Immobilization of biogenic metal nanoparticles on sustainable materials – green approach applied to wastewater treatment: a systematic review
Verónica
Rocha
 *a,
Ana
Lago
a,
Bruna
Silva
ab,
Óscar
Barros
a,
Isabel C.
Neves
*a,
Ana
Lago
a,
Bruna
Silva
ab,
Óscar
Barros
a,
Isabel C.
Neves
 ac and
Teresa
Tavares
ac and
Teresa
Tavares
 ab
ab
aCEB – Centre of Biological Engineering, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: vrocha@ceb.uminho.pt
bLABBELS – Associate Laboratory, Braga/Guimarães, Portugal
cCQ-UM – Centre of Chemistry, Department of Chemistry, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal
First published on 7th December 2023
Abstract
The application of the principles of green chemistry to the synthesis of metal nanoparticles (MNP) is a new emerging issue concerning sustainability. Together with the green nanotechnology, this is an evolving approach with innovative, reliable and sustainable solutions for applications in all fields of life. Those principles may be used on greener bio-inspired materials applied for the synthesis of MNP as well as for their immobilization on solid supports. This review is carried out for its relevance, covering the available literature on the green fabrication of MNP from different bio-resources, ranging from micro- to macromolecular levels (bacteria, fungi, yeasts, algae and plants) acting as reducing and capping agents and enabling the immobilization of biogenic MNP on sustainable materials to improve their performance when applied to wastewater treatment. Materials derived from renewable and earth-abundant sources (clays, natural zeolites, sediments, sands, rocks and volcanic glasses) or residues from food, industry and agro-forestry activity, activated carbon and biochar were found as sustainable supports with diverse applications such as disinfection of water or removal/degradation of various pollutants of wastewater. For the first time, a thorough review of the literature available on this topic has been carried out and the synthesis and immobilization of biogenic MNP on sustainable materials are reported, through two main routes: (i) bio-inspired fabrication of MNP, with the consideration of the adverse effects of their suspension status and (ii) supported MNP obtained with sustainable supports, with focus on natural or on waste materials, activated carbon and biochar, as well as on the obtained modified sustainable materials. Finally, an economic and environmental perspective as well as the challenges on the use of supported MNP will be presented.
Environmental significanceCurrently, there is growing effort on investigating the environmental pollution abatement issue. With the increasing need to develop sustainable materials/methods for the elimination or effective reduction of pollutants in wastewater and drinking water, nanotechnology appears as an innovative tool with excellent demonstrated potential for the purpose through more effective strategies than previously explored ones. Various bio-resources have shown their effectiveness in the biosynthesis of metal nanoparticles (MNP). However, the utilization of powder MNP in reactors shows several limitations, including the challenge of filtering the final suspensions due to the small particle size and their agglomeration. The immobilization of MNP on solid supports could be used to address these issues. |
1. Introduction
The continuous growth of the global population combined with industrialization and urbanization has resulted in increased demand for chemicals, materials and energy.1 The release of unwanted wastes into the environment from numerous anthropogenic sources has been inevitably accompanied by a lower quality of environmental systems.2 There is an urgent need for the development of sustainable, efficient and cost-effective materials/methods for the elimination or effective reduction of pollutants and microorganisms in wastewater and drinking water.3In recent years, nanotechnology has appeared as a new innovative tool with excellent potential for wastewater treatment through more effective strategies than previously explored ones.4 Nanotechnology is an evolving field with the creation and utilization of materials with structural features of bulk materials with at least one dimension in the nanoscale.5 Over the past few years, researchers have been developing and characterizing unique nanostructural morphologies such as nanowires, nanotubes, nanospheres, nanoparticles (NP), etc. with various practical applications in engineering, materials science, chemistry and biology.6 Among the enormous variety of nanostructured materials that have emerged, metal nanoparticles (MNP) are considered as the basic building blocks of nanotechnology and act as bridges between bulk materials and atomic or molecular structures.7,8 MNP have gained prominence in technological advancements due to their tunable physicochemical characteristics such as melting point, wettability, electrical and thermal conductivity, catalytic activity, light absorption and scattering and surface area to volume ratio resulting in enhanced performance over their bulk counterparts.9 The nanostructured materials find their pioneering applications in diverged areas ranging from medicine, agriculture, electronic element fabrication, environmental waste remediation to several industrial domains.10
The fabrication of MNP can be broadly categorized into two approaches: top-down and bottom-up.11 The top-down approach involves crushing or cutting the bulk materials into fine particles at the nanoscale, using processes such as arc discharge, ball milling, pulsed laser ablation, etc.5,9 In the bottom-up approach, MNP are formed by assembling atom by atom, molecule by molecule or cluster by cluster.5,12 This last approach includes numerous methods of MNP synthesis: coprecipitation, chemical reduction of metal salts, electrochemical and green synthesis methods.13,14 In the chemical synthesis of MNP, the chemical reduction of metal ions from salt solution in the presence of strong bases (reducing agents), sodium borohydride (NaBH4) or sodium hydroxide (NaOH) was achieved, followed by the addition of a stabilizing agent, also called the capping agent.8 However, the reagents employed as reducing agents and the solvents used to dissolve the stabilizers are commonly toxic substances that have adverse and deleterious health and environmental effects.15,16 The physical MNP synthesis is mainly a top-down approach in which the material is reduced in size by various physical approaches.17 These conventional methods of MNP synthesis have various limitations such as the need for a huge amount of energy, specific and costly equipment, the production of flammable hydrogen gas and the use of toxic chemicals such as NaBH4, organic solvents, and stabilizing and dispersing agents.18
The development of an environmentally friendly, renewable, easy-to-implement and cost-effective means of MNP synthesis has become one of the foremost demands in the field of environmental remediation.1 The bio-inspired fabrication is cost-effective, easy to implement, reduces the chemical load into the environment and eliminates unnecessary processing during synthesis.19,20
Although the green synthesis mitigates the environmental impact in production of MNP, these biogenic procedures still suffer from certain limitations. In the past years, solid materials have been used as supports for MNP to prevent agglomeration and to overcome the obstacles associated with stability and recovery. Additionally, the accumulation of MNP in the environment, after their intended use, also generates persistent MNP waste.21 The development of an environmentally friendly method for the synthesis of MNP with good dispersion and stability and their immobilization on effective and sustainable supports is highly desirable and it has progressively focused on green materials applied as supports for MNP for sustainable development.22,23 The interest in sustainable materials applied to support the biosynthesized MNP in the field of wastewater treatment has become attractive.
The simultaneous use of bio-resources and sustainable materials as reducing/capping agents and supporting materials to obtain supported MNP is a significant green approach applied to wastewater treatment. Bearing this in mind, in the present study a systematic review was carried out to assess the literature currently available on the performance increase of biogenic MNP applied to wastewater treatment. In the following qualitative analysis of all research articles published in this field, the literature focusing on the use of sustainable materials as supports for biogenic MNP was thoroughly reviewed for the first time.
This systematic review focused on biogenic MNP immobilized on sustainable materials is divided into two different goals:
(1) Green fabrication of MNP from different bio-resources ranging from micro- to macromolecular levels (bacteria, fungi, yeasts, algae and plants – Fig. 1) acting as reducing and capping agents and a brief overview of the adverse effects of suspensions of MNP, emphasizing the need for solid-supported MNP.
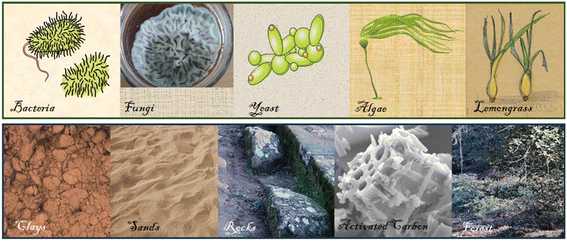 | ||
| Fig. 1 Bio-resources: bacteria, fungi, yeast, algae and plants and some examples of sustainable materials: clays, sands, rocks, activated carbon and residues from forest. | ||
(2) Immobilization of biogenic MNP on sustainable materials derived from earth-abundant and renewable materials (clays, natural zeolites, sediments, sands, rocks and volcanic glass), residues from food, industry and agro-forestry activities, activated carbon or biochar derived from wastes and their use as green nanocomposites with diverse applications for removal or degradation of various pollutants or disinfection of water (Fig. 1).
2. Methodology for literature search
Using the search strings listed in Table 1, a literature search was performed on the Scopus database, according to the Preferred Reporting Items of Systematic Reviews and MetaAnalyses (PRISMA) 2020 statement.| Search string | Document results |
|---|---|
| TITLE-ABS-KEY (biogenic OR green OR biosynthesis AND nanoparticles OR composite OR nanocomposite OR bionanocomposite AND supported OR loaded OR attached OR imprinted AND “natural material”) AND (LIMIT-TO (DOCTYPE, “ar”)) | 9 |
| TITLE-ABS-KEY (biogenic OR green OR biosynthesis AND nanoparticles OR composite OR nanocomposite OR bionanocomposite AND supported OR loaded OR attached OR imprinted AND clay) AND (LIMIT-TO (DOCTYPE, “ar”)) | 79 |
| TITLE-ABS-KEY (biogenic OR green OR biosynthesis AND nanoparticles OR composite OR nanocomposite OR bionanocomposite AND supported OR loaded OR attached OR imprinted AND zeolite) AND (LIMIT-TO (DOCTYPE, “ar”)) | 61 |
| TITLE-ABS-KEY (biogenic OR green OR biosynthesis AND nanoparticles OR composite OR nanocomposite OR bionanocomposite AND supported OR loaded OR attached OR imprinted AND sand) AND (LIMIT-TO (DOCTYPE, “ar”)) | 11 |
| TITLE-ABS-KEY (biogenic OR green OR biosynthesis AND nanoparticles OR composite OR nanocomposite OR bionanocomposite AND supported OR loaded OR attached OR imprinted AND waste) AND (LIMIT-TO (DOCTYPE, “ar”)) | 264 |
| TITLE-ABS-KEY (biogenic OR green OR biosynthesis AND nanoparticles OR composite OR nanocomposite OR bionanocomposite AND supported OR loaded OR attached OR imprinted AND residues) AND (LIMIT-TO (DOCTYPE, “ar”)) | 70 |
| TITLE-ABS-KEY (biogenic OR green OR biosynthesis AND nanoparticles OR composite OR nanocomposite OR bionanocomposite AND supported OR loaded OR attached OR imprinted AND “activated carbon”) AND (LIMIT-TO (DOCTYPE, “ar”)) | 141 |
| TITLE-ABS-KEY (biogenic OR green OR biosynthesis AND nanoparticles OR composite OR nanocomposite OR bionanocomposite AND supported OR loaded OR attached OR imprinted AND biochar) AND (LIMIT-TO (DOCTYPE, “ar”)) | 40 |
Briefly, the literature search combined the terms “biogenic” or “green” or “biosynthesis” and “nanoparticles” or “composite” or “nanocomposite” or “bionanocomposite” and “supported” or “loaded” or “attached” or “imprinted” with the terms for different sustainable materials (e.g., clay, zeolite, sand, waste, activated carbon, biochar, etc.). This literature search was performed from September 2021 to January 2023. Titles and abstracts of the retrieved articles were screened for relevance considering the following eligibility criteria: use of sustainable materials as supports for biogenic MNP with application in wastewater treatment and original studies. Exclusion criteria: full text in a language other than English, lack of access to the full article, articles without application in wastewater treatment or articles with MNP supported on non-sustainable materials. The classification of sustainable materials was performed according to our detailed analysis of the reported results.
2.1 Results
A total of 675 results were obtained by conducting a literature search on the Scopus database, based on the strings described in Table 1 (Fig. 2A).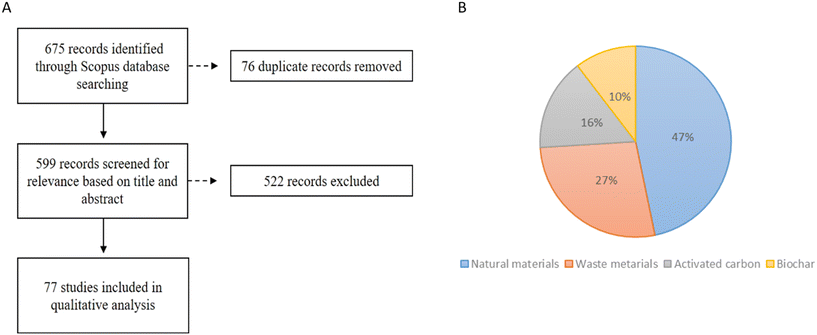 | ||
| Fig. 2 (A) Flowchart considered in the process of the study selection and (B) data collected from the 77 studies selected from qualitative analysis based on the criteria summarized in A. | ||
The titles and abstracts of those articles were screened for relevance and duplicates were removed, leading to the selection of 77 articles for full-text reading. The type of sustainable material used as a support for biogenic MNP was analyzed first and the results showed that 48% of sustainable materials are from renewable sources (clays, natural zeolites, sediments, sands, rocks and volcanic glass), 27% are residues, 16% are activated carbon and biochar only represents 10% (Fig. 2B). Until 2017, few works were found on the application of these sustainable materials. Since then, the application of these materials has aroused the interest of researchers and an increase in the number of publications in this field is expected.
3. Bio-inspired fabrication of metal nanoparticles
In recent years, interest has grown for the implementation of environment friendly and economic remediation methods that guarantee low secondary impacts. Several green methods have been proposed and implemented for MNP synthesis. This field has received great attention due to its capability to design alternative, harmless, energy-efficient and less toxic routes towards the synthesis of these MNP. These routes have been associated with the rational utilization of various substances and limited use of toxic precursors consequently reducing the quantity of impurities and by-products.24The bio-inspired fabrication of MNP involves the use of prokaryotic/eukaryotic cells or extracted biomolecules that act as reducing, stabilizing and capping agents.13 The green synthetic processes comprise the use of either microorganisms such as bacteria,10,25 fungi26,27 and yeasts,28,29 as well as algae30,31 and plant biomass or plant extract.32,33
Plants have been reported to contain a huge variety of biomolecules that work in harmony to obstruct cellular components from oxidative damage, thereby resulting in metal ion reduction to MNP.13 The first work on plants employed in the synthesis of MNP used Medicago sativa (alfalfa) which was able to synthesize Au-NP and Ag-NP.34 Since then, more attention has been paid to plants. Most of the studies confer the production of MNP by plants that are known to be more stable than MNP synthesized by microorganisms.35 Biosynthesis can be achieved by plant tissues, extract of leaves, stems, roots, fruits, bark peels and flowers, being from a sustainable perspective, i.e. the utilization of a renewable feedstock as a precursor material. The usage of plant extracts for the synthesis of MNP has drawn special attention due to their rapid, low-cost, biogenic and single-step method involved, making it an interesting and reliable alternative to conventional methodologies.6 Currently, the number of publications on the synthesis of MNP produced by plant extracts is increasing exponentially. Extracts derived from diverse plant species such as Psidium guajava (guava),36Cymbopogon citratus (lemongrass),37Trigonella foenumgraceum (fenugreek),38Aloe vera (cactus),39Hibiscus rosa sinensis (hibiscus),40Citrus sinensis (orange),41Saussurea costus (costus)42 or Eucalyptus globulus (eucalyptus)43 are some examples of successful usage in the green synthesis of MNP.
3.1 Adverse effects of suspension nanoparticles
Suspensions of MNP have been widely applied for the removal and/or degradation of various poisonous aqueous pollutants or the disinfection of water. However, the accumulation of MNP in the environment, after their intended use, also generates persistent pollution and can provoke harmful effects on living organisms.21MNP disposal by incineration or by land fill processes leads to the accumulation of the NP in land or in subaquatic sediments or to dispersion in the atmosphere.44 Shi et al.45 studied the outcome of titanium dioxide (TiO2) NP in wastewater treatment plants (WWTP) and although a part of the NP (>74%) was removed by the activated sludge, a significant concentration of them (27 to 43 μg L−1) was found in the effluents, which is higher than natural background levels (less than 5 μg L−1). After being released into the environment, the MNP can suffer different transformations such as agglomeration, sedimentation, oxidation, reduction, sulphidation, photochemical and biological mediated reactions.46–48
The toxicity effects of citrate-coated Ag-NP in adult zebrafish in vivo are reported by Osborne et al.49 The authors show that the particle size and dissolution of Ag+ ions from Ag-NP determined the toxic impact in the grills and intestines of zebrafish. Martin et al.50 studied the effect of Ag-NP on a boreal lake, where the Ag accumulation was monitored in the tissues of yellow perch (Perca flavescens) and of northern pike (Esox lucius). It was found that both species accumulated Ag in their tissues for 2 years following exposure, with higher concentration detected in the liver. The toxic effects of Ag-NP of different sizes and surface functionalities were further investigated by Hou et al.51 on aquatic crustacean Daphnia magna. Shi et al.45 also reported the presence of Ti in various fish tissues.
