefore, using the dibromo ether crosslinker BBEE eliminates the issue of the cyclic carbonate side product formation from the reaction with CO2 and results in a high degree of crosslinking.
To investigate the stability of the coating formulation over time, the molecular weight distribution of the polymer was monitored. Crosslinking would lead to higher molecular weight polymers which can be monitored via SEC analysis. The coating solution was aged for 1 month with the crosslinker. The SEC analysis was conducted to determine if there were any changes in the molecular weight distribution and compared with the fresh solution (polymer in carbonated water in the presence of the crosslinks without aging). Fig. 4 shows the molecular weight distributions of the two samples. A slight shift in molecular weight distribution was observed in the lower molecular weight region, however, there is no evidence of a shift at the higher molecular weight region by the crosslinking reaction. In addition, the solution remained transparent with no sign of the presence of the solid phase, indicating that there is little to no crosslinking taking place during the storage of the carbonated formulation.
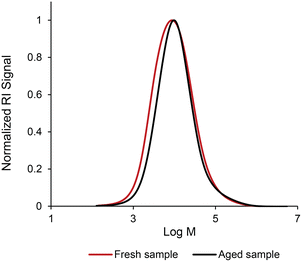 | ||
| Fig. 4 The molecular weight distribution of p(DMAEMA-co-BMA) dissolved in carbonated water in the presence of the crosslinking agent, aged for 1 month (aged sample) and without aging (fresh sample). | ||
Swelling test
To examine the solvent resistance of the coatings after crosslinking, the swelling ratio was measured by exposing the dried free coatings to various solvents. The film's swelling ratios were measured after 24 h of shaking the samples in excess solvents at room temperature (Table 2). For the films crosslinked with 1 mole BBEE per 10 moles DMAEMA repeating unit, solvents such as acetone, THF, toluene, and MEK swelled the network with ratios of 0.41 to 0.56. Increasing the crosslinking agent to 5 moles BBEE per 10 moles DMAEMA repeating unit decreased the swelling ratios to 0.15 to 0.23. Higher crosslinking density decreased the solvent penetration to the crosslinked polymeric networks thereby decreasing swelling ratio values. The film cast in the absence of a crosslinking agent was susceptible to dissolution in all solvents except for water. Therefore, the introduction of the dibromide crosslinker significantly enhanced the solvent resistance of the coating. The obtained swelling ratios are lower than the reported swelling ratios of latexes crosslinked by conventional crosslinking systems.37–42 As a comparison, crosslinked latexes using a 1K crosslinking system with the adipic acid dihydrazide (ADH) crosslinker showed swelling ratios between 3.16 and 6.48 in MEK (the weight ratio of the films after and before being immersed in solvent for 24 h).37 Another study on self-crosslinking acrylate containing fluorine and N-hydroxymethyl acrylamide (NMA) as the crosslinker reported swelling ratios of 7.01–9.81 in acetone, 11.08–16.75 in THF, and 0.55–0.78 in methanol (the weight ratio of the films after being immersed in solvents minus the dried film weight over the weight before immersion in solvent for 48 h).41The greatest swelling ratio was observed when the crosslinked films were immersed in acetic acid. The crosslinked films made with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 5
10 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 molar ratios (BBEE
10 molar ratios (BBEE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAEMA repeating unit) demonstrated swelling ratios of 2.45, and 0.92 in acetic acid, respectively. The pH of 1 M acetic acid is 2, which impacts the swelling ratio by protonating the tertiary amine units. As a comparison, the equilibrium swelling ratio of the crosslinked p(DEAEMA-co-BMA) (60
DMAEMA repeating unit) demonstrated swelling ratios of 2.45, and 0.92 in acetic acid, respectively. The pH of 1 M acetic acid is 2, which impacts the swelling ratio by protonating the tertiary amine units. As a comparison, the equilibrium swelling ratio of the crosslinked p(DEAEMA-co-BMA) (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 mole ratio) using the ethylene glycol dimethacrylate crosslinker in a buffer solution of pH 4 is reported to be approximately 12 at 25 °C.43
40 mole ratio) using the ethylene glycol dimethacrylate crosslinker in a buffer solution of pH 4 is reported to be approximately 12 at 25 °C.43
In neutral water, the swelling ratios (water uptake) of the crosslinked films with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 and 5
10 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 molar ratios of the BBEE
10 molar ratios of the BBEE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAEMA repeating unit are 0.24, and 0.16, respectively. These ratios are greater in carbonated water (0.25, and 0.32) than in neutral water due to the acidic environment of the carbonated water (pH of approximately 4) that can protonate tertiary amine groups. The uncrosslinked films dissolved in carbonated water under the same conditions.
