Open Access Article
Open Access ArticleEnantioselective construction of quaternary stereocenters via organocatalytic arylation of isoxazolin-5-ones with o-quinone diimides†
Ricardo
Torán
 ,
Eduardo
Portillo
,
Amparo
Sanz-Marco
,
Eduardo
Portillo
,
Amparo
Sanz-Marco
 ,
Carlos
Vila
,
Carlos
Vila
 and
Gonzalo
Blay
and
Gonzalo
Blay
 *
*
Departament de Química Orgànica-Facultat de Química, Universitat de València, C/Dr. Moliner 50, 46100-Burjassot, València, Spain. E-mail: gonzalo.blay@uv.es
First published on 24th October 2023
Abstract
A bifunctional squaramide derived from Cinchona alkaloid catalyzes the enantioselective arylation of isoxazolin-5-ones with o-quinone diimides (o-QDIs) to give isoxazolin-5-ones featuring an arylated quaternary stereocenter in high yields and excellent enantioselectivities. To the best of our knowledge, this is the first reported enantioselective arylation of isoxazol-5-ones and the first application of o-QDIs as arylating reagents in asymmetric catalysis.
Introduction
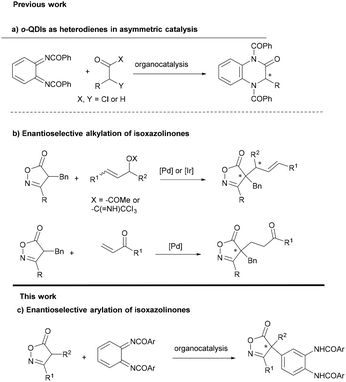
Carbonyl and related compounds featuring an arylated quaternary stereocenter at the α-position represent important structural motifs often found in natural products, drugs and bioactive molecules.1 The construction of these highly congested stereocenters in an enantioselective fashion constitutes a formidable challenge that has garnered increasing attention from synthetic organic chemists.2 With this regard, catalytic enantioselective α-arylation of carbonyl or related compounds appears as a convenient strategy to access optically active α-aryl carbonyl compounds.3 Two major approaches have been devised to reach this goal: (a) the catalytic asymmetric cross-coupling of electrophilic α-halocarbonyl compounds with nucleophilic aryl species and (b) the catalytic enantioselective α-arylation of nucleophilic α-carbonyl enolates with arylating electrophiles. Approach (a) has found success in generating tertiary stereocenters, although its application in constructing quaternary stereocenters has been limited.4 On the other hand, there exists a wide array of reactions enabling the arylation of α,α-disubstituted enolates (approach b). Aryl halides and aryl triflates have been extensively used as arylating reagents under metal catalysis by different authors.5 Nucleophilic aromatic substitution with α-substituted esters has been achieved using nitroaryl fluorides or fluoroarene chromium complexes under PTC conditions,6 while oxindole has been arylated with diaryliodonium salts.7 Finally, quinone derivatives have been employed as electrophilic arylating agents to construct various chiral functionalized compounds under a variety of organocatalytic conditions.8 Despite these remarkable achievements, the asymmetric catalytic arylation of α,α-disubstituted enolates is still to be developed and there are many limitations in terms of available arylating reagents and the scope of enolate precursors that limit the synthetic applicability. Moreover, many of the reported methods require the use of expensive chiral ligands and precious metals and need harsh conditions, evidencing a shortage of metal-free approaches. Therefore, the development of new reactions based on organocatalytic methods with new arylating agents and other weakly acidic carbon pro-nucleophiles is of great interest to synthetic chemists.o-Quinone diimides (o-QDIs) are the diaza analogues of o-quinones, featuring a unique 1,2-diiminocyclohexa-3,5-diene structure. They are synthesized via the oxidation of 1,2-diaminobenzamides. o-QDIs have gained significant atention for their utility as heterodienes in asymmetric formal [4 + 2] cycloaddition reactions9 that are triggered by aromatization (Scheme 1a). However, there are no antecedents in the literature involving the participation of quinone diimides as arylating reagents in asymmetric reactions.10
 | ||
| Scheme 1 Enantioselective reactions with o-QDIs and C–C bond formation at C4 in 4-substituted isoxazolinones. | ||
Isoxazolinones are structural constituents of a wide range of drugs and natural products.11 They are synthetically versatile building blocks for the synthesis of β-amino acids and other heterocycles.12 Despite this, asymmetric methods for the alkylation of isoxazolin-5-ones are scarce, compared with other related five-membered heterocycles.13 This holds particularly true for reactions that produce quaternary stereocenters. Peters described in 2015 the enantioselective alkylation of isoxazol-5-ones with vinyl ketones14 and later two methods of C4-allylation using iridium or palladium catalysis (Scheme 1b).15 Recently, the group of Li and Li have achieved an organocatalytic addition of isoxazolinones to alkynyl iminoesters providing chiral tetrasubstituted α-amino allenoates.16
Herein we report the asymmetric organocatalytic arylation of isoxazolin-5-ones with o-QDIs to provide isoxazol-5-ones bearing an arylated quaternary stereocenter with excellent enantioselectivity (Scheme 1c). To the best of our knowledge this is the first time that o-QDIs are used as arylating reagents17 in enantioselective catalysis and the first enantioselective C4-arylation of isoxazolinones.