Studies to evaluate the phytotoxic impacts of suspended MNP have been also reported by Pradas Del Real et al.52 who studied the effect on wheat plants exposed to Ag in its different forms (Ag-NP, Ag2S-NP and Ag+ ions). Various chemical transformations were observed on the epidermis and inside of the roots after the exposure.
The wide application of MNP is limited by many obstacles such as the difficulty of manipulating the suspension, the ultimate health risks of the dissipation of MNP into the environment and the instability of size due to aggregation.53 Synthesis of MNP on a solid support has emerged as a prospective solution that may prevent the accumulation of MNP waste in the ecosystem through a recycling process.21
4. Supported metal nanoparticles as a green approach
The bio-inspired fabrication of MNP on solid supports has emerged as a novel strategy for the sustainable development of nanotechnology. The easy recovery and reuse of the supported MNP avoid their accumulation into the environment and provide stability to the MNP. It further prevents aggregation and leaching of metal ions and thus reduces the expected toxicity of MNP.21 Moreover, MNP powder is widely used in laboratory experiments for wastewater treatment, but for large-scale water treatment, immobilized MNP are much more desired.54,55A credible support-based material candidate should have high charge carrier mobility and thermal stability, high specific surface area, as well as a biocompatible nature.56–58 Moreover, they should not be strong oxidant materials to restrict the production of harmful disinfectant by-products. The nature of the support will influence the performance kinetics and the leaching rate of the MNP.59,60 Generally, supporting the MNP either by the usual chemical process or by green methods into suitable carriers enhances the surface area, adsorption capacity and mechanical stability.61,62
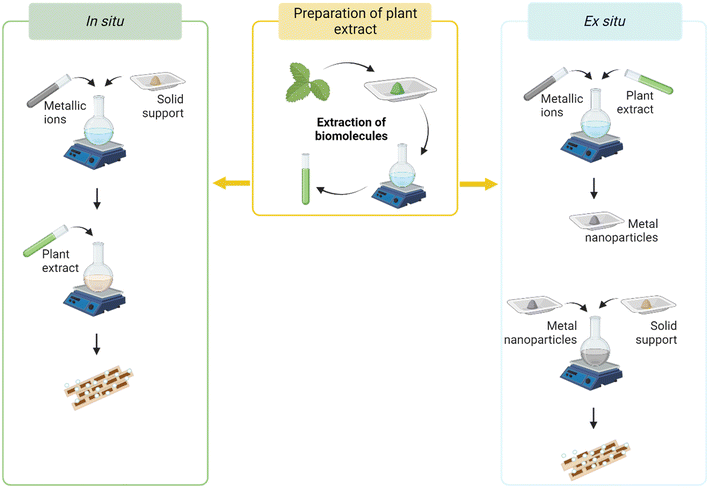
The attachment of MNP to the supporting medium can be performed by: (1) in situ fabrication that involves the formation of a bond between metallic ions and the negatively charged functional groups of the supporting material, with the gradual reduction of the first that start nucleating on the solid support (Fig. 3) or (2) ex situ fabrication which includes the mixing of freshly pre-synthesized MNP with the supporting material by an electrostatic interaction between them (Fig. 3).21,63,64
4.1 Synthetic supports
Different types of solid supports can be used for the immobilization of biogenic MNP and the most common are polymer-based membranes,65,66 glass,67 silica gel,68 commercial activated carbon,69 synthetic zeolites,70–72 resins,73 graphene oxides (GO)74 and reduced graphene oxide (rGO).75,76Amongst the different available synthetic supports, polymer-based membranes have received special attention due to their open structure and high internal surface area which ensure a high MNP loading and easy active site accessibility.77 Recently, Kalaivizhi et al.65 described the removal process of Congo red and methylene blue by using biogenic ZnONP immobilized on polysulfone/polyurethane membranes. Arif et al.66 studied the application of TiO2-NP synthesized through a green route using a polyvinylidene fluoride polymer embedded in the extract of Cajanus cajan to develop a photocatalytic membrane. This membrane supporting biogenic TiO2 acted as a synergistic separator to reject chromium (Cr6+) and further reduce the ion toxicity by photocatalytic reduction of the concentrated Cr6+, achieving >90% rejection and >85% reduction of Cr6+.
Zeolites acting as supports have been receiving considerable attention due to their high specific surface area, high catalytic activity, high thermal stability and exceptional structural properties with uniform distribution of pores and channels.72 Nizam et al.70 reported the use of biosynthesized magnetic iron(III) oxide (Fe2O3)-NP immobilized in situ on 13X zeolite (Fe2O3/MS) in the photocatalytic degradation of methylene blue. The results demonstrated excellent performance of Fe2O3/MS on the photodegradation of the dye, with 99% efficiency achieved in 150 min. The authors observed an enhanced photocatalytic performance of Fe2O3/MS when compared to the pure synthesized Fe2O3-NP. Rostami-Vartooni et al.71 investigated the use of magnetically recoverable Fe3O4/HZSM-5 as a support for Ag-NP, synthesized in situ in the presence of Juglans regia L. leaf extract. The authors found that the Ag/Fe3O4/HZSM-5 nanocomposite showed high activity for the reduction of methyl orange and 4-nitrophenol, being reused three times without loss of activity. Also, Tajbakhsh et al.72 studied the efficiency of the Ag/HZSM-5 nanocomposite for the reduction of different organic dyes such as methylene blue, Congo red, rhodamine B and 4-nitrophenol. The green synthesized Ag/HZSM-5 nanocomposite prepared in situ using Euphorbia heterophylla leaf extract was found to be an efficient nanocatalyst with high regeneration potential.
Another class of materials, GO and rGO, has been extensively used as promising supports for the immobilization of green MNP. These materials have many advantages such as a large specific surface area, high adsorption capacity, good electrical conductivity and mechanical stability.75,78 Naghdi et al.74 reported the use of Cuscuta reflexa leaf extract for the biosynthesis of the Cu/GO/MnO2 nanocomposite as a heterogeneous catalyst for the reduction of 4-nitrophenol, 2,4-dinitrophenylhydrazine, Congo red, methyl orange, methylene blue and rhodamine B. These authors reported a high catalytic activity of the Cu/GO/MnO2 nanocomposite (100% degradation attained instantly) as a recyclable heterogeneous catalyst for the reduction of all organic dyes and nitro compounds tested. Xue et al.75 evaluated the use of rGO-supported bimetallic Fe/Ni-NP (Fe/Ni–rGO) for the simultaneous removal of rifampicin and Pb2+ from aqueous solution. It is stated that Fe/Ni–rGO nanocomposites prepared by green synthesis using green tea extract were effective for the removal of mixed Pb2+ and rifampicin, with removal efficiencies of 81.9% and 94.3%, respectively. Ranjith et al.76 synthesized a hybrid rGO–TiO2/Co3O4 nanocomposite and studied the photocatalytic degradation of methylene blue and crystal violet. The results revealed that the presence of rGO significantly induced higher photocatalytic ability and the degradation of both dyes was more extensive under visible light irradiation.
For the sake of comparison and although the selected support is not a common material, the work of Harjati et al.79 should be referred as they were able to synthesize green Fe2O3 supported on TUD-1 (Technische Universiteit Delft), using Parkia speciosa hassk pod extract as a bioreductor agent. The support is, in fact, a three-dimensional mesoporous silicate with a high specific surface area and with Brønsted acidic behavior. These biosynthesized Fe2O3-NP were immobilized on TUD-1 via the sol–gel method and their photocatalytic activity on bromophenol blue and methyl violet degradation was assessed.
Most of these nanocomposites were developed with expensive and sometimes not-readily available materials, which makes it difficult to scale-up their production to technologically relevant amounts. Alternative and sustainable supports have been recently employed on the preparation of supported MNP.
4.2 Sustainable supports
From the perspective of circular economy and green development, the application of natural materials, organic wastes, activated carbon or biochar as solid supports for biogenic MNP, in the treatment of wastewater is the most attractive path for environmental researchers to incorporate the principles of Green Chemistry.Recently, several works in immobilized green MNP for environmental pollution remediation have diverted their focus on the direct or indirect immobilization of MNP on a number of sustainable materials. There is a variety of sustainable materials, meaning materials in high abundance and are renewable, agro-forest, food and industrial residues, which can either be utilized in pristine or in modified structures for supporting MNP. In this section, an exhaustive overview of the application of sustainable materials supporting biogenic MNP is presented, as well as their application in wastewater treatment for the degradation of various pollutants and for the inactivation of some microorganisms.
| Support | Biogenic MNP | Bioreductor | Immobilization method | Shape and size | Wastewater application and recyclability | Ref. |
|---|---|---|---|---|---|---|
| Nano zerovalent iron (nZVI); room temperature (RT); not reported (n.r.). | ||||||
| Kaolin | nZVI | Ruellia tuberosa leaf extract | In situ with stirring | Spherical | Adsorption of reactive black 5 | 84 |
| 20–40 nm | 5 cycles | |||||
| TiO2/ZnWO4 | Carica papaya seeds and Musa paradisiaca peels biomass | In situ with mechanical grinding for 15 min and calcined | Spherical | Photocatalytic degradation of ampicillin and sulfamethoxazole; artemether | 3 | |
| 62–257 nm | 5 cycles | |||||
| Ag/AgCl | Teucrium polium extract | In situ with stirring for 24 h | Spherical | Catalytic reduction of 4-nitrophenol | 83 | |
| 19–22 nm | n.r. | |||||
| Montmorillonite | Ag | Sida acuta leaf extract | In situ with stirring at room temperature for 24 h | n.r. | Adsorption of methylene blue | 89 |
| 15–20 nm | n.r. | |||||
| nZVI | Green tea extract | In situ with stirring | Spherical | Adsorption of Cr6+ | 86 | |
| 15–30 nm | n.r. | |||||
| Ag/ZnO | Urtica dioica leaf extract | In situ with stirring at 80 °C for 4 h | Spherical | Photocatalytic degradation of methylene blue | 87 | |
| 2–4 nm | 4 cycles | |||||
| Bentonite | Ag | Euphorbia larica extract | In situ with constant stirring at 80 °C for 4 h | Spherical | Catalytic reduction of 4-nitrophenol, Congo red, methylene blue and rhodamine B | 92 |
| 32 nm | 5 cycles | |||||
| Fe | Different extracts | In situ with stirring for 30 min or overnight | n.r. | Adsorption of chlorfenapyr | 93 | |
| Ag | n.r. | |||||
| Cu | Thymus vulgaris leaf extract | In situ with constant stirring at 80 °C for 4 h | Spherical | Catalytic reduction of methylene blue and Congo red | 94 | |
| 23–94 nm | 5 cycles | |||||
| Spherical | Catalytic reduction of 4-nitrophenol | 95 | ||||
| 56 nm | 5 cycles | |||||
| SnO2 | Ziziphus jujuba fruit extract | In situ with vigorous stirring at RT for 30 min and calcined | Spherical | Photocatalytic degradation of methylene blue and eriochrome black T | 90 | |
| 18 nm | 3 cycles | |||||
| Pd | Gardenia taitensis leaf extract | In situ with constant stirring at 80 °C for 4 h | Spherical | Catalytic reduction of Cr(VI), 4-nitrophenol and 2,4-dinitrophenylhydrazine | 96 | |
| 76–97 nm | 5 cycles | |||||
| ZnO | Jujube fruit extract | In situ with constant stirring at 80 °C for 5 h and calcinated | Spherical | Photocatalytic degradation of methylene blue and eriochrome black-T | 97 | |
| 10–40 nm | 3 cycles | |||||
| nZVI | Green tea extract | In situ with constant stirring at RT | Spherical | Adsorption of phosphate | 98 | |
| 40–60 nm | n.r. | |||||
| In situ with constant stirring at RT | n.r. | Adsorption of Cr6+ | 99 | |||
| 40–80 nm | n.r. | |||||
| In situ with constant stirring at RT for 15 min | Irregular spherical | Fenton-like oxidation of reactive blue 238 | 100 | |||
| <50 nm | n.r. | |||||
| In situ with stirring for 1 h | n.r. | Adsorption of malachite green | 101 | |||
| 50–60 nm | 2 cycles | |||||
| Eucalyptus leaf extract | In situ with stirring for 3 h | Irregular | Catalytic reduction of 4-nitrophenol | 91 | ||
| 10–60 nm | 5 cycles | |||||
| Black tea extract | In situ with constant stirring at RT for 15 min | Irregular spherical | Fenton-like oxidation of reactive blue 238 | 102 | ||
| <50 nm | n.r. | |||||
| nZVI-Cu | Pomegranate rind extract | In situ with continuous purging of nitrogen gas and continuous stirring | Spherical | Adsorption of tetracycline | 81 | |
| 60 nm | 5 cycles | |||||
| Ag/Fe3O4 | Salix aegyptiaca leaf extract | In situ with stirring at RT for 1 h | Spherical | Catalytic reduction of rhodamine B, methylene blue and methyl orange | 103 | |
| Pd/Fe3O4 | 15–65 nm | 4 cycles | ||||
| Fe/Pd | Pomegranate peel extract | In situ with nitrogen gas atmosphere | Mostly spherical | Adsorption of tetracycline | 104 | |
| 20–60 nm | 3 cycles | |||||
| Fe/Ni | Spherical | Adsorption of tetracycline and Cu2+ | 105 | |||
| 70 nm | 4 cycles | |||||
| Sand | Fe/Ni | Punica granatum peel extract | In situ with orbital shaker for 1 h | Spherical | Adsorption of tetracycline | 113 |
| 161 nm | 4 cycles | |||||
| Silty | nZVI | Green tea leaf extract | In situ with stirring for 1 h | Cubical | Adsorption of phenol | 114 |
| 100 nm | n.r. | |||||
| Diatomite | Ag | Alocasia macrorrhiza leaf extract | In situ with stirring at 80 °C for 4 h | Spherical | Catalytic reduction of 2,4-dinitrophenylhydrazine, nigrosin, 4-nitrophenol, methyl orange and Congo red | 115 |
| 32 nm | 5 cycles | |||||
| Pt | Cinnamomum camphora leaf extract | In situ with stirring at 90 °C for 1 h | n.r. | Catalytic oxidation of benzene | 116 | |
| 3.5 nm | n.r. | |||||
| Perlite | Ag | Hamamelis virginiana leaf extract | In situ with stirring at 60 °C for 1 h | Spherical | Catalytic reduction of 4-nitrophenol and Congo red | 117 |
| 8–25 nm | 4 cycles | |||||
| Cu | Euphorbia esula L. leaf extract | Ex situ with stirring at 100 °C for 15 h | Spherical | Catalytic reduction of 4-nitrophenol | 118 | |
| 32 nm | 3 cycles | |||||
| Pd | Euphorbia neriifolia L. leaf extract | In situ with continuous stirring and heated under reflux conditions for 3 h | Spherical | Catalytic reduction of 2,4-dinitrophenylhydrazine, 4-nitrophenol, rhodamine B, methyl orange and Congo red | 119 | |
| <18 nm | 5 cycles | |||||
| Clinoptilolite | Ag | Vaccinium macrocarpon fruit extract | In situ with constant stirring at 60–70 °C for 30 min | Spherical | Catalytic reduction of rhodamine B, methylene blue, methyl orange and Congo red | 107 |
| 15–30 nm | 6 cycles | |||||
| CuO | Rheum palmatum L. root extract | In situ with constant stirring at 70 °C for 10 min | Spherical | Catalytic reduction of 4-nitrophenol, rhodamine B, methylene blue and methyl orange | 109 | |
| 30 nm | 5 cycles | |||||
| Natrolite | Ag | Euphorbia prolifera leaf extract | In situ with stirring at 60 °C for 2 h | Semispherical | Catalytic reduction of 4-nitrophenol, rhodamine B, methylene blue, methyl orange and Congo red | 108 |
| 15 nm | 7 cycles | |||||
| Cu | Anthemis xylopoda flower extract | In situ with stirring at 100 °C for 15 h | Spherical | Catalytic reduction of 4-nitrophenol, Congo red and methylene blue | 110 | |
| 20 nm | 5 cycles | |||||
| Pd | Piper longum fruit extract | In situ with stirring at 100 °C for 15 h | Spherical | Catalytic reduction of 4-nitrophenol, rhodamine B, methylene blue, methyl orange and Congo red | 111 | |
| 12.5 nm | 7 cycles | |||||
| Natural zeolite | nZVI | Eucalyptus leaf extract | Ex situ with stirring | Spherical | Adsorption of ammonia and phosphate | 112 |
| 60 nm | n.r. | |||||
Clays are layered phyllosilicate minerals that occur naturally in the earth's crust and are important constituents of soils. These inorganic materials have gained significant interest as solid supports for MNP due to their natural abundance, non-toxic nature, porosity, low cost, layered morphology, chemical inertness and mechanical stability.80,81 Kaolin, which is one of the most common clay minerals, is low-cost, easily available, biogenic and has high thermal and mechanical stability.82 These properties make it a significant candidate for an MNP solid support. Alfred et al.3 developed an alternative method for biofabrication of kaolinite–TiO2 nanocomposites doped with ZnWO4 with effective activity on the photodegradation of artemether, ampicillin and sulfamethoxazole in water. These nanocomposites were prepared by biomass assisted synthesis routes, using Carica papaya seeds or Musa paradisiaca peels. The authors performed a pre-screening to evaluate the influence of TiO2 and the impact of the different biomasses on the photocatalytic efficiency of the nanocomposites. The results showed that the presence of both biomass and TiO2 is crucial for the photocatalytic efficiency of the nanocomposites. The nanocomposite prepared from Musa paradisiaca peels at 500 °C showed the highest efficiency for the degradation of the substrates in water, due to its relatively small particle size (62 nm), high porosity and consequent large surface area, with a high number of active sites needed for photocatalysis.3 Recently, Rakhshan et al.83 produced a three-component Ag/AgCl/kaolinite bionanocomposite and a four-component Ag/AgCl/Fe3O4/kaolinite using the aqueous extract of Teucrium polium. Both showed relevant catalytic activity for the reduction of 4-nitrophenol to 4-aminophenol, with a surplus for the four-component nanocomposite that can be easily separated with the aid of an external magnet. Kaolin was used as a support for nano zerovalent iron (nZVI) synthesized via a green method using the leaf extract of Ruellia tuberosa as a reducing agent.84 The synthesized green nanocomposite was used for the degradation of reactive black 5 with high decolorization efficiency in acidic medium.