DMAEMA repeating unit are 0.24, and 0.16, respectively. These ratios are greater in carbonated water (0.25, and 0.32) than in neutral water due to the acidic environment of the carbonated water (pH of approximately 4) that can protonate tertiary amine groups. The uncrosslinked films dissolved in carbonated water under the same conditions.
Conclusions
The superior performance of solvent-based coatings compared to conventional water-based systems (latexes) results from the full dissolution of the polymeric binder in a solvent. However, in response to environmental and health considerations, it is essential to replace solvent-based coatings with water-based alternatives due to the elimination of VOCs. In this research, we enhanced the solvent resistance of a zero-VOC water-based coating made of a CO2-responsive polymer. The CO2-switchable polymeric binder exhibits hydrophobic properties in the neutral state but becomes hydrophilic in the presence of CO2 and water forming a homogeneous solution. This approach combines the performance advantages and eco-friendliness of both systems.The solvent resistance of the coating was successfully improved by introducing a 1K self-crosslinking system triggered by CO2 removal. The coating formulation consists of p(DMAEMA-co-BMA) and a dibromide compound crosslinking agent. In this study, the reaction between different small molecule amines and a bromo alcohol compound, mimicking the pendant group of polymeric amines and a bromide crosslinking agent respectively, was initially investigated. The 1H NMR study of model compounds suggested that the non-bulky tertiary amine (DMAE) reacts quickly with BPO under air and the reaction is inhibited under CO2. This met the requirements of a 1K crosslinking system where the crosslinking agent is present in the formula and the reaction does not take place until the time of application.
The addition of the BBEE crosslinker in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 molar ratio (BBEE
10 molar ratio (BBEE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMAEMA repeating unit) resulted in a 99% gel fraction to the crosslinked coatings when dried at room temperature. The swelling tests indicated that the uncrosslinked films were soluble in the different organic solvents, whereas the crosslinked films remained intact and insoluble even after 24 hours of shaking in excess amounts of different solvents (acetone, THF, toluene, methanol, acetic acid, and carbonated water) indicating a significant improvement in the solvent resistance of the coatings after crosslinking.
DMAEMA repeating unit) resulted in a 99% gel fraction to the crosslinked coatings when dried at room temperature. The swelling tests indicated that the uncrosslinked films were soluble in the different organic solvents, whereas the crosslinked films remained intact and insoluble even after 24 hours of shaking in excess amounts of different solvents (acetone, THF, toluene, methanol, acetic acid, and carbonated water) indicating a significant improvement in the solvent resistance of the coatings after crosslinking.
The prototype formulation does not crosslink during storage because the bromide crosslinker cannot react with the protonated amine. Storing the polymer in a carbonated water solution showed no evidence of higher molecular weight chain formation due to the crosslinking reaction in the higher molecular weight region. Moreover, the solution remained liquid and transparent over the time of storage.
The proposed approach for enhancing the solvent resistance of coatings made from CO2-switchable polymers should lead to the development of a new environmentally friendly and high performance coating and contribute significantly to reducing VOC emissions arising from the paints and coatings industry. Furthermore, the crosslinking chemistry that relies on CO2 switching could bring significant advantages to the field of CO2-switchable polymers beyond paints and coatings applications.