Results and discussion
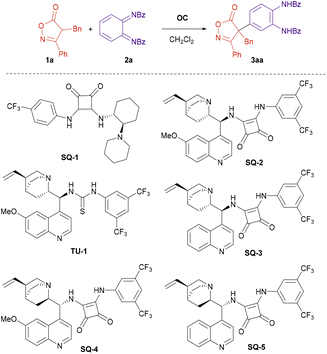
In the onset of our research we tested the reaction of isoxazolinone 1a and diimide 2a in the presence of different organocatalysts (Scheme 2 and Table 1). Cinchona alkaloid-derived organocatalyst SQ-2 gave better results than cyclohexane diamine-derived analogue SQ-1 (Table 1, entry 1 vs. entry 2), and squaramides gave better results than thioureas (Table 1, entry 2 vs. entry 3). Several Cinchona squaramides were tested at 0 °C, SQ-3 derived from cinchonidine providing the best result (Table 1, entry 5). Replacing dichloromethane by toluene resulted in lower yield and enantioselectivity (Table 1, entry 8). On the other hand, lowering the reaction temperature to −20 °C permitted to further increase the ee of compound 3aa to 93% keeping the high yield (Table 1, entry 9). Finally, increasing the concentration had a deleterious effect on the yield and enantioselectivity (Table 1, entry 10).| Entry | OC | Solvent | T (°C) | Yield 3aab (%) | eec (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.11 mmol), 2 (0.10 mmol), OC (0.01 mmol), solvent (10 mL), 3 h. b Yield of 3aa after column chromatography. c Determined by HPLC; different sign indicates opposite enantiomer. d 5 mL of dichloromethane were used. | |||||
| 1 | SQ-1 | CH2Cl2 | rt | 55 | −33 |
| 2 | SQ-2 | CH2Cl2 | rt | 98 | 78 |
| 3 | TU-1 | CH2Cl2 | rt | 83 | 54 |
| 4 | SQ-2 | CH2Cl2 | 0 | 84 | 83 |
| 5 | SQ-3 | CH2Cl2 | 0 | 93 | 90 |
| 6 | SQ-4 | CH2Cl2 | 0 | 79 | −89 |
| 7 | SQ-5 | CH2Cl2 | 0 | 83 | −80 |
| 8 | SQ-3 | Toluene | 0 | 90 | 83 |
| 9 | SQ-3 | CH2Cl2 | −20 | 92 | 93 |
| 10d | SQ-3 | CH2Cl2 | −20 | 90 | 83 |
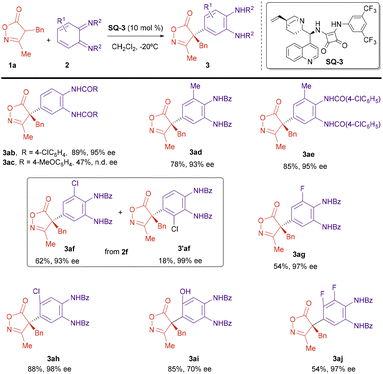
Next, we explored the applicability of the reaction under the optimized conditions. The scope of the isoxazolinone component was first studied (Scheme 3). A number of 3-methylisoxazolones bearing benzyl derivatives attached to C4 reacted with diimide 2a to give the corresponding arylated compounds with high yields and excellent enantiomeric excesses, above 90% (3aa–3ha). Electron-donating (MeO) or electron-withdrawing (Cl) were groups at different positions of the aromatic ring in the benzyl substituents were allowed without a big effect on the enantioselectivity of the reaction. The substituent at C3 of the isoxazolinone was also amenable to variation. Isoxazolinones 1i (R1 = Bn, R2 = Ph) and 1j (R1 = Bn, R2 = cyclopropyl) reacted also with good yields and enantiomeric excesses near 90%. However, introduction of an alkyl (Me) group at C4 provided the reaction products 3ka and 3la with lower ee.