Montmorillonite is a very promising support for the preparation of nanocomposites, due to its particular characteristics such as high ion exchange capacity, swelling, intercalation and layered nanostructure that make it a suitable solid support for the green synthesis of MNP.53,85–89 Yang et al.86 successfully prepared a nanocomposite of nZVI/montmorillonite using a low-cost and environment friendly green synthesis via tea leaf extract. Although few studies propose the nanocomposite formation mechanism, the authors suggest that during the synthesis with green tea extract, the unique structure of montmorillonite with isolated exchangeable cations of Fe(III) results in nZVI particle formation, covered by organic matter (green tea extract) in the clay interlayer associated with negative charges (Fig. 4A).86 The bio-synthesized composite showed a great removal capacity for Cr6+ and sufficient mobility under different soil conditions. Also, Sohrabnezhad and Seifi used montmorillonite to support green bimetallic Ag/ZnO-NP.87 The attachment of MNP on the surface of montmorillonite and the presence of aluminum atoms in this clay structure not only prevent the loss of the photocatalyst during recovery but also assist in electron–hole separation during the photocatalytic process.87
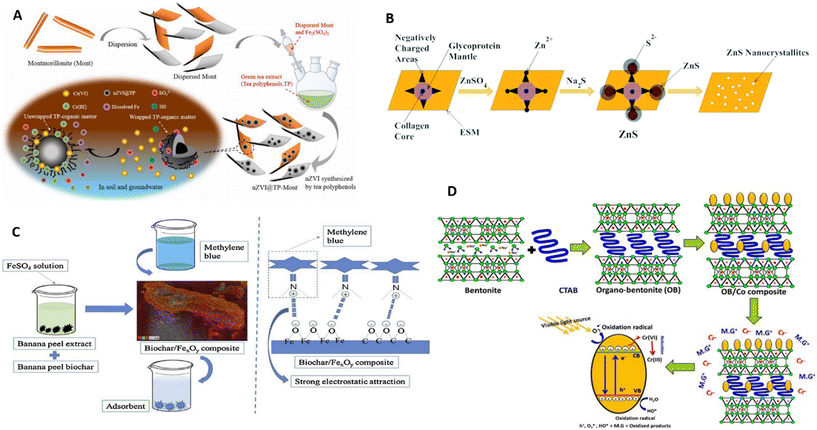 | ||
| Fig. 4 Mechanism of composite synthesis: (A) nZVI/montmorillonite, reproduced from ref. 86 with permission from Elsevier, copyright 2021. (B) ZnS/eggshell membrane (ESM), reproduced from ref. 120 with permission from Royal Society of Chemistry, copyright 2014, (C) FexOy/biochar, reproduced from ref. 121 with permission from Elsevier, copyright 2020, and (D) Co/modified bentonite, reproduced from ref. 122 with permission from American Chemical Society, copyright 2020. | ||
Bentonite is a member of the smectite family, which is found in many countries and originates from the activities of volcanoes.90 This clay has an ordered structure, uniform pore volume, high exchange capacity, high specific surface area, thermal, chemical and mechanical stability and it is low-cost, which makes it a promising support.91 Bentonite is widely used as a support for green MNP such as Ag-NP,92,93 Cu-NP,94,95 SnO2-NP,90 Pd-NP,96 ZnO-NP,97 nZVI-NP,91,98–102 bimetallic nZVI/Cu-NP,81 Ag/Fe3O4 and Pd/Fe3O4-NP,103 Fe/Pd-NP104 and Fe/Ni-NP.105 The efficiency of tetracycline adsorption by green nZVI/Cu-NP in suspension and by bentonite supported green nZVI/Cu using pomegranate rind extract was studied by Gopal et al.81 The aggregation of bimetal NP was reduced and their dispersion onto bentonite increased. The increased surface area could be assigned to the certainty of bentonite as a supporting material that effectively reduces the aggregation of nZVI/Cu-NP and leads to equal distribution of bimetal NP on its surface.3 Issaabadi et al.94 described the preparation of bentonite/Cu-NP using the aqueous extract of the leaves of Thymus vulgaris as a reducing agent and efficient stabilizer and studied the catalytic efficiency of the nanocomposite in the degradation of methylene blue and Congo red. They observed that the Thymus vulgaris leaf extract allows a fast and convenient preparation of the bentonite/Cu-NP and can reduce Cu2+ ions into Cu-NP without severe conditions. All the syntheses using green tea extract to produce bentonite supporting nZVI (ref. 98–101) were performed at RT. The biofabrication performed by Hassan et al.100 was carried out in a short reaction period (15 min), but NaOH solution was added to adjust the pH, which makes the process less sustainable.
Zeolites are a particular group of aluminosilicate and microporous materials that have very good adsorption properties. The microporous structure with varying pore diameter can retain the substances to be separated depending on their molecular size and thus acts as molecular sieves. This attribute of zeolites qualifies them as reliable, cost-effective and eco-friendly supports for binding the MNP.106 Natural zeolites have been used as sustainable supports for biogenic MNP such as Ag-NP,107,108 Cu-NP,109,110 Pd-NP111 and nZVI-NP.112 In a recent study, the green nZVI-NP dispersed onto zeolite produced with eucalyptus leaf extracts was applied to concurrently eliminate ammonia and phosphate from aqueous solutions.112 This nanocomposite eliminated 43.3% of NH4+ and 99.8% of PO43−, at a primary concentration of 10 mg L−1 for each of the 2 co-existing ions. After optimization, the conditions for maximum adsorption capacity of the produced material for NH4+ and PO43− were 3.47 and 38.91 mg g−1, respectively.
Other natural materials such as sand,113 sediments,114 rocks115,116 and volcanic glass117–119 have also been reported as solid supports for green MNP. Perlites are small pebbles of natural glass which contain a small amount of occluded water and are found in volcanic deposits.117 The reduction of nitroarenes and organic dyes in water was evaluated using green Pd-NP dispersed on perlite.119 The green Pd/perlite nanocomposite can be recovered and recycled several times without significant loss of activity. Likewise, Nasrollahzadeh et al.118 used perlite to immobilize Cu-NP synthesized using an aqueous extract of the leaves of E. esula L. Ex situ fabrication involves the mixing of freshly pre-synthesized Cu-NP along with perlite for 15 h. The particles exhibited spherical morphology with a low tendency to agglomeration. The Cu-NP/perlite shows favorable activity and separability on the catalytic reduction of 4-nitrophenol and can be reused several times without a decrease in the catalytic activity.
The in situ fabrication is widely used to synthesize composites since it warrants more uniformity of the NP on the support. Only two studies were performed ex situ using natural materials as solid supports and both reported a low tendency of NP to agglomerate.112,118
| Support | Biogenic MNP | Bioreductor | Immobilization method | Shape and size | Wastewater application and recyclability | Ref. |
|---|---|---|---|---|---|---|
| Room temperature (RT); not reported (n.r.); not applicable (n.a.). | ||||||
| Eggshell | Cu | Orchis mascula L. leaf extract | In situ with vigorous shaking at 70 °C for 3 h | Spherical | Catalytic reduction of 4-nitrophenol, rhodamine B, methylene blue, methyl orange and Congo red | 124 |
| Fe3O4 | ||||||
| Cu/Fe3O4 | 5–15 nm | 7 cycles | ||||
| Pd | Barberry fruit extract | In situ with vigorous stirring and refluxed for 3 h | Spherical | Catalytic reduction of 4-nitrophenol, methylene blue, methyl orange and Congo red | 126 | |
| <20 nm | 6 cycles | |||||
| SnO2/ZnO | Teucrium polium leaf extract | In situ with vigorous stirring at 70 °C for 8 h | Spherical | Adsorption of Hg2+ | 125 | |
| 20–25 nm | 3 cycles | |||||
| CuO | Pomegranate peel extract | Ex situ with reflux condition at 80 °C for 24 h | Spherical | Adsorption of aromatic compound from crude oil | 127 | |
| 10 cycles | ||||||
| 30 nm | Catalytic reduction of 4-nitrophenol | |||||
| 6 cycles | ||||||
| Ag | Cacumen platycladi extract | In situ with impregnation for 6 h and reacted for 48 h | Spherical | Catalytic reduction of 4-nitrophenol | 128 | |
| 5 cycles | ||||||
| 60 nm | Antibacterial activity against E. coli and S. aureus | |||||
| n.a. | ||||||
| ZnO | Ferulago macrocarpa extract | In situ with stirring for 24 h and calcined | Spherical | Photocatalytic degradation of diazinon | 129 | |
| n.r. | ||||||
| 25 nm | Antibacterial activity against both human and fish pathogenic bacteria | |||||
| n.a. | ||||||
| Membrane of eggshell | MnO2 | Membrane of eggshell | In situ with stirring at RT for 35 min | Spherical | Adsorption of tetracycline | 130 |
| 4.8 nm | n.r. | |||||
| ZnS | In situ with liquid impregnation method | Spherical | Photocatalytic degradation of methyl orange | 120 | ||
| 40 nm | 4 cycles | |||||
| Au | Lagerstroemia speciose leaf extract | In situ with impregnation for 3 h | Spherical | Catalytic reduction of 4-nitrophenol | 131 | |
| 20 nm | 10 cycles | |||||
| Almond shell | Pd | Almond hull extract | In situ with stirring at 100 °C | Spherical | Catalytic reduction of methylene blue, methyl orange and rhodamine 6G | 132 |
| <20 nm | 10 cycles | |||||
| Ag | Ruta graveolens sleeve extract | In situ with stirring at 70 °C for 2 h | Spherical | Catalytic reduction of 4-nitrophenol, rhodamine B and methylene blue | 133 | |
| 10–15 nm | 5 cycles | |||||
| Walnut shell | Pd | Equisetum arvense L. leaf extract | In situ with vigorous shaking at 70 °C for 3 h | Spherical | Catalytic reduction of 4-nitrophenol, rhodamine B, methylene blue, methyl orange and Congo red | 22 |
| 5–12 nm | 7 cycles | |||||
| Peach kernel shell | Ag | Achillea millefolium L. extract | In situ with reflux conditions at 70 °C for 1.5 h | Spherical | Catalytic reduction 4-nitrophenol, methyl orange and methylene blue | 4 |
| <20 nm | 5 cycles | |||||
| Seashell | Ag | Bunium persicum seed extract | In situ with stirring at 70 °C for 1 h | Spherical | Catalytic reduction of 4-nitrophenol, methylene blue, methyl orange and Congo red | 140 |
| 11 nm | 5 cycles | |||||
| CuO | Rumex crispus seed extract | In situ with stirring at 60 °C for 20 min and calcination | Spherical | Catalytic reduction of 4-nitrophenol and Congo red | 141 | |
| 8–60 nm | 5 cycles | |||||
| Pistachio shell | Ag | Cichorium intybus L. leaf extract | In situ with stirring at 75 °C for 2 h | Spherical | Catalytic reduction of 4-nitrophenol, methylene blue, methyl orange and Congo red | 134 |
| 20–50 nm | 5 cycles | |||||
| Cu | Pistacia vera L. hull extract | In situ with sonicator bath at 60 °C | Spherical | Catalytic reduction of 4-nitrophenol, rhodamine B, methylene blue, and methyl orange | 135 | |
| 15–45 nm | 5 cycles | |||||
| Hazelnut shell | Ag | Origanum vulgare leaf extract | In situ with stirring at 70 °C for 2 h | Spherical | Catalytic reduction of methyl orange and Congo red | 136 |
| <30 nm | 5 cycles | |||||
| Wheat bran | Fe | Different extracts | In situ with stirring for 30 min or overnight | n.r. | Adsorption of chlorfenapyr | 93 |
| Rice bran | Ag | n.r. | ||||
| Coconut palm spathe | Fe–TiO2 | Cymbopogon citratus aqueous extract | Ex situ with doctor blade technique | Spherical | Photocatalytic degradation of cypermethrin | 139 |
| 13–16 nm | n.r. | |||||
| Elaeagnus angustifolia seed | Ag@AgCl | Elaeagnus angustifolia leaf extract | In situ with stirring at 60 °C | Spherical | Photocatalytic degradation of methylene blue | 137 |
| 40 nm | 3 cycles | |||||
| Luffa sponge | Zn | Peroxidase enzymes from Euphorbia amygdaloides | Ex situ with ultrasonic batch for 30 min and lyophilized | n.a. | Adsorption of trypan blue | 142 |
| 20 nm | n.r. | |||||
Eggshell waste is among the most abundant waste materials coming from food processing industries and it is mainly composed of calcium carbonate (CaCO3).123 The eggshell waste has been applied as a reliable support for biosynthesized Cu/Fe2O3-NP,124 SnO2/ZnO-NP,125 Pd-NP,126 Cu-NP,127 Ag-NP128 and ZnO-NP.129 Nasrollahzadeh et al.124 used for the first time waste materials as a support for biogenic MNP with catalytic activity for pollutant reduction. These authors produced Cu/eggshell, Fe3O4/eggshell and Cu/Fe3O4/eggshell nanocomposites from waste chicken eggshells using the aqueous extract of the leaves of Orchis mascula L. as a stabilizing and reducing agent. The immobilization of Cu and Fe3O4-NP increased the specific surface area, the number of pores on the surface of the eggshell and the catalytic activity compared with the MNP in suspension. Also, eggshell was used as an economic and environmentally friendly support for the preparation of the supported Pd-NP through a green and simple in situ reduction method using barberry fruit extract as a reducing and stabilizing agent.126 The authors observed that Pd-NP were immobilized on the eggshell surface, which indicates the perfect combination of the Pd-NP and eggshell.
The eggshell membrane is the innermost portion of the eggshell and is mainly composed of a thin inner and a thick outer membrane which includes proteins such as collagen, sialoprotein, osteopontin, and a small amount of saccharides with an interwoven fibrous structure.120 The use of the eggshell membrane as a support for biogenic MNP was found for MnO2-NP,130 ZnS-NP120 and Au-NP.131 Wang et al.130 used the eggshell membrane acting as both a support and reducing agent for MnO2-NP. The clean eggshell membrane was cut into slices, soaked into potassium permanganate solution and stirred at room temperature. This nanocomposite showed a good capacity for decontamination of tetracycline hydrochloride and can be separated easily from the bulk solution by taking the membrane out to stop the degradation, instead of centrifugation or filtration. Zhang et al.120 proposed a mechanism to explain the production of ZnS supported on the eggshell membrane. The authors show that when the membrane of the eggshell is immersed into Zn-precursor solution, Zn2+ can be tightly adsorbed by negative functional groups on the glycoprotein mantle of the eggshell membrane. By consecutive soaking of the new eggshell membrane into Na2S solution, S2− is moved toward active positions to achieve in situ nucleation of the ZnS nanocrystallites. With the repetition of this cycle, the ZnS-NP self-assembled spheroid-like nanocrystallites on the eggshell membrane do grow (Fig. 4B).