Future research to further improve paints and coatings formulation made from CO2-responsive polymers will involve increasing the solid content of the formulation by decreasing the molecular weight of the polymers while maintaining low viscosity. An increase in molecular weight can be achieved post-coating application through a crosslinking reaction during the drying phase. CO2-switchable polymers with a lower amine content will also be explored to mitigate the risk of yellowing in clear coatings.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Natural Sciences and Engineering Research Council of Canada (NSERC) (CREATE/543366-2020, Discovery RGPIN-2023-05700 (Jessop), Discovery RGPIN-2020-04361 (Cunningham) and the Canada Research Chairs Program (Jessop) are acknowledged for financial support. The authors thank the NSERC-funded Centre for Innovation and Research on Carbon Utilization in Industrial Technologies (CIRCUIT) for financial support.References
- ESIG Newsletter “Solutions” Spring 1997. Available online: https://www.esig.org/uploads/ModuleXtender/Publications/122/Solutions%201%20(EN).pdf CrossRef CAS PubMed (accessed on 21 July 2015). As cited in: J. H. Clark, T. J. Farmer, A. J. Hunt and J. Sherwood, Int. J. Mol. Sci., 2015, 16, 17101–17159 CrossRef CAS PubMed.
- J. Liu and G. Zheng, Waste Manage., 2020, 106, 193–202 CrossRef CAS PubMed.
- T.-D.-H. Vo, C. Lin, C.-E. Weng, C.-S. Yuan, C.-W. Lee, C.-H. Hung, X.-T. Bui, K.-C. Lo and J.-X. Lin, J. Environ. Manage., 2018, 217, 327–336 CrossRef CAS.
- Waterborne coatings market size, share & trends analysis report by resin (acrylic, PU, epoxy, polyester), by application (architectural, general industrial, marine), by region, and segment forecasts, 2023–2030, Available online: https://www.grandviewresearch.com/industry-analysis/waterborne-coatings-market, (accessed October 2, 2023).
- Y. Yang, J. He, Y. Zhang, Y. Hong and X. Wang, Appl. Surf. Sci., 2022, 579, 152239 CrossRef CAS.
- F. Lecomte, J. Siepmann, M. Walther, R. J. MacRae and R. Bodmeier, Pharm. Res., 2004, 21, 882–890 CrossRef CAS PubMed.
- P. A. Steward, J. Hearn and M. C. Wilkinson, Adv. Colloid Interface Sci., 2000, 86, 195–267 CrossRef CAS PubMed.
- J. L. Keddie, Mater. Sci. Eng., R, 1997, 21, 101–170 CrossRef.
- A. F. R. Joseph and L. Keddie, Fundamentals of Latex Film Formation: Processes and Properties, Springer, Gildford, 2010 Search PubMed.
- M. J. Gibbons, S. Nikafshar, T. Saravi, K. Ohno, S. Chandra and M. Nejad, Coatings, 2020, 10, 1013 CrossRef CAS.
- J. Ho, B. Mudraboyina, C. Spence-Elder, R. Resendes, M. F. Cunningham and P. G. Jessop, Green Chem., 2018, 20, 1899–1905 RSC.
- S. Lin and P. Theato, Macromol. Rapid Commun., 2013, 34, 1118–1133 CrossRef CAS PubMed.
- A. Darabi, P. G. Jessop and M. F. Cunningham, Chem. Soc. Rev., 2016, 45, 4391–4436 RSC.
- P. G. Jessop, D. J. Heldebrant, X. Li, C. A. Eckert and C. L. Liotta, Nature, 2005, 436, 1102–1102 CrossRef CAS PubMed.
- M. F. Cunningham and P. G. Jessop, Eur. Polym. J., 2016, 76, 208–215 CrossRef CAS.
- Y. J. Park, M. J. Monteiro, S. Van Es and A. L. German, Eur. Polym. J., 2001, 37, 965–973 CrossRef CAS.
- A. Pathania, R. K. Arya and S. Ahuja, Prog. Org. Coat., 2017, 105, 149–162 CrossRef CAS.
- K. Dušek and M. Dušková-Smrčková, Prog. Polym. Sci., 2000, 25, 1215–1260 CrossRef.
- G. Tillet, B. Boutevin and B. Ameduri, Prog. Polym. Sci., 2011, 36, 191–217 CrossRef CAS.
- T. M. Vogel and M. Reinhard, Environ. Sci. Technol., 1986, 20, 992–997 CrossRef CAS PubMed.
- W. C. Bray, J. Am. Chem. Soc., 1910, 32, 932–938 CrossRef CAS.
- K. Zhang, W. Feng and C. Jin, MethodsX, 2020, 7, 100779 CrossRef CAS PubMed.
- Standard Practice for Assessing the Solvent Resistance of Organic Coatings Using Solvent Rubs, ASTM D5402-19.
- H. T. Turan, S. Brickel and M. Meuwly, J. Phys. Chem. B, 2022, 126, 1951–1961 CrossRef CAS PubMed.
- A. Dasgupta, D. Das and P. K. Das, Biochimie, 2005, 87, 1111–1119 CrossRef CAS.
- A. K. Alshamrani, J. R. Vanderveen and P. G. Jessop, Phys. Chem. Chem. Phys., 2016, 18, 19276–19288 RSC.
- T.-T. Wang and T.-C. Huang, Chem. Eng. J, Biochem. Eng. J., 1993, 53, 107–113 CrossRef CAS.
- K. Banert, M. Heck, A. Ihle, J. Kronawitt, T. Pester and T. Shoker, J. Org. Chem., 2018, 83, 5138–5148 CrossRef PubMed.
- G. Ou, B. He and P. Halling, Biochim. Biophys. Acta, Gen. Subj., 2016, 1860, 1404–1408 CrossRef CAS PubMed.
- Y. Ju and R. S. Varma, Green Chem., 2004, 6, 219 RSC.
- X. Wang, N. G. Akhmedov, Y. Duan and B. Li, Energy Fuels, 2015, 29, 3780–3784 CrossRef CAS.
- M. F. Cunningham and P. G. Jessop, Eur. Polym. J., 2016, 76, 208–215 CrossRef CAS.
- S. G. Khokarale and J. P. Mikkola, RSC Adv., 2019, 9, 34023–34031 RSC.
- M. R. Reithofer, Y. N. Sum and Y. Zhang, Green Chem., 2013, 15, 2086 RSC.
- X. Zhou, X. Yang, T. Chen, Y. Zhang and G. Wang, Chin. J. Catal., 2009, 30, 7–8 CrossRef.
- S. Oi, K. Nemoto, S. Matsuno and Y. Inoue, Macromol. Rapid Commun., 1994, 15, 133–137 CrossRef CAS.
- C. Koukiotis and I. D. Sideridou, Prog. Org. Coat., 2010, 69, 504–509 CrossRef CAS.
- G. L. Shoaf and R. R. Stockl, Polym. React. Eng., 2003, 11, 319–334 CrossRef CAS.
- M. Huang, Y. Liu, G. Yang, J. Klier and J. D. Schiffman, ACS Appl. Polym. Mater., 2019, 1, 657–663 CrossRef CAS.
- H. Mohd Ghazaly, E. S. Daniels, V. L. Dimonie, A. Klein, L. H. Sperling and M. S. El-Aasser, J. Appl. Polym. Sci., 2003, 88, 42–49 CrossRef.
- Y. Chen, C. Zhang, Y. Wang, S. Cheng and P. Chen, J. Appl. Polym. Sci., 2003, 90, 3609–3616 CrossRef CAS.
- G. Wicha, K. Intharaprasit, E. Wimolmala, T. Markpin and K. Saenboonruang, IOP Conf. Ser.: Mater. Sci. Eng., 2020, 773, 012010 CAS.
- A. Emileh, E. Vasheghani-Farahani and M. Imani, Eur. Polym. J., 2007, 43, 1986–1995 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3lp00186e |
| This journal is © The Royal Society of Chemistry 2024 |