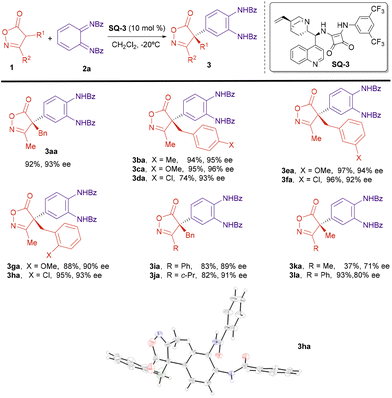 | ||
| Scheme 3 Enantioselective arylation; isoxazolinone scope. Reaction conditions: 1 (0.11 mmol), 2a (0.10 mmol), SQ-3 (0.01 mmol), CH2Cl2 (10 mL), −20 °C, 3 h. Yields of compounds 3 after column chromatography. ee determined by HPLC. X-Ray structure of compound 3ha [Flack parameter 0.042(10)].† | ||
Next, we studied the scope of the diimide 2 (Scheme 4). The benzoic amide group can be substituted by a p-chlorobenzoic amide without affecting yield or enantioselectivity (3ab). A p-methoxybenzoic group is also possible, although in this case the reaction took place with lower yield and the ee could not be determined under any of the chromatographic conditions available (3ac). Next, we studied the effect of substitution on the cyclohexane ring of the diimide 2. A methyl group at position 3 was tolerated and the reaction proceeded regioselectively, with excellent yield and enantioselectivity (3ad and 3ae). However, when an electron-withdrawing Cl group was present at position 3, the reaction with oxazolinone 1a provided a ca. 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of two regioisomers 3af and 3′af with good overall yield and excellent enantioselectivity for both regioisomers (for structure determination of compounds 3ad–3ag and differentiation between regioisomers 3af and 3′af, see ESI†). On the other hand, the reaction with the related fluoro-substituted diimide 2g, provided only one regioisomer 3ag, in moderated yield and excellent enantioselectivity. Diimides substituted at position 4 were also proper reaction partners. Better enantioselectivities were obtained when this substituent was an electronegative halogen (3ah) while a hydroxyl group led to lower enantioselectivity (3ai). Finally, we performed the reaction with the 3,4-difluoride substituted imine 2j that provided the reaction product 3aj with excellent ee, although in moderated yield.
1 mixture of two regioisomers 3af and 3′af with good overall yield and excellent enantioselectivity for both regioisomers (for structure determination of compounds 3ad–3ag and differentiation between regioisomers 3af and 3′af, see ESI†). On the other hand, the reaction with the related fluoro-substituted diimide 2g, provided only one regioisomer 3ag, in moderated yield and excellent enantioselectivity. Diimides substituted at position 4 were also proper reaction partners. Better enantioselectivities were obtained when this substituent was an electronegative halogen (3ah) while a hydroxyl group led to lower enantioselectivity (3ai). Finally, we performed the reaction with the 3,4-difluoride substituted imine 2j that provided the reaction product 3aj with excellent ee, although in moderated yield.