Agriculture wastes can be used as supports for biogenic MNP as is the case of the shells of almonds,132,133 walnuts,22 peaches,4 pistachios,134,135 hazelnuts,136 wheat bran and rice bran93 and seeds of Russian olives.137 The catalytic performance of green Pd-NP supported on modified almond shells was studied.153 The surface of almond shells was chemically modified using captopril and it was observed that the surface to volume ratio increased and the immobilized NP were more stable. Walnut shell is a low-cost, easily available biomaterial, which has an intrinsic pore structure and is composed of cellulose, hemicelluloses and lignin.138 This material was used for the first time by Bordbar and Mortazavimanesh,22 in the in situ fabrication of Pd-NP by reduction of Pd ions adsorbed on the surface of the walnut shell using the aqueous extract of the leaves of E. arvense L. as a reducing agent. These nanocomposites showed high catalytic activity in the reduction of 4-nitrophenol, Congo red, methylene blue and rhodamine B in the presence of aqueous NaBH4 at room temperature. Also, the pistachio shell powder is an effective support for immobilization and dispersion of Cu-NP using pistachio hull extract.135 The nanocomposite of Cu-NP/pistachio shell has shown high catalytic activity in the reduction of organic dyes and could be easily recovered and reused several cycles.
Forestry wastes such as coconut palm spathe have been used as supports for green Fe–TiO2-NP and used for photocatalytic degradation of cypermethrin, reaching more than 80% degradation.139
Natural seashell waste was utilized to support Ag-NP by a simple and green method using B. persicum seed extract.140 The Ag-NP/seashell nanocomposite showed great catalytic reduction of different organic dyes. Also, Rostami-Vartooni used seashell waste to support CuO-NP using Rumex crispus seed extract as a chelating and capping agent.141 This nanocomposite was reused five times for 100% reduction of 4-nitrophenol.
Luffa sponge is the dried vascular bundle from the fruit of a widely distributed cucurbit and it is an environmentally friendly and pollution-free support with a large specific surface area and was used to immobilize ZnO-NP to remove trypan blue azo dye.142 The ZnO-NP were obtained by using the peroxidase enzyme from Euphorbia amygdaloides plants and then were immobilized on the fiber of the luffa sponge by incubation in an ultrasonic bath for 30 min. The optimum removal of the dye was found at pH 7 and the equilibrium was attained within 30 min.
| Support | Biogenic MNP | Bioreductor | Immobilization method | Shape and size | Wastewater application and recyclability | Ref. |
|---|---|---|---|---|---|---|
| Nano zerovalent iron (nZVI); room temperature (RT); not reported (n.r.); not applicable (n.a.). | ||||||
| Biochar from carbonaceous material from sewage sludge | Ag | Camellia sinensis leaf extract | In situ with stirring for 12 h | Non-spherical | Catalytic reduction of methylene blue | 145 |
| 26 nm | n.r. | |||||
| Biochar from Spartina alterniflora | ZnO | Spartina alterniflora extract | Ex situ with a water bath at 80 °C for 2 h and carbonized | Near-rodlike | Photocatalytic degradation of malachite green | 146 |
| 25–40 nm | n.r. | |||||
| Biochar from jackfruit peel | Fe | Polysaccharide extract from mushroom | Ex situ with microwave irradiation | Spherical | Adsorption of phosphate and nitrate | 147 |
| 100 nm | 5 cycles | |||||
| Biochar from oak wood | nZVI | Tea polyphenol | In situ with stirring for 30 min | Spherical | Adsorption of Cr6+ | 148 |
| 90–200 nm | n.r. | |||||
| Biochar from banana peel | Fe | Banana peel extract | In situ with continuous sonication for 1.5 h | Irregular | Adsorption of methylene blue | 121 |
| n.r. | 5 cycles | |||||
| Biochar from kenaf bar | nZVI | Green tea extract | In situ with N2 atmosphere | Spherical | Catalytic reduction and oxidation of Cu2+ and bisphenol A | 149 |
| 100 nm | 3 cycles | |||||
| Biochar from green tea residues | n.r. | Adsorption of p-nitrophenol | 150 | |||
| 5 cycles | ||||||
| Biochar from N. lappaceum peel | Fe | Nephelium lappaceum peel extract | In situ with stirring at RT for 30 min | Spherical | Adsorption of organochlorine pesticides | 151 |
| 20–80 nm | 5 cycles | |||||
| Activated carbon from Hildegardia barteri leaves | Ag | Ocimum gratissimum leaf extract | Ex situ with stirring for 2 h | Spherical | Adsorption of Congo red | 153 |
| 22–43 nm | 5 cycles | |||||
| Activated carbon from Corchorus olitorius stems | SnO2 | Saccharum officinarum juice | In situ with constant stirring for 15 min and autoclaved | Irregular | Photocatalytic degradation of naproxen | 154 |
| 3 nm | 5 cycles | |||||
| Activated carbon from Delonix regia | ZnO | Tithonia diversifolia leaf extract | Ex situ with stirring at 150 °C for 1 h | Spherical | Adsorption of methylene blue | 155 |
| 9–29 nm | 5 cycles | |||||
| Activated carbon from palm coconut shell | Cu | Moringa oleifera leaf extract | In situ with stirring at RT for 36 h | n.r. | Adsorption of nitrate | 156 |
| 66–61 | n.r. | |||||
| Activated carbon from lemon pomace | Fe/Zn | Lemon leaf extract | In situ with stirring for 3 h | Spherical | Fenton-like oxidation of reactive red 2 | 157 |
| 126 nm | n.r. | |||||
| Activated carbon from corn straws | S-nZVI | Green tea extract | In situ with stirring for 2 h under nitrogen-flowing atmosphere | n.r. | Adsorption of Pb2+ | 158 |
| n.r. | ||||||
| Activated carbon from worn tires | ZnO | Walnut peel extract | Ex situ with stirring for 2 h | Spherical | Adsorption of eosin Y and erythrosine B | 160 |
| 31–35 nm | 5 cycles | |||||
| Walnut shell extract | Ex situ with stirring for 10 h | n.r. | Adsorption of reactive blue 19 and reactive black-5 | 159 | ||
| n.r. | ||||||
| Activated carbon from pomegranate peel | nZVI | Pomegranate peel extract | Ex situ with stirring for 2 h and placed in the oven for 10 h at 95 °C | Non-uniform | Adsorption of furfural | 161 |
| 11–35 nm | 5 cycles | |||||
| Activated carbon from sawdust | Cu | Green tea extract | Ex situ with stirring for 12 h at RT and then heated at 80 °C | n.r. | Adsorption of acridine orange | 162 |
| 200 nm | n.r. | |||||
| Activated carbon from spent mushroom compost | Ag | Corncob extract | Ex situ with magnetic stirring and sonication | Spherical | Adsorption of levofloxacin | 163 |
| n.r. | ||||||
| 10–25 nm | Antibacterial activity against both E. coli and S. aureus | |||||
| n.a. | ||||||
| Activated carbon from the spent home filtration system filters | Ag | Azadirachta indica leaf extract | Ex situ with vigorous stirring overnight at RT | Spherical | Catalytic reduction of 4-nitrophenol | 164 |
| n.r. | ||||||
| ZnO | 20–300 nm | Antibacterial activity against 16 pathogenic bacteria | ||||
| Ag/ZnO | n.a. | |||||
Biochar is an attractive and low-cost carbon-rich material derived from biomass and produced by pyrolysis under limited oxygen or by hydrothermal carbonization at high pressure. In order to produce biochar the pyrolysis temperature generally ranges from 300 to 1000 °C.144 A variety of raw materials have been tested as precursors of biochar such as carbonaceous materials from sewage sludge,145 invasive plants,146 jackfruit peel,147 oak wood,148 banana peel,121 kenaf bar,149 green tea150 and rambutan peel,151 to serve as a support for biogenic MNP. The synthesis of a novel biochar-supported nZVI through a green method with green tea aqueous extract has been reported by Liu et al.149
The nanocomposite showed high performance on the simultaneous removal of Cu2+ and bisphenol A by a combination of biochar–nZVI with a persulfate system, reaching removal rates of 96% and 98% within 60 min, respectively. The authors proposed a possible reaction mechanism for the synergistic reduction and oxidation. For the Cu2+ removal process, Cu2+ species could be firstly adsorbed and then suffer co-precipitation and complexation processes, being reduced into Cu+ and Cu0 species at the end through one electron reduction process. For the bisphenol A removal process, this could also be adsorbed on the biochar–nZVI surface, while Fe2+ would be released from the nanocomposite under acidic conditions which could activate the persulfate system for the production of SO42−. Subsequently, bisphenol A could be degraded into a series of products such as p-isopropenyl phenol, 4-isopropylphenol, 4-hydroxyacetophenone, p-hydroquinone, fumaric acid and 2-hydroxypropionic acid.149 The synthesis of a green biochar/iron oxide composite, using a facile approach involving banana peel extract and biochar from the same banana peel, was achieved with an enhanced adsorption ability for methylene blue (862 mg g−1).121 The authors proposed a mechanism in which the banana peel biochar with reductive biomolecules reacts with FeSO4 to form a biochar/nZVI composite. Formerly, under the action of dissolved oxygen in water, nZVI is oxidized and hydrolyzed to form a biochar/FexOy composite (Fig. 4C).
Also Jing et al.146 synthesized ZnO-NP using S. alterniflora extract loaded on biochar derived from S. alterniflora by a one-step carbonization method and showed 98.38% photocatalytic degradation of malachite green. Nayak et al.147 fabricated a novel biocompatible nanocomposite by ultrasonication-assisted extraction of natural polysaccharides from mushrooms followed by its use in the synthesis of biogenic magnetite NP immobilized onto the biochar of jackfruit peel with a good adsorption capacity for phosphate (7.95 mg g−1) and nitrate (5.26 mg g−1).
Activated carbon is produced from environmental wastes with high carbon content.152 Lignocellulosic and coal materials such as tree,153 jute stick,154 flamboyant pods,155 palm coconut,156 lemon pomace,157 corn straws,158 worn tires,159,160 pomegranate peel,161 sawdust,162 spent mushroom compost163 and spent filters from home filtration systems164 have been used as raw materials planned for the manufacture of activated carbon to act as a support for biogenic MNP.
The preparation of an efficient heterogeneous nanocomposite based on the immobilization of biogenic SnO2-NP on activated carbon from Corchorus olitorius stems was reported and showed excellent photocatalytic activity towards degradation of naproxen.154 Taha et al.164 have reported the green synthesis of Ag-, ZnO- and Ag/ZnO-NP with posterior immobilization on activated carbon from the spent filters from home filtration systems and evaluated the antibacterial and the catalytic activity of the synthesized nanocomposites. The results showed that Ag-NP loaded on activated carbon have the best catalytic activity compared to the other nanocomposites, which was attributed to the good dispersion of Ag-NP on the surface of activated carbon.
4.3 Modified sustainable materials
Modified sustainable materials as efficient solid supports for the immobilization of biogenic MNP have been applied on the removal of organic or inorganic water contaminants.The modification of natural bentonite by different chemical and physical processes including alkaline treatment, acid leaching, thermal treatment, polymer intercalation and organic modifications resulted in hybrid materials with high basal spacing, high surface area, high adsorption capacity and with more active functional groups with high affinity for organic pollutants.165 Abdel Salam et al.122 modified natural bentonite by direct intercalation of its layers by cetyltrimethylammonium bromide (CTAB) and applied it as a carrier for green fabricated cobalt oxide-NP. The authors showed that the mechanism involved the intercalation of bentonite layers with CTAB chains, producing organically modified bentonite of high basal spacing and extended surface area, with different types of functional groups. Afterward, the green decoration of the modified clay by Co3O4-NP results in the formation of individual nanograins of cobalt oxide distributed on the surface of the clay, defining other functional groups to increase the overall adsorption capacity (Fig. 4D). This organic modification improved the bentonite surface area and its adsorption capacity, especially for the organic pollutant fixation, compared to those of the raw phase, which is considered a vital step for effective photocatalytic degradation and reduction processes. In addition, natural bentonite was modified by interchanging the Al polycation with Na+ and Ca2+ in the interlayer of the clay through a pillarization process followed by calcination.166 The resulting pillared bentonite has a relatively large surface area and interlayer distance, has uniform pores and is not fluffy (i.e., it is non-swelling) which renders it thermally stable and allows it to be used as a catalyst.
The natural low-cost montmorillonite has proven to exhibit excellent properties in wastewater treatment. The modification of montmorillonite is performed to increase the surface area and to generate pores enabling it to act as a good support and stabilizer in the synthesis of MNP. Saikia et al.167 modified montmorillonite (purified bentonite) with hydrochloric acid to increase the surface area by pore generation, in order to immobilize Pd-NP, using Ocimum sanctum leaf extract as a reducing agent. The supported Pd-NP were used in the catalytic hydrodechlorination of 4-chlorophenol in water under base free conditions and showed conversions up to 98%.
Natural zeolite is an abundant resource of aluminosilicate materials with a high cation exchange capacity. Kim et al.168 tested the adsorption of Pb2+, Cd2+ and Zn2+ by immobilizing biogenic manganese oxide on natural zeolite pretreated with NaCl or NaOH to increase the adsorption ability.
Perlite, a naturally occurring glassy volcanic siliceous rock with both unique physical and chemical properties, has attracted a great deal of attention in different areas, especially when it is heated rapidly at 760–1100 °C. Shirkhodaie et al.169 used expanded perlite supporting Fe2O3-NP with yellow pea as a reducing agent and ibuprofen as an organic agent. With perlite being heated, it expands up to 20 times its original volume and forms a light white mineral. It is chemically inert and nontoxic with different types of silanol groups and alumina hydrous oxide on its surface.
5. Economic and environmental perspective
In order to guarantee the highest quality drinking water, it is necessary to introduce new advanced technologies in this area. The ability to integrate different properties, resulting in multifunctional systems, is a major benefit for the use of nanomaterials as compared with traditional water technology.170 In fact, the development of sustainable, efficient and cost-effective methods for the elimination or effective reduction of pollutants and microorganisms in wastewater and drinking water is highly required.3Recently, the application of bio-resources for the development of green methods for the synthesis of MNP has gained widespread attention. Using non-toxic, biocompatible and environmentally benign methods for the synthesis of MNP associated with sustainable materials that may be recovered and reused has become of great interest. Various high-value products may be obtained with low economic and environmental impact and nature provides many useful materials with hidden potential to be applied in innovative processes. This environmental approach has been reflected in increased interest in clay minerals because they are abundant, easily found everywhere and present unique properties to act as MNP supports. They have a significant ability to immobilize pollutants, either by adsorption or ion exchange mechanisms, and usually present excellent mechanical stability.81 Among the more than four thousand known minerals, only a small part has been tested as a support for green NP. Clays such as sepiolite,171 rectorite,172 chrysotile173 and halloysite174 are found in the literature as sustainable supports for commercial MNP or chemically synthesized MNP.
Amongst natural materials, cork (the bark of the evergreen oak Quercus suber L.) is a particularly interesting and promising option not yet found as a support for biogenic MNP.175,176 The use of cork is sustainable, as the tree is not harmed during the harvesting of the bark (the harvesting is performed every 9–13 years).177
Wastes have been used as valuable and environmentally friendly supports for the immobilization of biogenic MNP. The concept of utilization of waste materials as a vector of circular economy introduces alternatives to maximize the reuse and recycling of wastes within the wastewater treatment process.178,179 At present, large quantities of wastes are discharged as the end of the line, resulting in considerable environmental issues. Food waste in the world is estimated at 1.6 billion tons per year as a result of population growth.178 For example, eggshell is one of the most common forms of food waste with a worldwide production of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per year.123 According to Food and Agriculture Organization (FAO) reports, almost 30 million tons of biomass from pistachio nuts are discarded annually in landfills by the pistachio nut processing industry.135 According to Welter et al.,180 more than 50% of fishing is thrown away as wastes resulting in around 20 million tons of residues being discarded worldwide each year from fisheries. These wastes, if not appropriately handled, will produce large quantities of greenhouse gases to be released into the atmosphere.181 Mostly, waste materials from the different sectors are disposed of by allowing them to rot at the source or in stockpiles.178 This lack of proper treatment of wastes severely affects the ecosystems.182 Furthermore, there are numerous wastes that can be applied as sustainable supports such as kitchen wipe sponge,183 palm trunk,184 industrial waste (lithium silicon powder),185 fly ash,186 wood187 and aloe vera.188 The use of wastes as sustainable supports for biogenic MNP only implies washing, drying and crushing. In addition, activated carbon and biochar have been produced from wastes as precursors, making this approach an effective and economically valuable solution for environmental pollution. Activated carbon has been identified as one of the most promising materials of the 21st century used in chemical industries thanks to its unique properties, including morphology, high mechanical strength, adsorption capacity, durability and good chemical stability.189 There is research effort to design and prepare affordable materials that may act as an alternative to the commercially available activated carbon.161,190
000 t per year.123 According to Food and Agriculture Organization (FAO) reports, almost 30 million tons of biomass from pistachio nuts are discarded annually in landfills by the pistachio nut processing industry.135 According to Welter et al.,180 more than 50% of fishing is thrown away as wastes resulting in around 20 million tons of residues being discarded worldwide each year from fisheries. These wastes, if not appropriately handled, will produce large quantities of greenhouse gases to be released into the atmosphere.181 Mostly, waste materials from the different sectors are disposed of by allowing them to rot at the source or in stockpiles.178 This lack of proper treatment of wastes severely affects the ecosystems.182 Furthermore, there are numerous wastes that can be applied as sustainable supports such as kitchen wipe sponge,183 palm trunk,184 industrial waste (lithium silicon powder),185 fly ash,186 wood187 and aloe vera.188 The use of wastes as sustainable supports for biogenic MNP only implies washing, drying and crushing. In addition, activated carbon and biochar have been produced from wastes as precursors, making this approach an effective and economically valuable solution for environmental pollution. Activated carbon has been identified as one of the most promising materials of the 21st century used in chemical industries thanks to its unique properties, including morphology, high mechanical strength, adsorption capacity, durability and good chemical stability.189 There is research effort to design and prepare affordable materials that may act as an alternative to the commercially available activated carbon.161,190
Alternative supports including sustainable natural biopolymers such as cellulose, chitosan, alginate, dextran and starch have been recently employed for the preparation of supported MNP.191,192 Biopolymers offer several advantages compared to traditional supports including low toxicity and cost, high biocompatibility, availability and abundance as well as delivering unique functions as per their use.193
These natural or cheap precursor materials, therefore, ensure that a reasonable quantity of nanocomposite can be produced and that there is potential for upscaling. This is a condition that is not typically the case in current nanocomposites because they are prepared from rather expensive reagents and often through more complex processes that are not generally amenable for developing countries.3
The circular economy nowadays is the goal for research that seeks improvement in sustainable development. It aims at minimizing human and industrial pollution by turning waste and by-products into added value products.194 From the perspective of circular economy and green development, the simultaneous utilization of low-cost materials and/or reuse of natural or organic waste materials as supports for green MNP in the treatment of wastewater is one of the most thrust areas for researchers and environmental scientists.182 This synergistic effect will enhance the reusability of material and eliminate the generation of secondary pollutants.
6. Applications in wastewater treatment
Green nanocomposites have been applied in wastewater treatment mainly as adsorbents and catalysts. Various studies propose the role of these nanocomposites in wastewater treatment.Qu et al.158 detailed the possible interactions associated with Pb(II) and sulfide-nZVI immobilized on activated carbon and proposed that the adsorption of metal occurred via the combination of electrostatic interactions, chemical precipitation, surface complexation and reductive actions (Fig. 5A). Soliemanzadeh and Fekri99 showed that Cr(VI) was attracted to the surface of green nZVI supported on bentonite by monolayer adsorption with specific force rather than a simple electrostatic attraction.
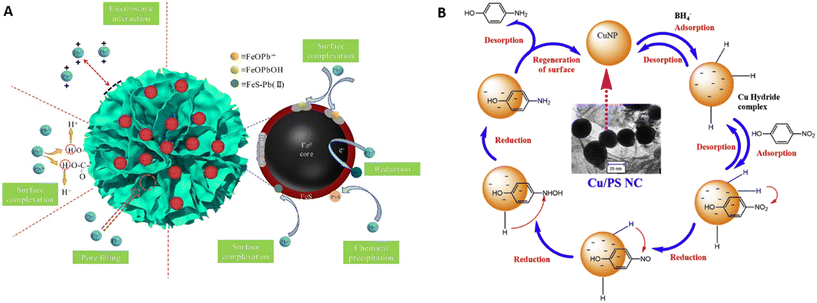 | ||
| Fig. 5 Mechanisms of green nanocomposite application in wastewater treatment. (A) Adsorption of Pb(II) by sulfide-nZVI supported in activated carbon, reproduced from ref. 158 with permission from Elsevier, copyright 2021, and (B) catalytic reduction of 4-nitrophenol by NaBH4 and CuNP supported on pistachio shell, reproduced from ref. 135 with permission from Elsevier, copyright 2018. | ||
Taghizadeh and Rad-Moghadam135 proposed a mechanism of catalytic reduction of 4-nitrophenol to 4-aminophenol by NaBH4 and CuNP supported on pistachio shell (Fig. 5B). Firstly, the formation of 4-hydroxylaminophenol due to dissociation of NaBH4 in the aqueous medium to generate borohydride ions is reported as well as the subsequent adsorption and diffusion of these ions on the surface of CuNP and adsorption of 4-nitrophenol onto the nanocomposite. The 4-hydroxylaminophenol is reduced to 4-aminophenol, then it departs from the surface of CuNP by physical desorption to create a free surface that enters into a new catalytic cycle.
Some studies were published with green nanocomposites applied as antibacterial agents.128,129,163,164 Lashkarizadeh129 demonstrated that green ZnONP immobilized on eggshells had a significant microbial growth inhibition compared with the ZnONP in suspension. Many studies reported that the reduced size of MNP leads to an increased surface area with a good affinity for both amino and carboxyl groups on the cell surface, facilitating penetration into the cells.164 A mechanism reported by Karadirek et al. indicates that the ions present in the MNP potentially leads to the destruction of the double helix chain structure of DNA.163
The ideal nanocomposite ought to be easily separated from the reaction medium, recycled and reused for other runs without marked loss of activity. The reuse efficiency of the nanocomposite is demonstrated in most studies. It should be noted that the highest recyclability is found in studies that use wastes as solid supports for MNP. Some composites were reused several times (10 fold) without any significant loss in the activity.127,131,132 In the case of the adsorption process, the reusability of nanocomposites implies the desorption of sorbate. Various authors reported the use of sodium hydroxide,81,105 hydrochloric acid,113 ethylenediaminetetraacetic acid,125 methanol,127 ammonium hydroxide,147 ethanol121 and n-hexane.151 Meanwhile in other wastewater applications, as catalysts, the reuse of the nanocomposite only implied the process of separation from the reaction mixture, which includes centrifugation,131,132 filtration127 and external magnetic separation.82
Although the use of green nanocomposites on water treatment is becoming more popular and the results seem to be promising in this research field, it still presents several challenges. First, the high variability of bio-resources to biosynthesize MNP influences the characteristics of NP, since different bio-resources contain different concentrations of organic reducing agents, which implies a previous optimization of the synthesis process. It is important to understand the specific biosynthesis mechanism to standardize the methodology. The green nanocomposite application in wastewater treatment is mostly assessed with dyes at the lab-scale. In the future, it is important to contemplate the application of these materials with other hazardous and persistent pollutants as well as to conduct research at the pilot-scale in order to evaluate the system efficiency in real effluents.3,81 In addition, the leaching rate of the green material and toxicity to the environment and human health are other challenges.195 Further research is needed to address the challenges posed by nanomaterials.
7. Conclusions
The principles of Green Chemistry can be used on greener bio-inspired materials applied on the synthesis of MNP, as well on their immobilization on solid supports. A wider application of MNP is limited by some issues such as the difficulty of suspension manipulation, the ultimate health risks of MNP dissipation into the environment and size instability due to aggregation. Synthesis of MNP on a solid support has emerged as a sustainable solution that may prevent the accumulation of MNP waste in the ecosystem through a recycling process. This review focusses on sustainable materials to act as supports of biosynthesized MNP in the field of wastewater treatment. Materials derived from renewable and earth-abundant sources, such as kaolin, montmorillonite and bentonite, are used as sustainable supports. Residues from the food industry, such as eggshells or almond shells, have been explored as MNP supports. The nanocomposites are applied in wastewater treatment as adsorbents, catalysts or antibacterial agents for removal or elimination of various xenobiotic pollutants or water disinfection. Constant efforts are made to use sustainable materials as pristine or in modified structures for supporting MNP and future studies should be focused on such new green methodologies and combined techniques for the reuse of sustainable materials.Abbreviations
| CTAB | Cetyltrimethylammonium bromide |
| FAO | Food and Agriculture Organization |
| GO | Graphene oxide |
| MNP | Metal nanoparticles |
| NP | Nanoparticles |
| nZVI | Nano zerovalent iron |
| rGO | Reduced graphene oxide |
| RT | Room temperature |
| TUD-1 | Technische Universiteit Delft |
| WWTP | Wastewater treatment plants |
Author contributions
Verónica Rocha: conceptualization, methodology, data curation, writing – original draft; Ana Elisa Lago: writing – original draft; Bruna Silva: writing – original draft; Óscar Barros: writing – original draft; Isabel C. Neves: conceptualization, methodology, supervision, writing – review & editing, Teresa Tavares: supervision, validation and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Verónica Rocha, Ana Lago and Óscar Barros thank FCT (Portuguese Foundation for Science and Technology) for their PhD grants (SFRH/BD/141073/2018, SFRH/BD/132271/2017, SFRH/BD/140362/2018, respectively). This research work has been funded by national funds funded through FCT/MCTES (PIDDAC) over the projects: UIDB/50020/2020, Centre of Chemistry (UID/QUI/0686/2020), CEB (UIDB/04469/2020) and LABBELS (LA/P/0029/2020), and project BioTecNorte (operation NORTE-01-0145-FEDER-000004), supported by the Northern Portugal Regional Operational Program (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (ERDF).References
- P. Rani, V. Kumar, P. P. Singh, A. S. Matharu, W. Zhang and K.-H. Kim, et al., Highly stable AgNPs prepared via a novel green approach for catalytic and photocatalytic removal of biological and non-biological pollutants, Environ. Int., 2020, 143, 105924 CrossRef CAS.
- H. Ali, E. Khan and I. Ilahi, Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation, J. Chem., 2019, 2019, 6730305 Search PubMed.
- M. O. Alfred, M. O. Omorogie, O. Bodede, R. Moodley, A. Ogunlaja and O. G. Adeyemi, et al., Solar-active clay-TiO2 nanocomposites prepared via biomass assisted synthesis: Efficient removal of ampicillin, sulfamethoxazole and artemether from water, Chem. Eng. J., 2020, 398, 125544 CrossRef.
- B. Khodadadi, M. Bordbar and M. Nasrollahzadeh, Achillea millefolium L. extract mediated green synthesis of waste peach kernel shell supported silver nanoparticles: Application of the nanoparticles for catalytic reduction of a variety of dyes in water, J. Colloid Interface Sci., 2017, 493, 85–93 CrossRef CAS PubMed.
- R. M. Tripathi and S. J. Chung, Biogenic nanomaterials: Synthesis, characterization, growth mechanism, and biomedical applications, J. Microbiol. Methods, 2019, 157, 65–80 CrossRef CAS.
- A. Rastogi, P. Singh, F. A. Haraz and A. Barhoum, Chapter 19 - Biological synthesis of nanoparticles: an environmentally benign approach, in Micro and Nano Technologies, ed. A. Barhoum and A. Hamdy Makhlouf, Elsevier, 2018, pp. 571–604 Search PubMed.
- A. SI, K. Pal, S. Kralj, G. S. El-Sayyad, F. G. de Souza and T. Narayanan, Sustainable preparation of gold nanoparticles via green chemistry approach for biogenic applications, Mater. Today Chem., 2020, 17, 100327 CrossRef CAS.
- D. Sharma, S. Kanchi and K. Bisetty, Biogenic synthesis of nanoparticles: A review, Arabian J. Chem., 2019, 12(8), 3576–3600 CrossRef CAS.
- J. Jeevanandam, A. Barhoum, Y. S. Chan, A. Dufresne and M. K. Danquah, Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations, Beilstein J. Nanotechnol., 2018, 9, 1050–1074 CrossRef CAS PubMed.
- J. Annamalai, S. B. Ummalyma, A. Pandey and T. Bhaskar, Recent trends in microbial nanoparticle synthesis and potential application in environmental technology: a comprehensive review, Environ. Sci. Pollut. Res., 2021, 28(36), 49362–49382 CrossRef CAS PubMed.
- N. Abid, A. M. Khan, S. Shujait, K. Chaudhary, M. Ikram and M. Imran, et al., Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review, Adv. Colloid Interface Sci., 2022, 300, 102597 CrossRef CAS PubMed.
- M. Shah, D. Fawcett, S. Sharma, S. K. Tripathy and G. E. J. Poinern, Green Synthesis of Metallic Nanoparticles via Biological Entities, Materials, 2015, 8(11), 7278–7308 CrossRef CAS PubMed.
- A. Rana, K. Yadav and S. Jagadevan, A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity, J. Cleaner Prod., 2020, 272, 122880 CrossRef CAS.
- P. Praveen Kumar, M. Laxmi Deepak Bhatlu, K. Sukanya, S. Karthikeyan and N. Jayan, Synthesis of magnesium oxide nanoparticle by eco friendly method (green synthesis)– A review, Mater. Today: Proc., 2020, 37, 3028–3030 Search PubMed.
- L. P. Silva, I. G. Reis and C. C. Bonatto, in Green Synthesis of Metal Nanoparticles by Plants: Current Trends and Challenges BT - Green Processes for Nanotechnology: From Inorganic to Bioinspired Nanomaterials, ed. V. A. Basiuk and E. V. Basiuk, Springer International Publishing, Cham, 2015, pp. 259–275 Search PubMed.
- S. Mohammadalinejhad, H. Almasi and M. Esmaiili, Simultaneous green synthesis and in-situ impregnation of silver nanoparticles into organic nanofibers by Lythrum salicaria extract: Morphological, thermal, antimicrobial and release properties, Mater. Sci. Eng., C, 2019, 105, 110115 CrossRef CAS PubMed.
- A. Gour and N. K. Jain, Advances in green synthesis of nanoparticles, Artif. Cells, Nanomed., Biotechnol., 2019, 47(1), 844–851 CrossRef CAS.
- A. Saravanan, P. S. Kumar, S. Karishma, D.-V. N. Vo, S. Jeevanantham and P. R. Yaashikaa, et al., A review on biosynthesis of metal nanoparticles and its environmental applications, Chemosphere, 2021, 264, 128580 CrossRef CAS.
- I. Khan, K. Saeed and I. Khan, Nanoparticles: Properties, applications and toxicities, Arabian J. Chem., 2019, 12(7), 908–931 CrossRef CAS.
- G. Marslin, K. Siram, Q. Maqbool, R. K. Selvakesavan, D. Kruszka and P. Kachlicki, et al., Secondary Metabolites in the Green Synthesis of Metallic Nanoparticles, Materials, 2018, 11(6), 940 CrossRef PubMed.
- T. Parandhaman, M. D. Dey and S. K. Das, Biofabrication of supported metal nanoparticles: exploring the bioinspiration strategy to mitigate the environmental challenges, Green Chem., 2019, 21(20), 5469–5500 RSC.
- M. Bordbar and N. Mortazavimanesh, Green synthesis of Pd/walnut shell nanocomposite using Equisetum arvense L. leaf extract and its application for the reduction of 4-nitrophenol and organic dyes in a very short time, Environ. Sci. Pollut. Res., 2017, 24(4), 4093–4104 CrossRef CAS PubMed.
- A. Kumar, G. Sharma, M. Naushad, A. H. Al-Muhtaseb, A. García-Peñas and G. T. Mola, et al., Bio-inspired and biomaterials-based hybrid photocatalysts for environmental detoxification: A review, Chem. Eng. J., 2020, 382, 122937 CrossRef CAS.
- H. Duan, D. Wang and Y. Li, Green chemistry for nanoparticle synthesis, Chem. Soc. Rev., 2015, 44(16), 5778–5792 RSC.
- A. Dieudonné, D. Pignol and S. Prévéral, Magnetosomes: biogenic iron nanoparticles produced by environmental bacteria, Appl. Microbiol. Biotechnol., 2019, 103(9), 3637–3649 CrossRef PubMed.
- E. A. Adebayo, M. A. Azeez, M. B. Alao, A. M. Oke and D. A. Aina, Fungi as veritable tool in current advances in nanobiotechnology, Heliyon, 2021, 7(11), e08480 CrossRef CAS PubMed.
- K. He, G. Chen, G. Zeng, Z. Huang, Z. Guo and T. Huang, et al., Applications of white rot fungi in bioremediation with nanoparticles and biosynthesis of metallic nanoparticles, Appl. Microbiol. Biotechnol., 2017, 101(12), 4853–4862 CrossRef CAS PubMed.
- A. Roychoudhury, Yeast-mediated Green Synthesis of Nanoparticles for Biological Applications, Indian J. Pharm. Biol. Res., 2020, 8, 26–31 CAS.