To show the robustness of the method, the reaction of 1a with 2a was performed at 1 mmol scale to provide the expected product 3aa with excellent yield and identical ee to that obtained in the reaction at 0.1 mmol scale (Scheme 5a). Next, the synthetic potential of the resulting products was shown by performing some chemical transformations (Scheme 5b). Thus, the carbonyl group of the isoxazolinone moiety in 3ab was selectively reduced upon treatment with sodium borohydride to give an 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 diastereomeric mixture of the corresponding lactol,16 which was acetylated to give compound 4. Transformation of the isoxazolinone ring into a pyrazolone ring 5 could be achieved by treatment with hydrazine in good yield.18 Finally, the N,N‘-bis(benzoyl)phenylene-1,2-diamine moiety was converted into benzoimidazole ring 6 upon cyclization in the presence of p-TsOH.19
13 diastereomeric mixture of the corresponding lactol,16 which was acetylated to give compound 4. Transformation of the isoxazolinone ring into a pyrazolone ring 5 could be achieved by treatment with hydrazine in good yield.18 Finally, the N,N‘-bis(benzoyl)phenylene-1,2-diamine moiety was converted into benzoimidazole ring 6 upon cyclization in the presence of p-TsOH.19
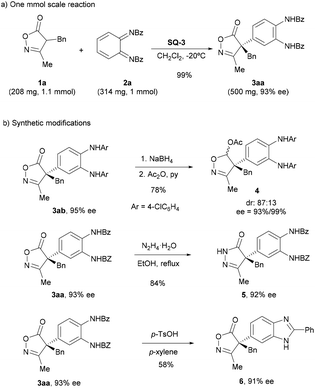 | ||
| Scheme 5 Synthesis of compound 3aa at 1 mmol scale and examples of synthetic transformations of compounds 3. | ||
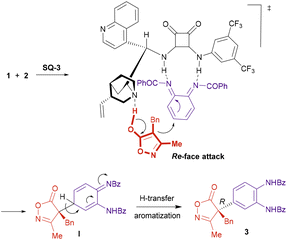
Scheme 6 illustrates a plausible mechanism and a stereochemical model for the arylation reaction. The bifunctional catalyst would be responsible for both the activation of the reactants and their orientation in the space. The isoxazolinone would be deprotonated by the tertiary amine of the catalyst, while the quinone diimide would be electrophilically activated through the formation of hydrogen bonds with the squaramide moiety. This arrangement would promote the conjugate addition of the isoxazolinone enolate from its Re-face to one of the C–C double bonds in the quinone diimide, resulting in the formation of intermediate I. Subsequent proton transfer and concomitant rearomatization would provide compound 3 with the observed R configuration at the quaternary stereocenter.
Conclusions
In conclusion, we have shown by the first time the application of o-QDIs as arylating reagents in asymmetric catalysis. A bifunctional squaramide catalyst derived from Cinchona alkaloid allowed the enantioselective arylation of isoxazolin-5-ones providing the corresponding products featuring an arylated quaternary stereocenter in high yields and excellent enantioselectivities for a broad range of substrates. To the best of our knowledge, this is the first reported enantioselective arylation of isoxazol-5-ones. The reaction can be scaled up and the synthetic potential has been shown by different synthetic transformations.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Grant PID2020-116944GB-100 funded by MCIN/AEI/10.13039/501100011033 and by the “European Union Next Generation EU/PRTR”. Grant CIAICO/2021/147 funded by Conselleria d'Innovació, Universitats, Ciència i Societat Digital. “Atracció de Talent” pre-doctoral contract to R. T. funded by the Universitat de València. Access to NMR and MS facilities from the Servei Central de Suport a la Investigació Experimental (SCSIE–UV) and Nanbiosis.References
- (a) A. B. Richon, M. E. Maragoudakis and J. S. Wasvary, Isoxazolidine-3,5-diones as lens aldose reductase inhibitors, J. Med. Chem., 1982, 25, 745 CrossRef CAS PubMed; (b) H. Koga, H. Sato, T. Dan and B. Aoki, Studies on uricosuric diuretics. 4. Three-dimensional structure-activity relationships and receptor mapping of (aryloxy)acetic acid diuretics, J. Med. Chem., 1991, 34, 2702 CrossRef CAS PubMed; (c) M. A. Mroue, K. L. Euler, M. A. Ghuman and M. Alam, Indole alkaloids of Haplophyton crooksii, J. Nat. Prod., 1996, 59, 890 CrossRef CAS; (d) D. Katayev and E. P. Kündig, Catalytic Enantioselective Synthesis of a 3-Aryl-3-benzyloxindole (= 3-Aryl-3-benzyl-1,3-dihydro-2H-indol-2-one) Exhibiting Antitumor Activity, Helv. Chim. Acta, 2012, 95, 2287 CrossRef CAS.
- Reviews: (a) L. Susse and B. M. Stoltz, Enantioselective Formation of Quaternary Centers by Allylic Alkylation with First Row Transition Metal Catalysts, Chem. Rev., 2021, 121, 4084 CrossRef CAS PubMed; (b) Z. Wang, Construction of all-carbon quaternary stereocenters by catalytic asymmetric conjugate addition to cyclic enones in natural product synthesis, Org. Chem. Front., 2020, 7, 3815 RSC; (c) E. V. Prusov, Construction of Quaternary Stereogenic Centers in the Total Synthesis of Natural Products, Angew. Chem., Int. Ed., 2017, 56, 14356 CrossRef CAS PubMed; (d) Y. Liu, S.-J. Han, W.-B. Liu and B. M. Stoltz, Catalytic Enantioselective Construction of Quaternary Stereocenters: Assembly of Key Building Blocks for the Synthesis of Biologically Active Molecules, Acc. Chem. Res., 2015, 48, 740 CrossRef CAS PubMed; (e) Y. Minko and I. Marek, Stereodefined acyclic trisubstituted metal enolates towards the asymmetric formation of quaternary carbon stereocentres, Chem. Commun., 2014, 50, 12597 RSC; (f) M. Bella and T. Gasperi, Organocatalytic formation of quaternary stereocenters, Synthesis, 2009, 1583 CrossRef CAS; (g) Quaternary Stereocenters: Challenges and Solutions for Organic Synthesis, ed. J. Christoffers and A. Baro, Wiley-VCH, Weinheim, Germany, 2006 Search PubMed.