- X. Zhang, Y. Qu, W. Shen, J. Wang, H. Li and Z. Zhang, et al., Biogenic synthesis of gold nanoparticles by yeast Magnusiomyces ingens LH-F1 for catalytic reduction of nitrophenols, Colloids Surf., A, 2016, 497, 280–285 CrossRef CAS.
- A. Mukherjee, D. Sarkar and S. Sasmal, A Review of Green Synthesis of Metal Nanoparticles Using Algae, Front. Microbiol., 2021, 12, 693899 CrossRef.
- S.-N. Li, R. Wang and S.-H. Ho, Algae-mediated biosystems for metallic nanoparticle production: From synthetic mechanisms to aquatic environmental applications, J. Hazard. Mater., 2021, 420, 126625 CrossRef CAS PubMed.
- R. G. Saratale, G. D. Saratale, H. S. Shin, J. M. Jacob, A. Pugazhendhi and M. Bhaisare, et al., New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: current knowledge, their agricultural and environmental applications, Environ. Sci. Pollut. Res., 2018, 25(11), 10164–10183 CrossRef CAS PubMed.
- S. Jadoun, R. Arif, N. K. Jangid and R. K. Meena, Green synthesis of nanoparticles using plant extracts: a review, Environ. Chem. Lett., 2021, 19(1), 355–374 CrossRef CAS.
- J. L. Gardea-Torresdey, E. Gomez, J. R. Peralta-Videa, J. G. Parsons, H. Troiani and M. Jose-Yacaman, Alfalfa Sprouts: A Natural Source for the Synthesis of Silver Nanoparticles, Langmuir, 2003, 19(4), 1357–1361 CrossRef CAS.
- A. M. Shafey, Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: A review, Green Processes Synth., 2020, 9(1), 304–339 Search PubMed.
- J. Singh, V. Kumar, K. H. Kim and M. Rawat, Biogenic synthesis of copper oxide nanoparticles using plant extract and its prodigious potential for photocatalytic degradation of dyes, Environ. Res., 2019, 177, 108569 CrossRef CAS PubMed.
- D. Patiño-Ruiz, L. Sánchez-Botero, L. Tejeda-Benitez, J. Hinestroza and A. Herrera, Green synthesis of iron oxide nanoparticles using Cymbopogon citratus extract and sodium carbonate salt: Nanotoxicological considerations for potential environmental applications, Environ. Nanotechnol., Monit. Manage., 2020, 14, 100377 Search PubMed.
- N. Akhlaghi, G. Najafpour-Darzi and H. Younesi, Facile and green synthesis of cobalt oxide nanoparticles using ethanolic extract of Trigonella foenumgraceum (Fenugreek) leaves, Adv. Powder Technol., 2020, 31(8), 3562–3569 CrossRef CAS.
- G. Nagaraj, D. Brundha, V. Kowsalya, C. Chandraleka, S. Sangavi and R. Jayalakshmi, et al., Biosynthesis of zinc doped Aloe Vera for green nanoparticles, Mater. Today: Proc., 2020, 43, 3354–3358 Search PubMed.
- H.-Y. Chai, S.-M. Lam and J.-C. Sin, Green synthesis of magnetic Fe-doped ZnO nanoparticles via Hibiscus rosa-sinensis leaf extracts for boosted photocatalytic, antibacterial and antifungal activities, Mater. Lett., 2019, 242, 103–106 CrossRef CAS.
- A. Fall, I. Ngom, M. Bakayoko, N. F. Sylla, H. Elsayed Ahmed Mohamed and K. Jadvi, et al., Biosynthesis of TiO2 nanoparticles by using natural extract of Citrus sinensis, Mater. Today: Proc., 2020, 36, 349–356 Search PubMed.
- A. R. M. Abd El-Aziz, A. Gurusamy, M. R. Alothman, S. M. Shehata, S. M. Hisham and A. A. Alobathani, Silver nanoparticles biosynthesis using Saussurea costus root aqueous extract and catalytic degradation efficacy of safranin dye, Saudi J. Biol. Sci., 2021, 28(1), 1093–1099 CrossRef CAS.
- A. Rana, N. Kumari, M. Tyagi and S. Jagadevan, Leaf-extract mediated zero-valent iron for oxidation of Arsenic (III): Preparation, characterization and kinetics, Chem. Eng. J., 2018, 347, 91–100 CrossRef CAS.
- P. Wang, N. W. Menzies, H. Chen, X. Yang, S. P. McGrath and F.-J. Zhao, et al., Risk of Silver Transfer from Soil to the Food Chain Is Low after Long-Term (20 Years) Field Applications of Sewage Sludge, Environ. Sci. Technol., 2018, 52(8), 4901–4909 CrossRef CAS PubMed.
- X. Shi, Z. Li, W. Chen, L. Qiang, J. Xia and M. Chen, et al., Fate of TiO2 nanoparticles entering sewage treatment plants and bioaccumulation in fish in the receiving streams, NanoImpact, 2016, 3–4, 96–103 CrossRef.
- Q. Abbas, B. Yousaf, Amina, M. U. Ali, M. A. M. Munir and A. El-Naggar, et al., Transformation pathways and fate of engineered nanoparticles (ENPs) in distinct interactive environmental compartments: A review, Environ. Int., 2020, 138, 105646 CrossRef CAS PubMed.
- C. Lei, Y. Sun, D. C. W. Tsang and D. Lin, Environmental transformations and ecological effects of iron-based nanoparticles, Environ. Pollut., 2018, 232, 10–30 CrossRef CAS PubMed.
- D. M. Mitrano, P. Limpiteeprakan, S. Babel and B. Nowack, Durability of nano-enhanced textiles through the life cycle: releases from landfilling after washing, Environ. Sci.: Nano, 2016, 3(2), 375–387 RSC.
- O. J. Osborne, S. Lin, C. H. Chang, Z. Ji, X. Yu and X. Wang, et al., Organ-Specific and Size-Dependent Ag Nanoparticle Toxicity in Gills and Intestines of Adult Zebrafish, ACS Nano, 2015, 9(10), 9573–9584 CrossRef CAS PubMed.
- J. D. Martin, P. C. Frost, H. Hintelmann, K. Newman, M. J. Paterson and L. Hayhurst, et al., Accumulation of Silver in Yellow Perch ( Perca flavescens) and Northern Pike ( Esox lucius) From a Lake Dosed with Nanosilver, Environ. Sci. Technol., 2018, 52(19), 11114–11122 CrossRef CAS PubMed.
- J. Hou, Y. Zhou, C. Wang, S. Li and X. Wang, Toxic Effects and Molecular Mechanism of Different Types of Silver Nanoparticles to the Aquatic Crustacean Daphnia magna, Environ. Sci. Technol., 2017, 51(21), 12868–12878 CrossRef CAS PubMed.
- A. E. Pradas Del Real, V. Vidal, M. Carriere, H. Castillo-Michel, C. Levard and P. Chaurand, et al., Ag nanoparticles and wheat roots: a complex interplay, Environ. Sci. Technol., 2017, 51, 5774–5782 CrossRef CAS PubMed.
- M. S. Abdel-Aziz, K. S. Abou-El-Sherbini, E. M. A. Hamzawy, M. H. A. Amr and S. El-Dafrawy, Green Synthesis of Silver Nano-particles by Macrococcus bovicus and Its Immobilization onto Montmorillonite Clay for Antimicrobial Functionality, Appl. Biochem. Biotechnol., 2015, 176(8), 2225–2241 CrossRef CAS PubMed.
- V. Porley and N. Robertson, 6 - Substrate and support materials for photocatalysis, in Micro and Nano Technologies, ed. R. Boukherroub, S. Ogale and N. Robertson, Elsevier, 2020, pp. 129–171 Search PubMed.
- R. Oblak, M. Kete, U. L. Štangar and M. Tasbihi, Alternative support materials for titania photocatalyst towards degradation of organic pollutants, J. Water Process. Eng., 2018, 23, 142–150 CrossRef.
- W. Zou, B. Gao, Y. S. Ok and L. Dong, Integrated adsorption and photocatalytic degradation of volatile organic compounds (VOCs) using carbon-based nanocomposites: A critical review, Chemosphere, 2019, 218, 845–859 CrossRef CAS PubMed.
- M. A. Mohd Adnan, N. Muhd Julkapli, M. N. I. Amir and A. Maamor, Effect on different TiO2 photocatalyst supports on photodecolorization of synthetic dyes: a review, Int. J. Environ. Sci. Technol., 2019, 16(1), 547–566 CrossRef CAS.
- N. M. Ainali, D. Kalaronis, E. Evgenidou, D. N. Bikiaris and D. A. Lambropoulou, Insights into Biodegradable Polymer-Supported Titanium Dioxide Photocatalysts for Environmental Remediation, Macromol, 2021, 1, 201–233 CAS.
- M. J. Ndolomingo, N. Bingwa and R. Meijboom, Review of supported metal nanoparticles: synthesis methodologies, advantages and application as catalysts, J. Mater. Sci., 2020, 55(15), 6195–6241 CrossRef CAS.
- J. Liu, H. Li, J. Harvey, G. Airey, S. Lin and S. L. J. Lee, et al., Study on leaching characteristics and biotoxicity of porous asphalt with biochar fillers, Transp. Res. D, 2023, 122, 103855 CrossRef.
- K. Vikrant, C. M. Park, K.-H. Kim, S. Kumar and E.-C. Jeon, Recent advancements in photocatalyst-based platforms for the destruction of gaseous benzene: Performance evaluation of different modes of photocatalytic operations and against adsorption techniques, J. Photochem. Photobiol., C, 2019, 41, 100316 CrossRef CAS.
- V. Tan and L. Vinh, Supported-Metal Oxide Nanoparticles-Potential Photocatalysts, 2020 Search PubMed.
- J. Y. Xu, X. C. Lei, R. Yang and Z. Z. Fan, In Situ Formation of Carbon Nanomaterials on Bulk Metallic Materials, J. Nanomater., 2014, 2014, 690630 Search PubMed.
- P. Tabrizian, W. Ma, A. Bakr and M. S. Rahaman, pH-sensitive and magnetically separable Fe/Cu bimetallic nanoparticles supported by graphene oxide (GO) for high-efficiency removal of tetracyclines, J. Colloid Interface Sci., 2019, 534, 549–562 CrossRef CAS.
- R. Kalaivizhi, B. Danagody and A. Yokesh, ACs@ZnO incorporated with a PSF/PU polymer membrane for dye removal, Mater. Adv., 2022, 3(23), 8534–8543 RSC.
- Z. Arif, N. K. Sethy, P. K. Mishra and B. Verma, Green approach for the synthesis of ultrafiltration photocatalytic membrane for tannery wastewater: modeling and optimization, Int. J. Environ. Sci. Technol., 2020, 17(7), 3397–3410 CrossRef CAS.
- J. A. Mazumder, M. Perwez, R. Noori and M. Sardar, Development of sustainable and reusable silver nanoparticle-coated glass for the treatment of contaminated water, Environ. Sci. Pollut. Res., 2019, 26(22), 23070–23081 CrossRef CAS PubMed.
- A. H. Gemeay, E. F. Aboelfetoh and R. G. El-sharkawy, Immobilization of Green Synthesized Silver Nanoparticles onto Amino-Functionalized Silica and Their Application for Indigo Carmine Dye Removal, Water, Air, Soil Pollut., 2017, 229, 1–17 Search PubMed.
- M. Bilal, S. Khan, J. Ali, M. Ismail, M. I. Khan and A. M. Asiri, et al., Biosynthesized silver supported catalysts for disinfection of Escherichia coli and organic pollutant from drinking water, J. Mol. Liq., 2019, 281, 295–306 CrossRef CAS.
- A. Nizam, V. G. Warrier, J. Devasia and N. Ganganagappa, Magnetic iron oxide nanoparticles immobilized on microporous molecular sieves as efficient porous catalyst for photodegradation, transesterification and esterification reactions, J. Porous Mater., 2021, 29, 119–129 CrossRef.
- A. Rostami-Vartooni and A. Moradi-Saadatmand, Green synthesis of magnetically recoverable Fe3 O4/HZSM-5 and its Ag nanocomposite using Juglans regia L. leaf extract and their evaluation as catalysts for reduction of organic pollutants, IET Nanobiotechnol., 2019, 13(4), 407–415 CrossRef PubMed.
- M. Tajbakhsh, H. Alinezhad, M. Nasrollahzadeh and T. A. Kamali, Green synthesis of the Ag/HZSM-5 nanocomposite by using Euphorbia heterophylla leaf extract: A recoverable catalyst for reduction of organic dyes, J. Alloys Compd., 2016, 685, 258–265 CrossRef CAS.
- A. Toli, C. Mystrioti, I. Avgoustidis and N. Papassiopi, Fixed-bed flow experiments with supported green nZVI for the remediation of contaminated waters: Effect of pH and background solution composition, Chemosphere, 2021, 279, 130472 CrossRef CAS PubMed.
- S. Naghdi, M. Sajjadi, M. Nasrollahzadeh, K. Y. Rhee, S. M. Sajadi and B. Jaleh, Cuscuta reflexa leaf extract mediated green synthesis of the Cu nanoparticles on graphene oxide/manganese dioxide nanocomposite and its catalytic activity toward reduction of nitroarenes and organic dyes, J. Taiwan Inst. Chem. Eng., 2018, 86, 158–173 CrossRef CAS.
- C. Xue, W. Cai, X. Weng, G. Owens and Z. Chen, A one step synthesis of hybrid Fe/Ni-rGO using green tea extract for the removal of mixed contaminants, Chemosphere, 2021, 284, 131369 CrossRef CAS PubMed.
- R. Ranjith, V. Renganathan, S.-M. Chen, N. S. Selvan and P. S. Rajam, Green synthesis of reduced graphene oxide supported TiO2/Co3O4 nanocomposite for photocatalytic degradation of methylene blue and crystal violet, Ceram. Int., 2019, 45(10), 12926–12933 CrossRef CAS.
- S. Leong, A. Razmjou, K. Wang, K. Hapgood, X. Zhang and H. Wang, TiO2 based photocatalytic membranes: A review, J. Membr. Sci., 2014, 472, 167–184 CrossRef CAS.
- M. F. R. Samsudin, A. Mahmood and S. Sufian, Enhanced photocatalytic degradation of wastewater over RGO-TiO2/BiVO4 photocatalyst under solar light irradiation, J. Mol. Liq., 2018, 268, 26–36 CrossRef CAS.
- F. Harjati, P. W. Citradewi, G. Purwiandono and I. Fatimah, Green synthesis of hematite/TUD-1 nanocomposite as efficient photocatalyst for bromophenol blue and methyl violet degradation, Arabian J. Chem., 2020, 13(11), 8395–8410 CrossRef CAS.
- C. Li, N. Zhu, S. Yang, X. He, S. Zheng and Z. Sun, et al., A review of clay based photocatalysts: Role of phyllosilicate mineral in interfacial assembly, microstructure control and performance regulation, Chemosphere, 2021, 273, 129723 CrossRef CAS PubMed.
- G. Gopal, H. Sankar, C. Natarajan and A. Mukherjee, Tetracycline removal using green synthesized bimetallic nZVI-Cu and bentonite supported green nZVI-Cu nanocomposite: A comparative study, J. Environ. Manage., 2020, 254, 109812 CrossRef CAS PubMed.
- T. Baran and M. Nasrollahzadeh, Facile fabrication of magnetically separable palladium nanoparticles supported on modified kaolin as a highly active heterogeneous catalyst for Suzuki coupling reactions, J. Phys. Chem. Solids, 2020, 146, 109566 CrossRef CAS.
- N. Rakhshan, M. Mansournia and F. Jookar, Plant Extract-Strategy Using Teucrium Polium Stems to Green Synthesize Ag/AgCl Bionanocomposite Imprinted on Fe3O4/kaolinite and Potentials in Catalytic and Chemosensor Applications, Arabian J. Chem., 2022, 103719 CrossRef CAS.
- U. Khunjan and P. Kasikamphaiboon, Green Synthesis of Kaolin-Supported Nanoscale Zero-Valent Iron Using Ruellia tuberosa Leaf Extract for Effective Decolorization of Azo Dye Reactive Black 5, Arabian J. Sci. Eng., 2021, 46(1), 383–394 CrossRef CAS.
- F. Moradi, S. Sedaghat, S. Arab-Salmanabadi and O. Moradi, Biosynthesis of silver-montmorillonite nanocomposites using Ocimum Basilicum and Teucrium Polium; a comparative study, Mater. Res. Express, 2019, 6(12), 125008 CrossRef CAS.
- J. Yang, S. Wang, N. Xu, Z. Ye, H. Yang and X. Huangfu, Synthesis of montmorillonite-supported nano-zero-valent iron via green tea extract: Enhanced transport and application for hexavalent chromium removal from water and soil, J. Hazard. Mater., 2021, 419, 126461 CrossRef CAS PubMed.
- S. Sohrabnezhad and A. Seifi, The green synthesis of Ag/ZnO in montmorillonite with enhanced photocatalytic activity, Appl. Surf. Sci., 2016, 386, 33–40 CrossRef CAS.
- S. Sohrabnezhad, M. Rassa and A. Seifi, Green synthesis of Ag nanoparticles in montmorillonite, Mater. Lett., 2016, 168, 28–30 CrossRef CAS.
- N. Choudhary, V. K. Yadav, K. K. Yadav, A. I. Almohana, S. F. Almojil and G. Gnanamoorthy, et al., Application of Green Synthesized MMT/Ag Nanocomposite for Removal of Methylene Blue from Aqueous Solution, Water, 2021, 13, 3206 CrossRef CAS.
- M. Honarmand, M. Golmohammadi and A. Naeimi, Green synthesis of SnO2-bentonite nanocomposites for the efficient photodegradation of methylene blue and eriochrome black-T, Mater. Chem. Phys., 2020, 241, 122416 CrossRef CAS.
- K. Sravanthi, D. Ayodhya and P. Y. Swamy, Green synthesis, characterization and catalytic activity of 4-nitrophenol reduction and formation of benzimidazoles using bentonite supported zero valent iron nanoparticles, Mater. Sci. Energy Technol., 2019, 2(2), 298–307 Search PubMed.
- S. M. Sajadi, K. Kolo, S. M. Hamad, S. A. Mahmud, A. A. Barzinjy and S. M. Hussein, Green Synthesis of the Ag/Bentonite Nanocomposite UsingEuphorbia larica Extract: A Reusable Catalyst for Efficient Reduction of Nitro Compounds and Organic Dyes, ChemistrySelect, 2018, 3(43), 12274–12280 CrossRef CAS.
- A. A. Romeh and R. A. Ibrahim Saber, Green nano-phytoremediation and solubility improving agents for the remediation of chlorfenapyr contaminated soil and water, J. Environ. Manage., 2020, 260, 110104 CrossRef CAS PubMed.
- Z. Issaabadi, M. Nasrollahzadeh and S. M. Sajadi, Green synthesis of the copper nanoparticles supported on bentonite and investigation of its catalytic activity, J. Cleaner Prod., 2017, 142, 3584–3591 CrossRef CAS.
- A. Rostami-Vartooni, M. Alizadeh and M. Bagherzadeh, Green synthesis, characterization and catalytic activity of natural bentonite-supported copper nanoparticles for the solvent-free synthesis of 1-substituted 1H-1,2,3,4-tetrazoles and reduction of 4-nitrophenol, Beilstein J. Nanotechnol., 2015, 6, 2300–2309 CrossRef CAS PubMed.
- M. Nasrollahzadeh, S. M. Sajadi, M. Maham and I. Kohsari, Biosynthesis, characterization and catalytic activity of the Pd/bentonite nanocomposite for base- and ligand-free oxidative hydroxylation of phenylboronic acid and reduction of chromium (VI) and nitro compounds, Microporous Mesoporous Mater., 2018, 271, 128–137 CrossRef CAS.
- M. Golmohammadi, M. Honarmand and A. Esmaeili, Biosynthesis of ZnO nanoparticles supported on bentonite and the evaluation of its photocatalytic activity, Mater. Res. Bull., 2022, 149, 111714 CrossRef CAS.
- A. Soliemanzadeh and M. Fekri, Synthesis of clay-supported nanoscale zero-valent iron using green tea extract for the removal of phosphorus from aqueous solutions, Chin. J. Chem. Eng., 2017, 25(7), 924–930 CrossRef CAS.
- A. Soliemanzadeh and M. Fekri, The application of green tea extract to prepare bentonite-supported nanoscale zero-valent iron and its performance on removal of Cr(VI): Effect of relative parameters and soil experiments, Microporous Mesoporous Mater., 2017, 239, 60–69 CrossRef CAS.
- A. K. Hassan, G. Y. Al-Kindi and D. Ghanim, Green synthesis of bentonite-supported iron nanoparticles as a heterogeneous Fenton-like catalyst: Kinetics of decolorization of reactive blue 238 dye, Water Sci. Eng., 2020, 13(4), 286–298 CrossRef.
- R. Abbassi, A. K. Yadav, N. Kumar, S. Huang and P. R. Jaffe, Modeling and optimization of dye removal using “green” clay supported iron nano-particles, Ecol. Eng., 2013, 61, 366–370 CrossRef.
- G. Al Kindi, A. Hassan, D. Yahya and H. Alhaidri, The nanoparticles zero-valent synthesis by black tea extract to remove rb 238 using synthetic and natural wastewater by packed bed reactor, IOP Conf. Ser. Earth Environ. Sci., 2021, 779, 12092 CrossRef.
- A. Rostami-Vartooni, L. Rostami and M. Bagherzadeh, Green synthesis of Fe3O4/bentonite-supported Ag and Pd nanoparticles and investigation of their catalytic activities for the reduction of azo dyes, J. Mater. Sci.: Mater. Electron., 2019, 30(24), 21377–21387 CrossRef CAS.
- G. Gopal, R. KVG, S. M, L. A. Angalene J, N. Chandrasekaran and A. Mukherjee, Green synthesized Fe/Pd and in-situ Bentonite-Fe/Pd composite for efficient tetracycline removal. Journal of Environmental, Chem. Eng., 2020, 8(5), 104126 CAS.
- G. Gopal, C. Natarajan and A. Mukherjee, Synergistic removal of tetracycline and copper (II) by in-situ B-Fe/Ni nanocomposite—A novel and an environmentally sustainable green nanomaterial, Environ. Technol. Innovation, 2022, 25, 102187 CrossRef CAS.
- B. Srikanth, R. Goutham, R. Badri Narayan, A. Ramprasath, K. P. Gopinath and A. R. Sankaranarayanan, Recent advancements in supporting materials for immobilised photocatalytic applications in waste water treatment, J. Environ. Manage., 2017, 200, 60–78 CrossRef CAS PubMed.
- B. Khodadadi, M. Bordbar, A. Yeganeh-Faal and M. Nasrollahzadeh, Green synthesis of Ag nanoparticles/clinoptilolite using Vaccinium macrocarpon fruit extract and its excellent catalytic activity for reduction of organic dyes, J. Alloys Compd., 2017, 719, 82–88 CrossRef CAS.
- A. Hatamifard, M. Nasrollahzadeh and S. M. Sajadi, Biosynthesis, characterization and catalytic activity of an Ag/zeolite nanocomposite for base- and ligand-free oxidative hydroxylation of phenylboronic acid and reduction of a variety of dyes at room temperature, New J. Chem., 2016, 40(3), 2501–2513 RSC.
- M. Bordbar, Z. Sharifi-Zarchi and B. Khodadadi, Green synthesis of copper oxide nanoparticles/clinoptilolite using Rheum palmatum L. root extract: high catalytic activity for reduction of 4-nitro phenol, rhodamine B, and methylene blue, J. Sol-Gel Sci. Technol., 2017, 81(3), 724–733 CrossRef CAS.
- M. Nasrollahzadeh, S. M. Sajadi, M. Maham and H. R. Dasmeh, In situ green synthesis of Cu nanoparticles supported on natural Natrolite zeolite for the reduction of 4-nitrophenol, congo red and methylene blue, IET Nanobiotechnol., 2017, 11(5), 538–545 CrossRef PubMed.
- A. Hatamifard, M. Nasrollahzadeh and J. Lipkowski, Green synthesis of a natrolite zeolite/palladium nanocomposite and its application as a reusable catalyst for the reduction of organic dyes in a very short time, RSC Adv., 2015, 5(111), 91372–91381 RSC.
- Q. Xu, W. Li, L. Ma, D. Cao, G. Owens and Z. Chen, Simultaneous removal of ammonia and phosphate using green synthesized iron oxide nanoparticles dispersed onto zeolite, Sci. Total Environ., 2020, 703, 135002 CrossRef CAS PubMed.
- K. V. G. Ravikumar, G. Debayan, P. Mrudula, N. Chandrasekaran and M. Amitava, In situ formation of bimetallic FeNi nanoparticles on sand through green technology: Application for tetracycline removal, Front. Environ. Sci. Eng., 2019, 14(1), 16 CrossRef.
- S. T. Kadhum, G. Y. Alkindi and T. M. Albayati, Eco friendly adsorbents for removal of phenol from aqueous solution employing nanoparticle zero-valent iron synthesized from modified green tea bio-waste and supported on silty clay, Chin. J. Chem. Eng., 2021, 36, 19–28 CrossRef CAS.
- M. Nasrollahzadeh, E. Mehdipour and M. Maryami, Efficient catalytic reduction of nitroarenes and organic dyes in water by synthesized Ag/diatomite nanocomposite using Alocasia macrorrhiza leaf extract, J. Mater. Sci.: Mater. Electron., 2018, 29(19), 17054–17066 CrossRef CAS.
- Q. Li, G. Zhai, Y. Xu, T. Odoom-Wubah, L. Jia and J. Huang, et al., Diatomite Supported Pt Nanoparticles as Efficient Catalyst for Benzene Removal, Ind. Eng. Chem. Res., 2019, 58(31), 14008–14015 CrossRef CAS.
- A. Rostami-Vartooni, M. Nasrollahzadeh and M. Alizadeh, Green synthesis of perlite supported silver nanoparticles using Hamamelis virginiana leaf extract and investigation of its catalytic activity for the reduction of 4-nitrophenol and Congo red, J. Alloys Compd., 2016, 680, 309–314 CrossRef CAS.
- M. Nasrollahzadeh, S. M. Sajadi, A. Rostami-Vartooni, M. Bagherzadeh and R. Safari, Immobilization of copper nanoparticles on perlite: Green synthesis, characterization and catalytic activity on aqueous reduction of 4-nitrophenol, J. Mol. Catal. A: Chem., 2015, 400, 22–30 CrossRef CAS.
- M. Maryami, M. Nasrollahzadeh, E. Mehdipour and S. M. Sajadi, Green synthesis of the Pd/perlite nanocomposite using Euphorbia neriifolia L. leaf extract and evaluation of its catalytic activity, Sep. Purif. Technol., 2017, 184, 298–307 CrossRef CAS.
- G. Zhang, C. Li, X. Zhang, X. Guo, Y. Liu and W. He, et al., Biogenic synthesis of photocatalytically active ZnS/ESM composites, RSC Adv., 2014, 4(26), 13569–13574 RSC.
- P. Zhang, D. O'Connor, Y. Wang, L. Jiang, T. Xia and L. Wang, et al., A green biochar/iron oxide composite for methylene blue removal, J. Hazard. Mater., 2020, 384, 121286 CrossRef CAS PubMed.
- M. Abdel Salam, M. R. Abukhadra and A. Adlii, Insight into the Adsorption and Photocatalytic Behaviors of an Organo-bentonite/Co(3)O(4) Green Nanocomposite for Malachite Green Synthetic Dye and Cr(VI) Metal Ions: Application and Mechanisms, ACS Omega, 2020, 5(6), 2766–2778 CrossRef CAS PubMed.
- M. Baláž, E. V. Boldyreva, D. Rybin, S. Pavlović, D. Rodríguez-Padrón and T. Mudrinić, et al., State-of-the-Art of Eggshell Waste in Materials Science: Recent Advances in Catalysis, Pharmaceutical Applications, and Mechanochemistry, Front. Bioeng. Biotechnol., 2020, 8, 612567 CrossRef PubMed.
- M. Nasrollahzadeh, S. M. Sajadi and A. Hatamifard, Waste chicken eggshell as a natural valuable resource and environmentally benign support for biosynthesis of catalytically active Cu/eggshell, Fe3O4/eggshell and Cu/Fe3O4/eggshell nanocomposites, Appl. Catal., B, 2016, 191, 209–227 CrossRef CAS.
- M. Honarmand, M. Mirzadeh and M. Honarmand, Green synthesis of SnO(2)-ZnO-eggshell nanocomposites and study of their application in removal of mercury (II) ions from aqueous solution, J. Environ. Health Sci. Eng., 2020, 18(2), 1581–1593 CrossRef CAS PubMed.
- M. Khazaei, A. Khazaei, M. Nasrollahzadeh and M. R. Tahsili, Highly efficient reusable Pd nanoparticles based on eggshell: Green synthesis, characterization and their application in catalytic reduction of variety of organic dyes and ligand-free oxidative hydroxylation of phenylboronic acid at room temperature, Tetrahedron, 2017, 73(38), 5613–5623 CrossRef CAS.
- S. M. Sajadi, K. Kolo, S. M. Abdullah, S. M. Hamad, H. S. Khalid and A. T. Yassein, Green synthesis of highly recyclable CuO/eggshell nanocomposite to efficient removal of aromatic containing compounds and reduction of 4-nitrophenol at room temperature, Surf. Interfaces, 2018, 13, 205–215 CrossRef CAS.
- X. Huang, L. Chang, Y. Lu, Z. Li, Z. Kang and X. Zhang, et al., Plant-mediated synthesis of dual-functional Eggshell/Ag nanocomposites towards catalysis and antibacterial applications, Mater. Sci. Eng., C, 2020, 113, 111015 CrossRef CAS PubMed.
- F. Lashkarizadeh, Green synthesis of ZnO/eggshell nanocomposite using ferulago macrocarpa extract and its photocatalytic and antimicrobial activity in water disinfection, Inorg. Nano-Met. Chem., 2021, 1–12 Search PubMed.
- Q. Wang, C. Ma, J. Tang, C. Zhang and L. Ma, Eggshell Membrane-Templated MnO2 Nanoparticles: Facile Synthesis and Tetracycline Hydrochloride Decontamination, Nanoscale Res. Lett., 2018, 13(1), 255 CrossRef PubMed.
- W. Chang, S. Liu, A. Qileng, W. Liu and Y. Liu, In-situ synthesis of monodispersed Au nanoparticles on eggshell membrane by the extract of Lagerstroemia speciosa leaves for the catalytic reduction of 4-nitrophenol, Mater. Res. Express, 2018, 6(1), 15002 CrossRef.
- M. Rashidi, M. R. Islami and A. M. Tikdari, Green synthesis of Pd nanoparticles supported on modified Nonpareil almond shell using almond hull extract: a beneficial nanocatalyst for convenient reduction of organic dyes, J. Mater. Sci.: Mater. Electron., 2019, 30(19), 18111–18122 CrossRef CAS.
- M. Bordbar, Biosynthesis of Ag/almond shell nanocomposite as a cost-effective and efficient catalyst for degradation of 4-nitrophenol and organic dyes, RSC Adv., 2017, 7(1), 180–189 RSC.
- M. Bordbar and N. Mortazavimanesh, Biosynthesis of waste pistachio shell supported silver nanoparticles for the catalytic reduction processes, IET Nanobiotechnol., 2018, 12(7), 939–945 CrossRef PubMed.
- A. Taghizadeh and K. Rad-Moghadam, Green fabrication of Cu/pistachio shell nanocomposite using Pistacia Vera L. hull: An efficient catalyst for expedient reduction of 4-nitrophenol and organic dyes, J. Cleaner Prod., 2018, 198, 1105–1119 CrossRef CAS.
- B. Khodadadi, Hazelnut shell as a valuable bio-waste support for green synthesis of Ag NPs using Origanum vulgare leaf extract: Catalytic activity for reduction of methyl orange and Congo red, Iran. J. Catal., 2017, 7, 111–119 CAS.
- M. Rashidi and M. R. Islami, Green synthesis of Ag@AgCl/Elaeagnus angustifolia seed nanocomposite using Elaeagnus angustifolia leaves: an amazing nanophotocatalyst with highly photocatalytic activity under sunlight irradiation, Environ. Sci. Pollut. Res., 2020, 27(17), 21455–21467 CrossRef CAS PubMed.
- A. Güngör, I. K. Akbay and T. Özdemir, Waste walnut shell as an alternative bio-based filler for the EPDM: mechanical, thermal, and kinetic studies, J. Mater. Cycles Waste Manage., 2019, 21(1), 145–155 CrossRef.
- R. A. Solano Pizzarro and A. P. Herrera Barros, Cypermethrin elimination using Fe-TiO2 nanoparticles supported on coconut palm spathe in a solar flat plate photoreactor, Adv. Compos. Lett., 2020, 29 DOI:10.1177/2633366X20906164.
- A. Rostami-Vartooni, M. Nasrollahzadeh and M. Alizadeh, Green synthesis of seashell supported silver nanoparticles using Bunium persicum seeds extract: Application of the particles for catalytic reduction of organic dyes, J. Colloid Interface Sci., 2016, 470, 268–275 CrossRef CAS PubMed.