- For an excellent review see: Y.-J. Hao, X.-S. Hu, Y. Zhou, J. Zhou and J.-S. Yu, Catalytic Enantioselective α-Arylation of Carbonyl Enolates and Related Compounds, ACS Catal., 2020, 10, 955 CrossRef CAS.
- (a) Friedel–Crafts reaction: J. Zhou, Z.-H. Li, L. Wang, J.-C. Kang, X.-H. Wang and S.-Y. Zhang, Base-Promoted Cobalt-Catalyzed Regio- and Enantioselective para-Friedel-Crafts Alkylation of Aniline Derivatives, Org. Lett., 2021, 23, 9353 CrossRef CAS PubMed; (b) Boronic esters: F.-L. Wang, L. Liu, C.-J. Yang, C. Luan, J. Yang, J.-J. Chen, Q.-S. Gu, Z.-L. Li and X.-Y. Liu, Synthesis of α-Quaternary β-Lactams via Copper-Catalyzed Enantioconvergent Radical C(sp3)-C(sp2) Cross-Coupling with Organoboronate Esters, Angew. Chem., Int. Ed., 2023, 62, e202214709 CrossRef CAS PubMed; (c) Aryl tin reagents: Y. Liang and G. C. Fu, Catalytic Asymmetric Synthesis of Tertiary Alkyl Fluorides: Negishi Cross-Couplings of Racemic α,α-Dihaloketones, J. Am. Chem. Soc., 2014, 136, 5520 CrossRef CAS PubMed; (d) Grignard reagents:, H. Yin and G. C. Fu, Mechanistic Investigation of Enantioconvergent Kumada Reactions of Racemic α-Bromoketones Catalyzed by a Nickel/Bis(oxazoline) Complex, J. Am. Chem. Soc., 2019, 141, 15433 CrossRef PubMed; (e) For an α-arylation of α-diazoesters see: X.-S. Liu, Z. Tang, Z.-Y. Si, Z. Zhang, L. Zhao and L. Liu, Enantioselective para-C(sp2)-H Functionalization of Alkyl Benzene Derivatives via Cooperative Catalysis of Gold/Chiral Bronsted Acid, Angew. Chem., Int. Ed., 2022, 61, e202208874 CrossRef CAS PubMed.
- Pioneering and selected examples: (a) T. Hamada, A. Chieffi, J. Åhman and S. L. Buchwald, An Improved Catalyst for the Asymmetric Arylation of Ketone Enolates, J. Am. Chem. Soc., 2002, 124, 1261 CrossRef CAS PubMed; (b) G. Chen, F. Y. Kwong, H. O. Chan, W.-Y. Yu and A. S. C. Chan, Nickel-catalyzed asymmetric α-arylation of ketone enolates, Chem. Commun., 2006, 1413 RSC; (c) X. Liao, Z. Weng and J. F. Hartwig, Enantioselective α-Arylation of Ketones with Aryl Triflates Catalyzed by Difluorphos Complexes of Palladium and Nickel, J. Am. Chem. Soc., 2008, 130, 195 CrossRef CAS PubMed; (d) J. Cornella, E. P. Jackson and R. Martin, Nickel-Catalyzed Enantioselective C-C Bond Formation through CSP2-O Cleavage in Aryl Esters, Angew. Chem., Int. Ed., 2015, 54, 4075 CrossRef CAS PubMed; (e) A. Ghosh, J. A. Walker Jr., A. Ellern and L. M. Stanley, Coupling Catalytic Alkene Hydroacylation and α-Arylation: Enantioselective Synthesis of Heterocyclic Ketones with α-Chiral Quaternary Stereocenters, ACS Catal., 2016, 6, 2673 CrossRef CAS; (f) X. Rao, N. Li, H. Bai, C. Dai, Z. Wang and W. Tang, Efficient Synthesis of (−)-Corynoline by Enantioselective Palladium-Catalyzed α-Arylation with Sterically Hindered Substrates, Angew. Chem., Int. Ed., 2018, 57, 12328 CrossRef CAS PubMed; (g) X. Xie, Y. Chen and D. Ma, Enantioselective Arylation of 2-Methylacetoacetates Catalyzed by CuI/trans-4-Hydroxy-L-proline at Low Reaction Temperatures, J. Am. Chem. Soc., 2006, 128, 16050 CrossRef CAS PubMed; (h) E. P. Kündig, T. M. Seidel, Y. X. Jia and G. Bernardinelli, Bulky chiral carbene ligands and their application in the palladium-catalyzed asymmetric intramolecular α-arylation of amides, Angew. Chem., Int. Ed., 2007, 46, 8484 CrossRef PubMed; (i) C. I. Jette, I. Geibel, S. Bachman, M. Hayashi, S. Sakurai, H. Shimizu, J. B. Morgan and B. M. Stoltz, Palladium-Catalyzed Construction of Quaternary Stereocenters by Enantioselective Arylation of γ-Lactams with Aryl Chlorides and Bromides, Angew. Chem., Int. Ed., 2019, 58, 4297 CrossRef CAS PubMed.
- (a) M. Bella, S. Kobbelgaard and K. A. Jørgensen, Organocatalytic Regio- and Asymmetric C-Selective SNAr Reactions-Stereoselective Synthesis of Optically Active Spiro-pyrrolidone-3,3′-oxoindoles, J. Am. Chem. Soc., 2005, 127, 3670 CrossRef CAS PubMed; (b) S. Shirakawa, K. Koga, T. Tokuda, K. Yamamoto and K. Maruoka, Catalytic asymmetric synthesis of 3,3′-diaryloxindoles as triarylmethanes with a chiral all-carbon quaternary center: Phase-transfer-catalyzed SNAr reaction, Angew. Chem., Int. Ed., 2014, 53, 6220 CrossRef CAS PubMed; (c) S. Shirakawa, K. Yamamoto and K. Maruoka, Phase-Transfer-Catalyzed Asymmetric SNAr Reaction of α-Amino Acid Derivatives with Arene Chromium Complexes, Angew. Chem., Int. Ed., 2015, 54, 838 CrossRef CAS PubMed.
- J. Guo, S. Dong, Y. Zhang, Y. Kuang, X. Liu, L. Lin and X. Feng, Chiral Scandium(III)-Catalyzed Enantioselective α-Arylation of N-Unprotected 3-Substituted Oxindoles with Diaryliodonium Salts, Angew. Chem., Int. Ed., 2013, 52, 10245 CrossRef CAS.
- (a) J. Alemán, B. Richter and K. A. Jørgensen, Organocatalytic highly enantioselective α-arylation of β-ketoesters, Angew. Chem., Int. Ed., 2007, 46, 5515 CrossRef PubMed; (b) W.-Y. Siau, W. Li, F. Xue, Q. Ren, M. Wu, S. Sun and H. Guo, Catalytic and Enantioselective α-Functionalization of Oxindoles Through Oxidative Reactions with Naphthoquinones, Chem. – Eur. J., 2012, 18, 9491 CrossRef CAS PubMed; (c) J.-S. Yu, F. Zhou, Y.-L. Liu and J. Zhou, Organocatalytic asymmetric Michael addition of unprotected 3-substituted oxindoles to 1,4-naphthoquinone, Beilstein J. Org. Chem., 2012, 8, 1360 CrossRef CAS PubMed; (d) G. Li, W. Sun, J. Li, F. Jia, L. Hong and R. Wang, Organocatalytic enantioselective formal arylation of azlactones using quinones as the aromatic partner, Chem. Commun., 2015, 51, 11280 RSC; (e) Y. Liu, J. Li, X. Ye, X. Zhao and Z. Jiang, Organocatalytic asymmetric formal arylation of benzofuran-2(3H)-ones with cooperative visible light photocatalysis, Chem. Commun., 2016, 52, 13955 RSC; (f) G. Zhu, G. Bao, Y. Li, J. Yang, W. Sun, J. Li and R. Hong, Chiral Phosphoric Acid Catalyzed Asymmetric Oxidative Dearomatization of Naphthols with Quinones, Org. Lett., 2016, 18, 5288 CrossRef CAS PubMed.
- (a) C. J. Abraham, D. H. Paull, M. T. Scerba, J. W. Grebinski and T. Lectka, Catalytic, Enantioselective Bifunctional Inverse Electron Demand Hetero-Diels-Alder Reactions of Ketene Enolates and o-Benzoquinone Diimides, J. Am. Chem. Soc., 2006, 128, 13370 CrossRef CAS PubMed; (b) R. Huang, X. Chen, C. Mou, G. Luo, Y. Li, X. Li, W. Xue, Z. Jin and Y. R. Chi, Carbene-Catalyzed α-Carbon Amination of Chloroaldehydes for Enantioselective Access to Dihydroquinoxaline Derivatives, Org. Lett., 2019, 21, 4340 CrossRef CAS PubMed; (c) S.-L. Zhou, J.-L. Li, L. Dong and Y.-C. Chen, Organocatalytic Sequential Hetero-Diels-Alder and Friedel-Crafts Reaction: Constructions of Fused Heterocycles with Scaffold Diversity, Org. Lett., 2011, 13, 5874 CrossRef CAS.
- Two examples of allylation reactions of o-QDIs using allyltri-n-butylstannane or allyltri-n-butylstannanes have been reported under achiral Lewis acid catalysis: (a) V. Nair, R. Dhanya, N. Vidya and S. Devipriya, Lewis acid catalyzed addition of allylsilane to o-quinonediimides: formal Diels-Alder reaction versus allylation, Synthesis, 2006, 107 CrossRef CAS; (b) V. Nair, R. Dhanya, C. Rajesh, M. M. Bhadbhade and M. Manoj, Lewis Acid-Promoted Annulation of o-Quinonediimines by Allylstannane: A Facile Synthesis of Quinoxaline Derivatives, Org. Lett., 2004, 6, 4743 CrossRef CAS.
- (a) P. Rozan, Y.-H. Kuo and F. Lambein, Amino acids in seeds and seedlings of the genus Lens, Phytochemistry, 2001, 58, 281 CrossRef CAS; (b) E. Hedner, M. Sjögren, S. Hodzic, R. Andersson, U. Göransson, P. R. Jonsson and L. Bohlin, Antifouling Activity of a Dibrominated Cyclopeptide from the Marine Sponge Geodia barretti, J. Nat. Prod., 2008, 71, 330 CrossRef CAS PubMed; (c) S. S. Mahajan, M. Scian, S. Sripathy, J. Posakony, U. Lao, T. K. Loe, V. Leko, A. Thalhofer, A. D. Schuler, A. Bedalov and J. A. Simon, Development of Pyrazolone and Isoxazol-5-one Cambinol Analogues as Sirtuin Inhibitors, J. Med. Chem., 2014, 57, 3283 CrossRef CAS PubMed; (d) B. Kafle, N. G. Aher, D. Khadka, H. Park and H. Cho, Isoxazol-5(4H)one Derivatives as PTP1B Inhibitors Showing an Anti-Obesity Effect, Chem. – Asian J., 2011, 6, 2073 CrossRef CAS PubMed; (e) C. Vergelli, I. A. Schepetkin, L. Crocetti, A. Iacovone, M. P. Giovannoni, G. Guerrini, A. I. Khlebnikov, S. Ciattini, G. Ciciani and M. T. Quinn, Isoxazol-5(2H)-one: a new scaffold for potent human neutrophil elastase (HNE) inhibitors, J. Enzyme Inhib. Med. Chem., 2017, 32, 821 CrossRef CAS PubMed.
- (a) A review: A. F. da Silva, A. A. G. Fernandes, S. Thurow, M. L. Stivanin and I. D. Jurberg, Isoxazol-5-ones as Strategic Building Blocks in Organic Synthesis, Synthesis, 2018, 2473 CAS; (b) M. L. Stivanin, M. Duarte, C. Sartori, N. M. R. Capreti, C. F. F. Angolini and I. D. Jurberg, An Aminocatalyzed Michael Addition/Iron-Mediated Decarboxylative Cyclization Sequence for the Preparation of 2,3,4,6-Tetrasubstituted Pyridines: Scope and Mechanistic Insights, J. Org. Chem., 2017, 82, 10319 CrossRef CAS; (c) N. Wannenmacher, C. Pfeffer, W. Frey and R. Peters, Enantioenriched γ-Aminoalcohols, β-Amino Acids, β-Lactams and Azetidines Featuring Tetrasubstituted Fluorinated Stereocenters via Palladacycle-Catalyzed Asymmetric Fluorination of Isoxazolinones, J. Org. Chem., 2022, 87, 670 CrossRef CAS PubMed.
- Enantioselective N-alkylation: (a) R. Torán, C. Vila, A. Sanz-Marco, M. C. Muñoz, J. R. Pedro and G. Blay, Organocatalytic Enantioselective 1,6-aza-Michael Addition of Isoxazolin-5-ones to p-Quinone Methides, Eur. J. Org. Chem., 2020, 627 CrossRef; (b) S.-S. Qi, Z.-H. Jiang, M.-M. Chu, Y.-F. Wang, X.-Y. Chen, W.-Z. Ju and D.-Q. Xu, Regioselective catalytic asymmetric N-alkylation of isoxazol-5-ones with para-quinone methides, Org. Biomol. Chem., 2020, 18, 2398 RSC; (c) F. Li, S. Liang, Y. Luan, X. Chen, H. Zhao, A. Huang, P. Li and W. Li, Organocatalytic regio-, diastereo- and enantioselective γ-additions of isoxazol-5(4H)-ones to β,γ-alkynyl-α-imino esters for the synthesis of axially chiral tetrasubstituted α-amino allenoates, Org. Chem. Front., 2021, 8, 1243 RSC. Enantioselective C-alkylation: (d) R. Torán, D. Puchan, A. Sanz-Marco, C. Vila, J. R. Pedro and G. Blay, Organocatalytic enantioselective Mannich reaction of isoxazol-5(4H)-ones to isatin-derived ketimines, Org. Biomol. Chem., 2022, 20, 8395 RSC; (e) W.-T. Meng, Y. Zheng, J. Nie, H.-Y. Xiong and J.-A. Ma, Organocatalytic asymmetric one-pot sequential conjugate addition/dearomative fluorination: synthesis of chiral fluorinated isoxazol-5(4H)-ones, J. Org. Chem., 2013, 78, 559 CrossRef CAS PubMed.
- T. Hellmuth, W. Frey and R. Peters, Regioselective Catalytic Asymmetric C-Alkylation of Isoxazolinones by a Base-Free Palladacycle-Catalyzed Direct 1,4-Addition, Angew. Chem., Int. Ed., 2015, 54, 2788 CrossRef CAS PubMed.
- (a) S. Rieckhoff, J. Meisner, J. Kästner, W. Frey and R. Peters, Double Regioselective Asymmetric C-Allylation of Isoxazolinones: Iridium-Catalyzed N-Allylation Followed by an Aza-Cope Rearrangement, Angew. Chem., Int. Ed., 2018, 57, 1404 CrossRef CAS PubMed; (b) X. Yu, L. Hu, W. Frey, G. Lu and R. Peters, Stereoretentive Regio- and Enantioselective Allylation of Isoxazolinones by a Planar Chiral Palladacycle Catalyst, Angew. Chem., Int. Ed., 2022, 61, e202210145 CrossRef CAS PubMed.
- F. Li, S. Liang, Y. Luan, X. Chen, H. Zhao, A. Huang, P. Li and W. Li, Organocatalytic regio-, diastereo- and enantioselective γ-additions of isoxazol-5(4H)-ones to β,γ-alkynyl-α-imino esters for the synthesis of axially chiral tetrasubstituted α-amino allenoates, Org. Chem. Front., 2021, 8, 1243 RSC.
- Recently, we have reported the non enantioselective arylation of isocyanoacetate esters with o-QDIs: A. Laviós, P. Martínez-Pardo, A. Sanz-Marco, C. Vila, J. R. Pedro and G. Blay, Synthesis of α,α-Diaryl-α-amino Acid Precursors by Reaction of Isocyanoacetate Esters with o-Quinone Diimides, Org. Lett., 2023, 25, 5608 CrossRef.
- T. Zhao, H. Zhang, L. Cui, C. Wang, J. Qu and B. Wang, A ZnCl2-Catalyzed Knoevenagel Condensation/1,5-Hydride Shift/Cyclization Sequence: Synthesis of Novel Spiroisoxazol-5-one Tetrahydroquinolines, ChemistrySelect, 2016, 1, 3713 CrossRef CAS.
- M. R. DeLuca and S. M. Kerwin, The para-toluenesulfonic acid-promoted synthesis of 2-substituted benzoxazoles and benzimidazoles from diacylated precursors, Tetrahedron, 1997, 53, 457 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2292242 (for 3ha). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qo01600e |
| This journal is © the Partner Organisations 2023 |