- A. Rostami-Vartooni, Green synthesis of CuO nanoparticles loaded on the seashell surface using Rumex crispus seeds extract and its catalytic applications for reduction of dyes, IET Nanobiotechnol., 2017, 11(4), 349–359 CrossRef PubMed.
- H. Nadaroglu, S. Cicek and A. A. Gungor, Removing Trypan blue dye using nano-Zn modified Luffa sponge, Spectrochim. Acta, Part A, 2017, 172, 2–8 CrossRef CAS PubMed.
- N. Hagemann, K. Spokas, H.-P. Schmidt, R. Kägi, M. A. Böhler and T. D. Bucheli, Activated Carbon, Biochar and Charcoal: Linkages and Synergies across Pyrogenic Carbon's ABCs, Water, 2018, 10, 182 CrossRef.
- A. K. Sakhiya, A. Anand and P. Kaushal, Production, activation, and applications of biochar in recent times, Biochar, 2020, 2(3), 253–285 CrossRef.
- A.-R. Vilchis-Nestor, J. Trujillo-Reyes, J. A. Colín-Molina, V. Sánchez-Mendieta and M. Avalos-Borja, Biogenic silver nanoparticles on carbonaceous material from sewage sludge for degradation of methylene blue in aqueous solution, Int. J. Environ. Sci. Technol., 2014, 11(4), 977–986 CrossRef CAS.
- H. Jing, L. Ji, Z. Wang, J. Guo, S. Lu and J. Sun, et al., Synthesis of ZnO Nanoparticles Loaded on Biochar Derived from Spartina alterniflora with Superior Photocatalytic Degradation Performance, Nanomaterials, 2021, 11, 2479 CrossRef CAS PubMed.
- A. Nayak, B. Bhushan, V. Gupta and S. Kotnala, Fabrication of microwave assisted biogenic magnetite-biochar nanocomposite: A green adsorbent from jackfruit peel for removal and recovery of nutrients in water sample, J. Ind. Eng. Chem., 2021, 100, 134–148 CrossRef CAS.
- Y. Zhang, X. Jiao, N. Liu, J. Lv and Y. Yang, Enhanced removal of aqueous Cr(VI) by a green synthesized nanoscale zero-valent iron supported on oak wood biochar, Chemosphere, 2020, 245, 125542 CrossRef CAS PubMed.
- C.-M. Liu, Z.-H. Diao, W.-Y. Huo, L.-J. Kong and J.-J. Du, Simultaneous removal of Cu2+ and bisphenol A by a novel biochar-supported zero valent iron from aqueous solution: Synthesis, reactivity and mechanism, Environ. Pollut., 2018, 239, 698–705 CrossRef CAS PubMed.
- B. Wang, C. Zhu, D. Ai and Z. Fan, Activation of persulfate by green nano-zero-valent iron-loaded biochar for the removal of p-nitrophenol: Performance, mechanism and variables effects, J. Hazard. Mater., 2021, 417, 126106 CrossRef CAS PubMed.
- S. Batool, A. A. Shah, A. F. Abu Bakar, M. J. Maah and N. K. Abu Bakar, Removal of organochlorine pesticides using zerovalent iron supported on biochar nanocomposite from Nephelium lappaceum (Rambutan) fruit peel waste, Chemosphere, 2022, 289, 133011 CrossRef CAS PubMed.
- A. Ahmad and T. Azam, in 4 - Water Purification Technologies, ed. A. Grumezescu and A. Holban, Woodhead Publishing, 2019, pp. 83–120 Search PubMed.
- K. S. Obayomi, S. Yon Lau, D. Akubuo-Casmir, M. Diekola Yahya, M. Auta and A. S. M. Fazle Bari, et al., Adsorption of endocrine disruptive congo red onto biosynthesized silver nanoparticles loaded on Hildegardia barteri activated carbon, J. Mol. Liq., 2022, 352, 118735 CrossRef CAS.
- S. Begum and M. Ahmaruzzaman, Biogenic synthesis of SnO2/activated carbon nanocomposite and its application as photocatalyst in the degradation of naproxen, Appl. Surf. Sci., 2018, 449, 780–789 CrossRef CAS.
- K. S. Obayomi, A. E. Oluwadiya, S. Y. Lau, A. O. Dada, D. Akubuo-Casmir and T. A. Adelani-Akande, et al., Biosynthesis of Tithonia diversifolia leaf mediated Zinc Oxide Nanoparticles loaded with flamboyant pods (Delonix regia) for the treatment of Methylene Blue Wastewater, Arabian J. Chem., 2021, 14(10), 103363 CrossRef CAS.
- C. R. Galan, M. F. Silva, D. Mantovani, R. Bergamasco and M. F. Vieira, Green synthesis of copper oxide nanoparticles impregnated on activated carbon using Moringa oleifera leaves extract for the removal of nitrates from water, Can. J. Chem. Eng., 2018, 96(11), 2378–2386 CrossRef CAS.
- Z. Oruç, M. Ergüt, D. Uzunoğlu and A. Özer, Green synthesis of biomass-derived activated carbon/Fe-Zn bimetallic nanoparticles from lemon (Citrus limon (L.) Burm. f.) wastes for heterogeneous Fenton-like decolorization of Reactive Red 2. Journal of Environmental, Chem. Eng., 2019, 7(4), 103231 Search PubMed.
- J. Qu, Y. Liu, L. Cheng, Z. Jiang, G. Zhang and F. Deng, et al., Green synthesis of hydrophilic activated carbon supported sulfide nZVI for enhanced Pb(II) scavenging from water: Characterization, kinetics, isotherms and mechanisms, J. Hazard. Mater., 2021, 403, 123607 CrossRef CAS PubMed.
- Y. Rashtbari, S. Afshin, A. Hamzezadeh, M. Abazari, Y. Poureshgh and M. Fazlzadeh, Application of powdered activated carbon coated with zinc oxide nanoparticles prepared using a green synthesis in removal of Reactive Blue 19 and Reactive Black-5: adsorption isotherm and kinetic models, Desalin. Water Treat., 2020, 179, 354–367 CrossRef CAS.
- Y. Rashtbari, S. Afshin, A. Hamzezadeh, A. Gholizadeh, F. J. Ansari and Y. Poureshgh, et al., Green synthesis of zinc oxide nanoparticles loaded on activated carbon prepared from walnut peel extract for the removal of Eosin Y and Erythrosine B dyes from aqueous solution: experimental approaches, kinetics models, and thermodynamic studies, Environ. Sci. Pollut. Res., 2022, 29(4), 5194–5206 CrossRef CAS PubMed.
- Y. Rashtbari, F. Sher, S. Afshin, A. Hamzezadeh, S. Ahmadi and O. Azhar, et al., Green synthesis of zero-valent iron nanoparticles and loading effect on activated carbon for furfural adsorption, Chemosphere, 2022, 287, 132114 CrossRef CAS PubMed.
- K. Chandrika, A. Chaudhary, T. Mareedu, U. Sirisha and M. Vangalapati, Adsorptive removal of acridine orange dye by green tea/copper-activated carbon nanoparticles (Gt/Cu-AC np), Mater. Today: Proc., 2021, 44, 2283–2289 CAS.
- Ş. Karadirek and H. Okkay, Ultrasound assisted green synthesis of silver nanoparticle attached activated carbon for levofloxacin adsorption, J. Taiwan Inst. Chem. Eng., 2019, 105, 39–49 CrossRef.
- A. Taha, M. Ben Aissa and E. Da'na, Green Synthesis of an Activated Carbon-Supported Ag and ZnO Nanocomposite for Photocatalytic Degradation and Its Antibacterial Activities, Molecules, 2020, 25(7), 1586 CrossRef CAS PubMed.
- T. de Mattos Amadio, D. Hotza, J. Batista Rodrigues Neto, M. Blosi, A. L. Costa and M. Dondi, Bentonites functionalized by impregnation with TiO2, Ag, Pd and Au nanoparticles, Appl. Clay Sci., 2017, 146, 1–6 CrossRef.
- M. Melvin, R. Bakri and Y. Yulizar, Green synthesis of gold nanoparticles supported in Al-pillared bentonite using aqueous leaf extracts of Dracaena angustifolia and their use for reductive degradation of methyl orange, J. Phys.: Conf. Ser., 2020, 1442, 12060 CrossRef CAS.
- P. K. Saikia, R. P. Bhattacharjee, P. P. Sarmah, L. Saikia and D. K. Dutta, A green synthesis of Pd nanoparticles supported on modified montmorillonite using aqueous Ocimum sanctum leaf extract: a sustainable catalyst for hydrodechlorination of 4-chlorophenol, RSC Adv., 2016, 6(111), 110011–110018 RSC.
- D.-G. Kim, T. T. Nhung and S.-O. Ko, Enhanced adsorption of heavy metals with biogenic manganese oxide immobilized on zeolite, KSCE J. Civ. Eng., 2016, 20(6), 2189–2196 CrossRef.
- M. Shirkhodaie, M. Hossein Beyki and F. Shemirani, Biogenic synthesis of magnetic perlite@iron oxide composite: application as a green support for dye removal, Desalin. Water Treat., 2016, 57(25), 11859–11871 CrossRef CAS.
- M. Nasrollahzadeh, M. Sajjadi, S. Iravani and R. S. Varma, Green-synthesized nanocatalysts and nanomaterials for water treatment: Current challenges and future perspectives, J. Hazard. Mater., 2021, 401, 123401 CrossRef CAS PubMed.
- B. Savun-Hekimoğlu, Z. Eren and N. H. Ince, Photocatalytic Destruction of Caffeine on Sepiolite-Supported TiO2 Nanocomposite, Sustainability, 2020, 12, 10314 CrossRef.
- S. Li, P. Zhou, W. Zhang, S. Chen and H. Peng, Effective photocatalytic decolorization of methylene blue utilizing ZnO/rectorite nanocomposite under simulated solar irradiation, J. Alloys Compd., 2014, 616, 227–234 CrossRef CAS.
- Q. Liu, H. Peng, X. Tian and J. Guo, Synthesis of chrysotile based nanocomposites for tuning band gap and photocatalytic property, Appl. Clay Sci., 2020, 199, 105885 CrossRef CAS.
- U. A. Khan, J. Liu, J. Pan, H. Ma, S. Zuo and Y. Yu, et al., Fabrication of flower-shaped hierarchical rGO QDs-Bi-Bi2WO6/EP floating photocatalyst: Eminent degradation kinetic under sun-like irradiation, Appl. Surf. Sci., 2019, 484, 341–353 CrossRef CAS.
- A. Quarta, R. M. Novais, S. Bettini, M. Iafisco, R. C. Pullar and C. Piccirillo, A sustainable multi-function biomorphic material for pollution remediation or UV absorption: Aerosol assisted preparation of highly porous ZnO-based materials from cork templates, J. Environ. Chem. Eng., 2019, 7(2), 102936 CrossRef CAS.
- N. H. Mohamad Idris, J. Rajakumar, K. Y. Cheong, B. J. Kennedy, T. Ohno and A. Yamakata, et al., Titanium Dioxide/Polyvinyl Alcohol/Cork Nanocomposite: A Floating Photocatalyst for the Degradation of Methylene Blue under Irradiation of a Visible Light Source, ACS Omega, 2021, 6(22), 14493–14503 CrossRef CAS PubMed.
- H. Pereira, The Rationale behind Cork Properties: A Review of Structure and Chemistry, BioResources, 2015, 10, 6207–6229 CrossRef CAS.
- N. Hossain, M. A. Bhuiyan, B. K. Pramanik, S. Nizamuddin and G. Griffin, Waste materials for wastewater treatment and waste adsorbents for biofuel and cement supplement applications: A critical review, J. Cleaner Prod., 2020, 255, 120261 CrossRef CAS.
- J. Hu, L. Zhao, J. Luo, H. Gong and N. Zhu, A sustainable reuse strategy of converting waste activated sludge into biochar for contaminants removal from water: Modifications, applications and perspectives, J. Hazard. Mater., 2022, 438, 129437 CrossRef CAS PubMed.
- N. Welter, J. Leichtweis, S. Silvestri, P. I. Z. Sánchez, A. C. C. Mejía and E. Carissimi, Preparation of a new green composite based on chitin biochar and ZnFe2O4 for photo-Fenton degradation of Rhodamine B, J. Alloys Compd., 2022, 901, 163758 CrossRef CAS.
- B. Richter, Knowledge and perception of food waste among German consumers, J. Cleaner Prod., 2017, 166, 641–648 CrossRef.
- R. Bushra, S. Mohamad, Y. Alias, Y. Jin and M. Ahmad, Current approaches and methodologies to explore the perceptive adsorption mechanism of dyes on low-cost agricultural waste: A review, Microporous Mesoporous Mater., 2021, 319, 111040 CrossRef CAS.
- D. Nagarajan and S. Venkatanarasimhan, Copper(II) oxide nanoparticles coated cellulose sponge-an effective heterogeneous catalyst for the reduction of toxic organic dyes, Environ. Sci. Pollut. Res., 2019, 26(22), 22958–22970 CrossRef CAS PubMed.
- M. Sboui, M. F. Nsib, A. Rayes, T. Ochiai and A. Houas, Application of solar light for photocatalytic degradation of Congo red by a floating salicylic acid-modified TiO2/palm trunk photocatalyst, C. R. Chim., 2017, 20(2), 181–189 CrossRef CAS.
- Y. Chen, M. Ran, Z. Zhou, X. Han, H. Zhu and J. Gu, Lanthanum/titanium dioxide immobilized onto industrial waste with enhanced photocatalytic activity, and the degradation of dimethyl phthalate, J. Cleaner Prod., 2021, 321, 129014 CrossRef CAS.
- G. Li, Q. Teng, B. Sun, Z. Yang, S. Liu and X. Zhu, Synthesis scaly Ag-TiO2 loaded fly ash magnetic bead particles for treatment of xanthate wastewater, Colloids Surf., A, 2021, 624, 126795 CrossRef CAS.
- L. Gao, W. Gan, S. Xiao, X. Zhan and J. Li, A robust superhydrophobic antibacterial Ag–TiO2 composite film immobilized on wood substrate for photodegradation of phenol under visible-light illumination, Ceram. Int., 2016, 42(2, Part A), 2170–2179 CrossRef CAS.
- B. Avinash, C. R. Ravikumar, M. R. A. Kumar, M. S. Santosh, C. Pratapkumar and H. P. Nagaswarupa, et al., NiO bio-composite materials: Photocatalytic, electrochemical and supercapacitor applications, Appl. Surf. Sci. Adv., 2021, 3, 100049 CrossRef.
- A. T. Adeleye, A. A. Akande, C. K. Odoh, M. Philip, T. T. Fidelis and P. I. Amos, et al., Efficient synthesis of bio-based activated carbon (AC) for catalytic systems: A green and sustainable approach, J. Ind. Eng. Chem., 2021, 96, 59–75 CrossRef CAS.
- Y. Dong, H. Yuan, D. Ge and N. Zhu, A novel conditioning approach for amelioration of sludge dewaterability using activated carbon strengthening electrochemical oxidation and realized mechanism, Water Res., 2022, 220, 118704 CrossRef CAS PubMed.
- W. B. Ayinde, W. M. Gitari, M. Munkombwe, A. Samie and J. A. Smith, Green synthesis of AgMgOnHaP nanoparticles supported on chitosan matrix: Defluoridation and antibacterial effects in groundwater, J. Environ. Chem. Eng., 2020, 8(5), 104026 CrossRef CAS.
- Z. Wang, L. Hu, M. Zhao, L. Dai, D. Hrynsphan and S. Tatsiana, et al., Bamboo charcoal fused with polyurethane foam for efficiently removing organic solvents from wastewater: experimental and simulation, Biochar, 2022, 4(1), 28 CrossRef CAS.
- B. Srikanth, R. Goutham, R. Badri Narayan, A. Ramprasath, K. P. Gopinath and A. R. Sankaranarayanan, Recent advancements in supporting materials for immobilised photocatalytic applications in waste water treatment, J. Environ. Manage., 2017, 200, 60–78 CrossRef CAS PubMed.
- I. H. Alsohaimi, A. M. Nassar, T. A. Seaf Elnasr and B. Cheba, A novel composite silver nanoparticles loaded calcium oxide stemming from egg shell recycling: A potent photocatalytic and antibacterial activities, J. Cleaner Prod., 2020, 248, 119274 CrossRef CAS.
- S. Zahmatkesh, M. Hajiaghaei-Keshteli, A. Bokhari, S. Sundaramurthy, B. Panneerselvam and Y. Rezakhani, Wastewater treatment with nanomaterials for the future: A state-of-the-art review, Environ. Res., 2023, 216, 114652 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |