Open Access Article
Open Access ArticleEco-friendly synthesis of carbon nanotubes and their cancer theranostic applications
Ebrahim
Mostafavi
 *ab,
Siavash
Iravani
*ab,
Siavash
Iravani
 *c,
Rajender S.
Varma
*c,
Rajender S.
Varma
 d,
Mehrdad
Khatami
ef and
Fatemeh
Rahbarizadeh
d,
Mehrdad
Khatami
ef and
Fatemeh
Rahbarizadeh
 f
f
aStanford Cardiovascular Institute, Stanford University School of Medicine, CA 94305, USA. E-mail: ebimsv@stanford.edu; ebi.mostafavi@gmail.com
bDepartment of Medicine, Stanford University School of Medicine, Stanford, CA 94305, USA
cFaculty of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences, 81746-73461, Isfahan, Iran. E-mail: siavashira@gmail.com
dRegional Centre of Advanced Technologies and Materials, Czech Advanced Technology and Research Institute, Palacky University in Olomouc, Slechtitelu 27, 783 71, Olomouc, Czech Republic
eNon-communicable Diseases Research Center, Bam University of Medical Sciences, Bam, Iran
fDepartment of Medical Biotechnology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
First published on 16th May 2022
Abstract
Carbon nanotubes (CNTs) with attractive physicochemical characteristics such as high surface area, mechanical strength, functionality, and electrical/thermal conductivity have been widely studied in different fields of science. However, the preparation of these nanostructures on a large scale is either expensive or sometimes ecologically unfriendly. In this context, plenty of studies have been conducted to discover innovative methods to fabricate CNTs in an eco-friendly and inexpensive manner. CNTs have been synthesized using various natural hydrocarbon precursors, including plant extracts (e.g., tea-tree extract), essential oils (e.g., eucalyptus and sunflower oil), biodiesel, milk, honey, and eggs, among others. Additionally, agricultural bio-wastes have been widely studied for synthesizing CNTs. Researchers should embrace the usage of natural and renewable precursors as well as greener methods to produce various types of CNTs in large quantities with the advantages of cost-effectiveness and environmentally benign features. In addition, multifunctionalized CNTs with improved biocompatibility and targeting features are promising candidates for cancer theranostic applications owing to their attractive optical, chemical, thermal, and electrical properties. This perspective discusses the recent developments in eco-friendly synthesis of CNTs using green chemistry-based techniques, natural renewable resources, and sustainable catalysts, with emphasis on important challenges and future perspectives and highlighting techniques for the functionalization or modification of CNTs. Significant and promising cancer theranostic applications as well as their biocompatibility and cytotoxicity issues are also discussed.
1. Introduction
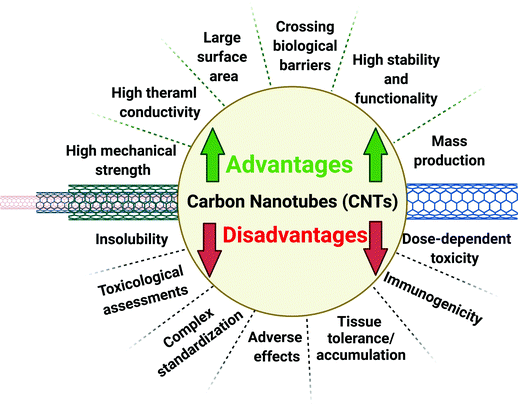
Single-walled (SW) and multi-walled (MW) carbon nanotubes (CNTs) are two main categories of CNTs, based on the number of graphene sheets comprising the cylindrical tubes. These cylindrical nanostructures can be employed for various pharmaceutical and biomedical applications owing to their distinct physicochemical properties and multiple functionalization capabilities (Fig. 1). CNTs exhibit attractive interfacial area with cellular membrane, multifunctional surfaces, and needle-like shapes, making them promising candidates for delivery of therapeutic and diagnostic agents.1,2 In this context, various natural resources and (bio)renewable materials such as plant biomass or animal bioproducts can be employed for the fabrication of nanostructures like CNTs.3 However, more explorations are still needed on green chemistry-based solutions to reduce or discontinue the use of toxic and harmful agents with environmental and human hazards. The greener synthesis and functionalization of CNT-based nanostructures are in their infancy stages, and some challenges such as optimization of conditions, biocompatibility improvements, (bio)renewability, biodegradation, pharmacokinetics, isolation/purification, toxicity/biosafety, and in vivo/in vitro analyses need to be systematically studied to obtain CNTs with higher purity standards and up-scalable potentials;4–7 these may be equally applicable to other carbon-based nanostructures such as graphene, graphene oxide, fullerenes, and nanodiamonds, among others.8,9 The standardization procedures are vital in transforming lab-scale into industrial and up-scalable production, taking into account environmentally friendly aspects and green chemistry principles.CNTs have been synthesized using assorted techniques like laser-ablation, spray pyrolysis, chemical vapor deposition, carbon arc discharge, and flame synthesis, among others.10 These synthesis methods have some limitations and drawbacks, such as dependence on expensive equipment, chemical catalysts, or high energy consumption, and inclusion of toxic chemical impurities in the ensuing CNTs.11,12 On the other hand, the application of greener techniques for the fabrication of CNTs with suitable properties has been explored to avoid the deployment of toxic/hazardous chemical materials and complicated instruments. Researchers have studied the utilization of low-cost and sustainable raw agricultural materials and biowastes to synthesize carbon-based nanostructures via simple and greener methods by preventing further pollutions/contaminants. For environmentally benign synthesis of CNTs with different sizes and structures, sustainable and natural carbon feedstocks/resources, sustainable catalysts, eco-friendly synthesis techniques should be applied, and purification procedures and gas emissions need to be within the tenets of the green chemistry principles.13
CNTs with good penetrability, nano-needle shapes, hollow monolithic structures, optico-electrical properties, and high drug loading capacity have shown promising applicability for targeted cancer therapy, photothermal ablation, and drug/gene delivery.14,15 These nanostructures with their unique sizes and morphologies as well as their high stability can be considered as attractive non-polymeric candidates for cancer nanotheranostics and anticancer nanocarriers applications.16,17 Also, their ability to easily penetrate cell membranes has provided an opportunity to consider them as efficient nanocarriers for drug delivery systems and theranostics.18 CNTs can effectively cross the biological barriers, allowing their applications in the delivery of therapeutically active molecules. The interaction between CNTs and cells is one of the important factors for evaluating their future biomedical potentials. Functionalized CNTs have exhibited a great potential to be taken up by a wide range of cells and can intracellularly traffic via different cellular barriers.19 Besides, unique mechanical, thermal, optical, and electric features such as good heat conduction, tensile strength, and flexibility make CNTs different from the other carbon-based nanomaterials.20 CNTs have also been explored for designing a variety of magnetic resonance imaging (MRI) contrast agents via the incorporation of gadolinium chelates.21 These carbon nanomaterials can also serve in cell imaging due to fluorescence in the near-infrared region; they do not affect the viability of the diagnosed cells. The strong absorption of CNTs in near-infrared regions can enable their additional deployments in photothermal therapy.22 These nanomaterials can transform the laser energy to acoustic signals and show excellent resonant Raman scattering and photoluminescence in near-infrared region, offering great opportunities in cancer diagnostics.23 One of the main differences between SWCNTs and MWCNTs is the suitable electrical features of the former; the properties of MWCNTs mainly depend on the number of layers.21 However, some disadvantages/limitations namely the poor solubility and dispersibility of CNTs need to be surmounted for their biological and biomedical applications.24,25
Surface multifunctionalization or modification tactics have been studied for improving the solubility/dispersibility and biocompatibility of CNTs.26–28 Thus, the biocompatibility of CNTs can be improved and their toxicity can be reduced by applying various functional groups via covalent or non-covalent bindings;29 the covalent functionalization create strong binding of groups with suitable biocompatibility on the surface of CNTs.30,31 Herein, recent advances on the synthesis of various types of CNTs have been covered with a focus on eco-friendly and sustainable synthesis techniques, natural renewable feedstocks, and green catalysts. The functionalization or modification of CNTs based on eco-friendly protocols are discussed as well as recent advances related to their cancer theranostic applications, focusing on important challenges and future perspectives encompassing biocompatibility and toxicity issues. The importance of sustainable methods with the explicit reduction in the usage of hazardous chemical agents and biocompatibility enhancement of ensued products is felt more than before. In the general domain of CNTs, however, there is a long way to go before realizing the benefits of greener synthesis and functionalization of these nanostructures.
2. Eco-friendly synthesis and functionalization
Greener synthesis and functionalization techniques can minimize the deployment of hazardous/toxic agents and expensive and rare materials/equipment to avoid adverse environmental impacts.32 Catalytic procedure has been broadly studied for increasing the yield of production and reducing the reaction temperature and time.33 The chemical vapor deposition technique with large-scale production potentials has been typically initiated by decomposition of hydrocarbon gases or organic solvents, when the nano-sized catalysts are generated in a heated furnace. However, this technique may suffer from high energy consumption and toxic/hazardous chemicals utilization; several techniques such as electrostatic spray-assisted or combustion chemical vapor deposition have been studied wherein safer reagents can be utilized. Via the optimization of the reaction conditions and applying renewable precursors/catalysts, different types of CNTs with unique properties can be achieved.342.1. Microwave (MW)-assisted synthesis
Several techniques such as conventional pyrolysis, hydrothermal carbonization pretreatment, cyclic oxidation, combustion method, and chemical vapor deposition have been reported for the synthesis of carbon materials from biomass, but are beleaguered by some noticeable disadvantages of high temperature usage, complex processes/intricacy of scale-up, low yield/efficiency, problems in process control, and likelihood of contaminations and emission of air pollutants during the synthesis procedure.35–38 On the other hand, the safer synthesis of CNTs, and the exact growth mechanisms of these ensued nanostructures from natural precursors/catalysts have been not entirely and analytically investigated.39,40 Microwave (MW)-assisted production approaches with mass production capabilities can be deployed for the assembly of CNTs with added benefits of lower cost, fast reaction time, simple/time-saving purification, and eco-friendliness;41 MW plasma chemical vapor deposition technique with advantages of non/low pollutions, significant reactivity, rapid heating, and controllability has garnered attention in the fabrication of CNTs.42 Despite these advantages, it still faces some important challenges regarding the quality of obtained CNTs under atmospheric pressure and low temperature that should be tuned. The effects of crucial factors such as the reacting gases (e.g., H2 pretreatment), nitrogen-containing additive (nitrogen-comprising gas), and appropriate MW power should be analytically evaluated.38,43 For instance, it was indicated that the higher ionization degree could be attained with elevated MW power; additional carbon atoms could be localized for thickening of CNTs.38 With an enhancement in the MW power and the subsequent increase in substrate temperature, the agglomeration of catalyst particles can transpire, thus culminating in the formation of CNTs with lager diameters. Nonetheless, in some instances, longer and thinner CNTs could be obtained by increasing the MW power due to the formation of significant amount of etching species (e.g., atomic hydrogen in the plasma) in the presence of too high MW power; outer walls of CNTs were etched away, thus reducing the diameter of ensued CNTs.44MWCNTs (∼10–40 nm) were synthesized via a MW-assisted technique with advantages of improved specific surface area in CNTs and enhanced yield of their production, providing optimal and inexpensive synthesis possibilities;41 MW irradiation has been deployed for the synthesis of CNTs with fewer imperfections compared to the conventional heating techniques.45 The effortless in-core heating of carbon-based sources can be performed under a MW field to allow their distortion via MW heating for acquiring newer carbon-based structures (e.g., CNTs).46 MWCNTs were rapidly synthesized on a graphite surface with ferrocene (as a catalyst),47 where deployment of MW technique highlighted the benefits of low temperature, and shorter reaction time, for decomposition of the ferrocene powder on abundant iron as catalyst and hydrocarbons (carbon source) to obtain CNTs.47
MWCNTs on biochar substrates were synthesized via MW-assisted chemical vapor deposition technique at 600 °C by applying nickel as a catalyst; temperature was much lower than the routine chemical vapor deposition techniques. The carbon-containing species found in the volatiles probably served as carbon sources originating the growth of CNTs on the biochar surfaces, wherein inorganic species in biomass structures functioned as catalysts.48 Further, gumwood biomass was employed to synthesize MWCNTs (∼50 nm) via a MW-induced technique, in which char particles and minerals (in char particles) represented as substrates and catalysts, respectively.49 The volatiles underwent the thermal and/or catalytic cracking procedure on the surface of char, generating the self-assembled amorphous carbon nanospheres onto MWCNTs structures under the MW irradiation conditions.49 Besides, the dissociation of biomass and formation of carbon nanomaterials significantly depended on the temperature and pressure. For instance, the rice husk powders produced the biogas including CH4 when they were irradiated by MW (∼200 °C) and plasma irradiation. This temperature could sufficiently crack the pyrolysis of CH4 to generate amorphous carbon layers as nucleation sites on the surface of nickel catalyst.50 Notably, physicochemical characteristics of CNTs can be affected by production temperature of biomass. As an example, CNTs were synthesized utilizing biochar (the precursor) under the MW irradiation wherein higher concentration of CNTs with smaller hydrodynamic diameter could be obtained from biochars prepared at 600 °C. Also, the prepared CNTs from biochar of wheat straw and hazelnut hulls exhibited a higher degree of wall graphitization, thus signifying high quality of CNTs.51
Typically, the heat transfer in conventional heating includes the process of conduction, convection, and radiation, but MW-assisted techniques stimulate the volumetric heating of the feedstock through bulk energy transfer. Additionally, the crucial advantages of MW-induced heating over conventional heating comprise volumetric/selective heating, low material pre-processing costs, uniform distribution of temperature, fast startup/shut down processes, shorter processing time, rapid heating and non-contact heating, low heat losses, and high efficiency of energy.20,22,23 In one study, MWCNTs were synthesized using MW-assisted pyrolysis technique in a bench scale pyrolysis reactor at 600 W power level which reached up to a temperature of 500 °C. MW pyrolysis of bagasse was performed with and without iron (Fe) and cobalt (Co) as susceptors/catalysts. As a result, by inserting Fe, high yield of H2 and CH4 gases could be obtained in addition to CO2 and CO.52 Compared to the conventional high temperature techniques (800–1000 °C) utilizing graphite/carbon fibers or hydrocarbon gases as precursors for manufacturing CNTs, MW-assisted technique featured relatively low temperature (400–500 °C) at medium MW power (600 W).52 Further, in this study, the authors stated that high temperature furnace treatment and chemical vapor deposition methods yielded better quality and finer MWCNTs (∼10–20 nm) compared to the MW-assisted pyrolysis technique. But, MW-assisted pyrolysis exhibited some important advantages such as short processing time (<15 min) and necessity of moderately low process temperatures. It appears that more elaborative studies are required for producing CNTs in high yields from lignocellulosic biomass via MW pyrolysis.52 Some important examples of CNTs prepared via MW-assisted chemical vapor deposition and MW pyrolysis techniques with advantages of uniform heating, higher yields, and improved mass transfer of volatiles are summarized in Table 1.
| CNTs | Catalysts | Synthesis method | Biomass | Ref. |
|---|---|---|---|---|
| MWCNTs (50–200 nm) | Nickel | MW-chemical vapor deposition | Rice husk | 50 |
| MWCNTs (17–100 nm) | Ferrocene | Oat hulls, hazelnut hulls, wheat straw, and rapeseed cake | 51 | |
| MWCNTs (50 nm) | Nickel (Ni) | Pine nutshell | 48 | |
| MWCNTs (50–100 nm) | Mineral matter in char particles from biomass | Gumwood | 49 | |
| MWCNTs (50–100 nm) | — | MW pyrolysis at low temperature (600 °C); self-extrusion of volatiles | Palm kernel shell cellulose | 53 |
| MWCNTs (∼20 ± 10 nm, 20 wt% Fe and 50 ± 20 nm, 33.3 wt% Fe) | Fe and Co | MW pyrolysis at low temperature (400–500 °C) | Sugarcane bagasse | 52 |
MW-assisted techniques can be deployed for converting renewable biomass resources to value-added CNTs with industrial potential. However, the mechanism of formation and transformation of biomass molecular structures into CNTs, e.g., the conversion of cellulose into CNTs and its interaction with MW, the evaluation of elemental composition of biomass, in-depth study on optimization and scale-up process (yield optimization and establishment of the MW heating efficiency), and the selection of appropriate catalysts need optimization in future to improve the present techniques for synthesizing CNTs without defects in structure, one of the main lingering challenges.36,37,53 MW-assisted synthesis techniques exhibit faster heating rates as the material couple strongly with the electromagnetic energy under MW irradiation.37,54,55 The fast heating rate under MW irradiation stimulates and enhances the thermal decomposition reactions.56 Dissimilar to the conventional heating, this can offer the selective heating due to the transformation of energy at a molecular scale. Additionally, MW heating generates hot spots that emerge from either the inhomogeneity of the MW field or the dielectric property and shape of feedstock particles. Consequently, the particle core is hotter than the particle surface temperature, as only the bulk temperature is measured; the size, shaper, and chemical structure of materials can affect the extent of MW absorption, the heating intensity, and hot spots. The production of hot spots has considerable influences on the composition and distribution of final products.37,54,55
After forming CNTs structures, they need to be purified and functionalized for further applications; MW-assisted protocols are favorable in view of rapid coupling of carbonaceous materials with MWs. The conventional techniques applied for the purification of CNTs include thermal, chemical, and sonication treatments.39,40 For instance, it has been revealed that physical properties such as thermal conductivity and diffusivity of the MWCNTs were highly enhanced by applying MW irradiation-based purification technique.57 The purification of MWCNTs via MW oxidation techniques can reduce the related structural defects and improve the dispersity of these nanostructures; higher purification efficiency could be realized compared to the typical oxidation technique.58 Furthermore, a two-step eco-friendly technique deployed MW irradiation for the purification of MWCNTs with the benefit of residual metal catalysts elimination;59 the functionalization of CNTs using MW irradiation can decrease the reaction time,60 and help improve their solubility.61
2.2. Vapor-assisted ozone treatment
A process for functionalizing of CNTs has been reported via a vapor-assisted ozone treatment; this technique is capable of introducing extensive level of oxygen groups with a dominant population of carboxyl groups at near ambient temperature.62 The oxidative modified CNTs were obtained through a simple ozone treatment process under the solvent vapors comprising H2O, H2O2, and C2H5OH; the oxygenated groups on CNTs were considerably enhanced by applying the suitable functionalization temperature (∼40 °C) and incremental partial vapor pressure. The ozone treatment under the vapor has been performed by applying compressed air as the carrier gas, providing environmentally benign functionalization procedure.62 Additionally, the suspension of CNTs was prepared deploying an ozone treatment to produce ultra-high performance concrete/CNT composites.63 This ozone treatment could improve the CNTs dispersion in aqueous media via the formation of oxygen-bearing carboxylic groups on the surfaces of CNTs. Therefore, the interfacial interaction between CNTs and ultra-high-performance concrete could be improved, instigating significant nucleation influence at initial ages. Ozone treatment offered multiple nucleation sites and double steric repulsion via the accelerated degree of CNTs dispersion, resulting in the enhanced hydration at initial ages and increased compressive strength at later ages.632.3. Ultrasonic irradiation
A process was reported for directly functionalization of SWCNTs using poly(ethylene glycol) grafted polymers in aqueous media via Diels–Alder click reaction at room temperature under ultrasonic irradiation.64 The prepared CNT-based nanosystems with improved drug loading capacity could successfully deliver doxorubicin to the targeted tumor cells; these nanosystems with pH-dependent behavior exhibited suitable drug release rate at acidic condition (pH = 5.5) compared to the physiological condition (pH = 7.4). The functionalized SWCNTs delivery system had no noticeable cytotoxicity against the normal cell lines, but demonstrated significant antitumor effects against HeLa cancerous cells, providing promising cancer/tumor-targeted chemotherapy options.64 Ultrasonic irradiation technique has been introduced for the chemical treatment of carboxylated MWCNTs–COOH with thiamine to generate poly(vinyl chloride)/thiamine-MWCNTs nanocomposites with significant thermal stability and improved mechanical properties.65 Besides, polymers could be grafted on the surface of MWCNTs using Diels–Alder reaction in water via heating-stirring and ultrasound-assisted method. As a result, significant reaction rate enhancement could be attained, which was ∼12 times higher than the one under the conventional heating-stirring condition without losing the efficiency of grafting process, thus offering a simple and environmentally benign direct functionalization technique applicable on larger scales.66MWCNTs have been synthesized via the sonication of CHCl3, CH2Cl2, and CH3I after the addition of silicon nanowires under ambient conditions. It was revealed that ultrasonic irradiation could facilitate the decomposition of solvent molecules and their subsequent reaction with the hydrogen-terminated silicon surfaces of the nanowires.67 Similarly, a sonochemical method was deployed for the synthesis of high-purity SWCNTs. The mixture solution was pulsed with high-intensity ultrasound for 20 min. Silica particles acted as nucleation sites and the ultrasound could facilitate the decomposition of ferrocene to produce Fe nanomaterials for catalyzing the growth of nanotubes.68 Although acceptable results can be found in limited studies, but still the quality of CNTs produced via ultrasound-assisted techniques did not match the superiority of CNTs obtained by traditional tactics. Therefore, it is unlikely that ultrasound-assisted production of CNTs will find industrial applications unless significant improvements and optimization can be attained; however, ultrasound-assisted techniques have been widely deployed for the surface modification and dispersion/solubilization of CNTs.69
3. Sustainable feedstocks and catalysts
Typically, carbon resources utilized in the synthesis of carbon nanomaterials are not renewable, and their production via conventional techniques often leads to the formation of hazardous or toxic waste/chemicals, generating possible adverse environmental impacts; innovative strategies and technologies are thus highly necessitated for the simpler, safer, low-cost, and sustainable fabrication of these nanostructures from renewable carbon resources with environmentally benign features, and lower sulfur/heavy metal levels, and greenhouse gas emissions.37,53 Carbon sources and synthesis techniques can affect the morphology and characteristics of CNTs, as well as their growth rate.39,70 Natural resources (e.g., plant-based hydrocarbons) and agricultural waste hydrocarbons have been explored for the eco-friendly synthesis of CNTs as low cost, renewable, and sustainable materials.71,72 Finding suitable and eco-friendly catalysts with low cost is crucial in producing high-quality CNTs.73 On the other hand, natural mineral-derived catalysts with advantages of sustainability should be further considered for fabricating carbon-based nanomaterials such as CNTs, because of their low cost, eco-friendliness, and abundant accessibility.74 For instance, MWCNTs were synthesized using a benign method comprising stone garnet sand (as low cost catalyst and support) and common household gas (as carbon source).75 Vertically aligned CNTs (∼4–30 nm) could be gotten in high yield utilizing Fe/vermiculite catalyst on a pilot plant fluidized bed reactor chemical vapor deposition.76 Natural clays have also been deployed as catalysts with the advantages of their inherently strong acidity, ion-exchange capability, adsorption, and large surface area.77 Siliceous breccia was utilized as a natural catalyst for manufacturing CNTs without any pretreatment process through the chemical vapor deposition technique (750 °C, 30 min);78 siliceous breccia with iron oxide-hydroxides functioned as catalysts for dissociating the precursors of hydrocarbon and producing the CNTs.78 Pumice (a volcanic rock) and laterite (a clay material) as natural catalysts have been evaluated for fabricating graphitized CNTs; these CNTs were employed as reinforcement constituents in nanocomposite materials.79 CNTs were also fabricated using biochar (a renewable and low-cost substrate) via MW-assisted chemical vapor deposition technique. Biochar produced via pyrolysis of biomass in the absence of oxygen demonstrated high porosity, allowing suitable dispersion of nanocatalysts on its surface thus stimulating the generation of CNTs.48Plant extracts as natural precursors can be deployed for sustainable synthesis of CNTs (Fig. 2).80 They serve as greener catalysts for fabricating CNTs with the advantages of cost-effectiveness, renewability, and abundancy,81 thereby avoiding the application of potentially hazardous metal catalysts (Table 2). Besides, plant extracts as green catalysts can be converted into porous and activated carbons with considerable nucleation sites for growing CNT-based nanostructures. The activated carbons with porous surfaces illustrated larger surface area than transition metals. Thus, nucleation sites for growing CNTs became larger, and the activated carbon could decompose hydrocarbons at a lower temperature; the growth of CNTs using plant extracts has been reported below ∼575 °C, whereas the typical reactions applying transition metal catalysts were performed at ∼700–1200 °C.40 By applying a wall-nut extract catalyst, high yield of CNTs production could be obtained at chemical vapor deposition temperature of 575 °C.80 Qu et al.82 reported the greener formation of CNTs-Cu/ZnO nanocomposites utilizing Brassica juncea as a plant source of Cu, Zn, and C. The prepared CNTs (∼80 nm) had middle-hollow structures and were not at all crystalline, with a few defects in the walls. They provided an innovative greener plant-based strategy for the development of CNTs; however, future investigations should be focused on their surface functionalization and optimization process.82 Besides, the nanostructures of MWCNTs (∼80–90 nm) were fabricated via chemical vapor deposition using Cocos nucifera Linn (coconut oil) where nitrogen gas was utilized as an inert atmosphere and a suitable carrier for the evaporated precursor.83
| Plant-based resources | CNT-Based nanostructures | Techniques | Sizes (nm) | Ref. |
|---|---|---|---|---|
| Olive oil (Olea europaea) | SWCNTs | Pyrolysis method | ∼27 | 84 |
| Brassica juncea L. | CNTs-Cu/ZnO nanocomposites | — | Outer diameter of ∼80 | 82 |
| Coconut oil (Cocos nucifera Linn.) | MWCNTs | Chemical vapor deposition | Diameter of ∼80–90 | 83 |
| Eucalyptus oil | SWCNTs | Spray pyrolysis method | Diameter of ∼0.79–1.71 | 86 |
| Natural palm oil | Vertically aligned CNTs; SWCNTs | Thermal catalytic chemical vapor method | SWCNTs: ∼0.6–1.2 | 32 |
| Fresh bamboo culms | MWCNTs | Chemical vapor deposition | Diameter of less than 20 | 87 |
| Rice straw (raw rice straw & neutral pulp) | MWCNTs | Pyrolysis method | Raw rice straw: ∼15–40 | 88 |
| Neutral pulp: 14.6–47.9 | ||||
| Turpentine oil (pine tree) | MWCNTs | Spray pyrolysis method | ∼15–40 | 89 |
| Sesame oil (Sesamum indicum) | Branched nitrogen (N)-doped CNTs | Spray pyrolysis-assisted chemical vapor deposition | ∼30–60 | 90 |
| Cinnamomum camphora (camphor) | MWCNTs | Chemical vapor deposition | ∼10 | 72 |
| Azadirachta indica (neem oil) | MWCNTs | Spray pyrolysis method | ∼15–30 | 91 |
| Cynodon dactylon, Rosa, Azadirachta indica, Juglans regia | MWCNTs | Chemical vapor deposition | ∼8–15 | 80 |
Olive (Olea europaea) and coconut oils served as natural carbon precursors and NiCl2 functioned as catalyst for the eco-friendly synthesis of CNTs (∼27–31 nm) through a simple and cost-effective pyrolysis technique at low temperatures. Consequently, uniformed SWCNTs from olive oil and the carbon rods from coconut oil were successfully obtained. The uniformity in SWCNTs was not reported by using coconut oil, because of high proportion of saturated fat content (∼82.5%) and less reactivity in comparison with the unsaturated hydrocarbons.84 These CNTs should be further evaluated for agricultural, biotechnological and biomedical applications.85
By applying a simple spray pyrolysis technique, SWCNTs (∼0.79–1.71 nm) were fabricated through catalytic decomposition of the eucalyptus oil on a high silica-zeolite support impregnated with Fe/Co catalyst at 850 °C.86 Suriani et al.32 synthesized vertically aligned CNTs with a diameter of ∼0.6–1.2 nm on silicon substrates via thermal catalytic-chemical vapor reactor technique utilizing natural palm oil (as carbon source); these CNTs had high purity of ∼90%.32 Additionally, the deployment of chemical vapor deposition technique was reported for synthesizing MWCNTs (<20 nm) using bamboo charcoals (as natural catalyst or substrate) derived from the heat-treated fresh bamboo culms at 1000–1500 °C. It was revealed that Mg2SiO4 and mostly calcium silicate were responsible for growing the MWCNTs.87 The minerals in the bamboo charcoal served as catalysts similar to the transition metals for nucleating CNTs. Basta et al.88 illustrated the eco-friendly fabrication of MWCNTs (∼14.6–47.9 nm) from hydrochars of rice straw via a simple pyrolysis technique. The pyrolysis of rice straw-alkaline pulp and sulfite pulp hydrochars resulted in almost needles-like CNTs produced in between graphene nanosheets, with diameters ranging from 2.5–6.8 nm and 4–8 nm, respectively.88 Besides, aligned CNTs (∼70–130 μm in length) were fabricated using turpentine oil (as a low-cost plant-based precursor) and ferrocene mixture via a simple spray pyrolysis technique. These CNTs with outer diameters between ∼15 and 40 nm should be further evaluated for biomedical applications.89
Sesame oil as a natural and low-cost hydrocarbon precursor was utilized for manufacturing aligned-stack of branched nitrogen-doped CNTs via a simple spray pyrolysis-assisted chemical vapor deposition technique; ferrocene (C10H10Fe) and acetonitrile (CH3CN) were utilized as the catalyst and nitrogen-doping agent, respectively for growing the N-doped aligned CNTs.90 Neem oil obtained from the seeds of Azadirachta indica was utilized as a carbon source to produce aligned CNTs nanostructures via a simple spray pyrolysis method. The main constituents of neem oil are hydrocarbons with less oxygen, offering the precursors in spray pyrolysis production of CNTs; the aligned CNT bundles were grown directly inside the quartz tubes. These CNTs were found to be multi-walled structures with an inner diameter of ∼15–30 nm.91
4. Carbon-based nanomaterials with biomedical potentials
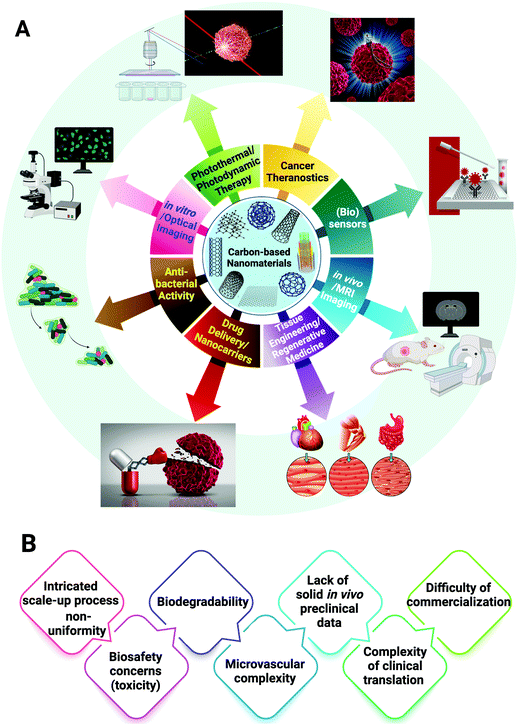
Overall, carbon-based nanomaterials with their attractive mechanical, optical, thermal, and electronic features can be simply functionalized;92 these materials have shown excellent potentials in bio- and nanomedicine because of their suitable electrical conductivity, biocompatibility, and large surface area in addition to their unique thermal and mechanical features.93,94 For instance, the electronic properties of CNTs mainly include high surface-to-volume ratio, the possibility of surface molecular absorption, fouling resistance, and stimulation ability to fast electron transfer kinetics.95 These nanomaterials can be loaded with targeted drugs or molecularly therapeutic agents via hydrophobic interactions or π–π stacking due to their inherent hydrophobic nature, providing promising drug delivery nanosystems with high sensitivity/selectivity, targeting properties, sustained drug release behaviors, stability, and bio-clearance. Carbon-based nanomaterials can be further conjugated with a variety of imaging agents or targeted moieties with high densities, offering great opportunities for solving the limitations of detection and sensitivity towards the cancerous cells, especially for multimodal cancer theranostics.96 These nanomaterials exhibited intrinsic properties that make them promising candidates for biomedical applications (Fig. 3A). As an example, CNTs exhibited unique optical absorption in the near-infrared region as well as easy functionalization, which can enhance their in vivo applications; they also showed bioimaging and tracing performances combined with drug delivery (Fig. 3A).97 However, since CNT-delivery systems with their needle (fiber)-like shapes could be bio-persistent and showed asbestos-like pathogenicity,98 the biosafety issues and potential toxicity of these carbon-based nanomaterials should be the priority of research, especially for clinical translations (Fig. 3B).97,99 Currently, various studies have focused on the improvement of their biocompatibility through surface functionalization as well as targeted bioecological interactions to provide biocompatible nanosystems with enough susceptibility for enzymatic degradation and non/low immune system reactions.100–102 In this section, we focus on cancer theranostic applications of CNTs.4.1. CNTs with cancer theranostic potentials
Nano-based structures and formulations with multifunctionality are being employed to produce innovative nano-delivery systems as the conventional drugs or drug delivery systems have suffered from low specificity, selectivity, efficiency, and high adverse effects.103,104 For cancer therapy, engineered nanostructures have been designed to specifically target cancerous cells and reduce the possible toxic effects of drugs.105–107 In this context, various innovative nanocarriers and nanosystems with targeted and controlled anticancer properties have been designed using CNTs (Tables 3 and 4).13,104,108–111 But, more elaborative studies are still required for ascertaining the biocompatibility, cytotoxicity, carcinogenicity, and pharmacokinetics of these CNT-based nanosystems.112 Wong et al.113 have reviewed the drug delivery potentials of CNTs, focusing on their drug loading and cellular uptake capacity as well as the other important features; the main challenges for the CNT-based delivery systems are hydrophobicity and potential toxicity (in biological systems). The surface functionalization of CNTs can enhance the biocompatibility and targeting features, which are crucial for cancer theranostics.114,115 For instance, a cancer therapy nanosystem was constructed by applying platinum nanoparticles supported on polybenzimidazole functionalized polymers and MWCNTs.116 Consequently, the prepared nanosystem demonstrated remarkable inhibitory activity on the epithelial–mesenchymal transition and cell cycle markers of cancerous cells; they had specific cytotoxicity on breast cancerous cells, but not on the adult stem cells.116 Further, hyaluronic acid as CD44 receptor targeting ligand and α-tocopheryl succinate for synergistic effects were employed for the functionalization of MWCNTs and the ensued structures were assessed for the targeted delivery of doxorubicin chemotherapeutic agent. Good biosafety and improved cellular placement as well as targeted anticancer effects could be obtained against breast cancerous cells.117 Significant cellular uptake, synergistic effects, growth inhibitory results, and total apoptotic ratio were reported by this nanoformulation.117| CNTs-based nanosystems | Properties | – Strong optical absorption |
| – The potential to convert the absorbed light into thermal heat | ||
| – Phototherapy agents | ||
| – High potential for generating reactive oxygen species (ROS) | ||
| – High potential for delivering therapeutic/diagnostic agents | ||
| Approaches | – CNTs-Based drug delivery in cancer therapy | |
| – CNTs mediated photothermal therapy | ||
| – CNTs mediated photodynamic therapy | ||
| – CNTs-Based combined photothermal therapy (photodynamic–photothermal, chemo–photothermal, and immune–photothermal therapy) | ||
| – CNTs for sonodynamic therapy | ||
| – CNTs with anti-metastatic properties and functionality | ||
| Anticancer pathways | – Stimulation of immune system | |
| – Inhibition of cancer/tumor cells | ||
| – Regulation of angiogenesis | ||
| – Photodynamic/photothermal effects | ||
| – Targeted/controlled anticancer therapeutics delivery |
| Type of CNTs | Anticancer drugs/agents | Advantages and properties | Ref. |
|---|---|---|---|
| SWCNTs | Doxorubicin | – Improved targeted delivery | 118 |
| – High intracellular accumulation of drug | |||
| – Suitable cytotoxicity effects with prolonged release behavior | |||
| – High loading potentials | |||
| Paclitaxel | – High efficacy against cell proliferation with enhanced apoptosis rate of anticancer drug in hypoxic environment | 119 | |
| – Improved chemotherapeutic effects of paclitaxel through hypoxia-inducible factor (HIF)-1a downregulation and apoptosis-related/autophagy-associated proteins upregulation | |||
| Camptothecin | – Improved anticancer therapeutic efficiency with selective inhibitory effects of αVβ3-expressed cancerous cells, while inducing low cytotoxicity to αVβ3-negative cancerous cell up to 3.78- and 3.02-fold in two and three dimensional culture | 120 | |
| Curcumin | – Improved blood concentration of curcumin (∼18-fold) with remarkable inhibitory effects against cancerous cells and related tumor growth | 121 | |
| – No noticeable toxicity | |||
| – Enhanced delivery features | |||
| HIF-1α small interfering RNA (siRNA) | – Effective suppression of tumor growth | 122 | |
| – High specificity/selectivity and low toxicity | |||
| – Selective inhibition of cellular HIF-1α performance | |||
| Cyclin A2 siRNA | – Improved reduction of cell proliferation with induction of apoptosis in chronic myelogenous leukaemia K562 cells | 123 | |
| – Depletion of cyclin A2 can inhibit the proliferation of targeted cells and stimulate apoptosis of them | |||
| p53 plasmids | – Induction of apoptosis | 124 | |
| – Over-expression and uptake of the P53 within the MCF-7 cells | |||
| MWCNTs | Methotrexate | – High selectivity with low toxicity | 125 |
| – pH-Responsive and prolonged release behaviour | |||
| – High efficacy and targeting properties | |||
| Gemcitabine | – Prolonged and pH dependent release behaviour | 126 | |
| – Low haemolytic toxicity | |||
| – High cytotoxicity against cancerous cells | |||
| – Improved biocompatibility and pharmacokinetics | |||
| Carboplatin | – High cellular uptake with specificity | 127 | |
| – High cytotoxicity against cancerous cells | |||
| Pemetrexed and quercetin | – Combinatorial therapeutic effects against pancreatic cancer cells | 110 | |
| – High efficiency for drug delivery | |||
| Recombined ricin A chain | – High induced cancerous cell death effects | 128 | |
| – High selectivity and targeting properties | |||
| Gold nanostars | – Improved photothermal effects with biocompatibility | 129 | |
| – High induced cancerous cell death | |||
| CREKA peptide (a tumor homing peptide) | – Targeted antitumor effects | 130 | |
| – High accumulation of CREKA peptide in tumors (∼6 fold) | |||
| Gemcitabine and lentinan | – Efficient crossing from the cell membrane | 131 | |
| – Targeted antitumor effects with synergetic activity | |||
| – Low toxicity | |||
| Tamoxifen and lentinan | – Significant inhibitory influences | 132 | |
| – High apoptosis rate and drug-loading capacity | |||
| Trastuzumab and pertuzumab | – High inhibitory activity against SK-BR-3 (a human breast cancer cell line) with targeting and selectivity properties | 133 | |
| Doxorubicin | – Efficient drug delivery | 134 | |
| – Good biocompatibility | |||
| – Internalization of hybrid-CNTs in MCF-7 (a breast cancer cell line) and MDA-MB-231 (a breast cancer cell line) | |||
| Serine/threonine-protein kinase (PLK1) siRNA | – High silencing of polo-like kinase 1 (PLK-1) in HeLa cells | 135 | |
| – Low toxicity | |||
| – Good biocompatibility | |||
| Hybrid (SWCNTs and MWCNTs) | Non-coding negative siRNA | – Antitumor proliferation effects | 136 |
| – Targeted cytotoxicity against cancerous cell lines |
The salient advantageous features for CNT-based drug delivery systems encompass pH-dependent behavior, specificity/selectivity, prolonged/controlled release features, reduced side effects, high drug loading capacity, and reduced administration dose/frequency.137 These materials with their unique architectures have been explored for targeted delivery of anticancer and chemotherapeutic agents (Table 5);138 the improved release behavior and multifunctionality could be attained especially by MWCNTs for cancer therapy and diagnosis owing to sustained drug release in related cancer tissues and high surface areas for functioning groups. In addition, pH sensitive polymers (e.g., chitosan) demonstrated high targeting delivery properties with the advantages of biocompatibility, high tumor selectivity, pH-responsive behaviour, and chemical versatility.125 Cirillo et al.125 fabricated methotrexate-loaded chitosan-MWCNTs nanohybrid platforms with pH-dependent, high sensitivity/selectivity, and sustained delivery features and evaluated their applicability against H1299 human lung cancerous cells compared to MRC-5 human lung fibroblast cells. In another study, an innovative nanoplatform comprised epirubicin-loaded magnetic-MWCNTs with controlled/sustained release and improved cytotoxicity effects.139 Besides, MWCNTs were derivatized with naringenin against lung cancerous cells.140 These functionalized CNTs demonstrated pH- dependency and prolonged release behaviour as well as low toxicity effects on non-malignant cells compared to free naringenin; efficient anticancer effects on malignant lung cells could be achieved, in vitro.140 In addition, the formulated chitosan–folate conjugated MWCNTs illustrated some important advantages of sustained release, biocompatibility, and biosafety features for targeted docetaxel delivery to the lung cancerous cells, in vitro (Fig. 4). This platform internalized into the cells via a folate receptor-mediated endocytic pathway with 89 fold more efficacy than the commercial docetaxel in A549 cells; the drug encapsulation efficacy was more than 79%. Histopathological analyses demonstrated no inflammatory or pathologic effects of this nanosystem.141
| Nanosystems | Anticancer agents | Targeting agents | Cancer/tumor | Ref. |
|---|---|---|---|---|
| Chitosan-modified SWCNTs | Doxorubicin | Folic acid | Liver cancer | 142 |
| MWCNTs functionalized with poly(acrylic acid) and decorated with iron oxide magnetic nanoparticles | Doxorubicin | Folic acid | Human glioblastoma cells | 143 |
| Polyoxyl 35 castor oil noncovalent modified SWCNTs | Doxorubicin | — | Sarcoma tumor | 144 |
| Polycitric acid–polyethylene glycol–polycitric acid functionalized MWCNTs | Cisplatin | — | Colon adenocarcinoma tumor | 145 |
| Functionalized MWCNTs | Gemcitabine | Magnetic particles | Cancer lymph node metastasis | 146 |
| Functionalized SWCNTs with piperazine–polyethylenimine derivative | siRNA | — | Human breast cancer cells | 147 |
| Poly(ethylene glycol) grafted polymers–SWCNTs | Doxorubicin | — | HeLa cancer cells | 64 |
| Radiolabelled SWCNTs | Radionuclide | Thiolated antibodies | Burkitt lymphoma | 148 |
| Transactivator of transcription (TAT) peptide–chitosan-MWCNTs | Doxorubicin | — | Bel-7402 cells | 149 |
| Functionalized SWCNTs | Taxoid | Biotin | Leukemia cells | 150 |
| –CONH–(CH2)6–NH3+Cl− functionalized SWCNTs | Telomerase reverse transcriptase siRNA- | — | Lewis lung tumors; human HeLa cells | 151 |
| MWCNTs–COOH | Pemetrexed and quercetin | — | Human tumor cells (breast and pancreatic cells) | 110 |
| MWCNTs coated with silica & chitosan | Doxorubicin | — | Breast cancer | 111 |
| Glycopolymer decorated MWCNTs | Doxorubicin | Folic acid | Breast cancer | 152 |
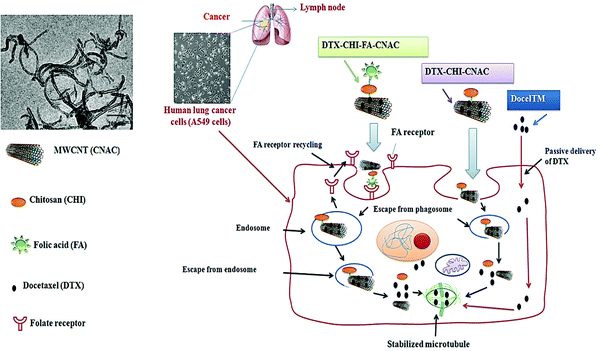 | ||
| Fig. 4 Chitosan–folate conjugated MWCNTs for targeted and sustained delivery of docetaxel anticancer drug to the lung. Reproduced with permission from ref. 141 Copyright 2017 Elsevier. | ||
Functionalized CNTs have shown appropriate target localization, which is vital for efficient and targeted cancer diagnosis and therapy. For instance, bisphosphonate–CNT conjugates have been constructed for targeting regions of active bone metabolism and were prepared by applying covalent functionalization or latently generating reactive polymer–nanotube complexes (Fig. 5A and B),153 which provided higher quality colloidal dispersions. Low cytotoxicity as well as proper biocompatibility profile could be achieved using both prepared bisphosphonate–CNT conjugates. These conjugates were radio-labelled with 99mTc in significant radiochemical yield (∼80–92%). The biodistribution evaluations illustrated that these complexes had speedy blood clearance after 1 h; interestingly, superb bone localization of latently reactive polymer–nanotube complexes could be attained in comparison to covalent functionalization.153 The functionalised MWCNTs were explored for gemcitabine drug delivery via a targeted lymph node system with magnetic properties, in vitro and in vivo. This nanosystem with high antitumor effects and drug loading capacity are suitable for the inhibition/regression of the metastasis in lymph node under the magnetic field with high efficacy and low side effects (Fig. 5C).146
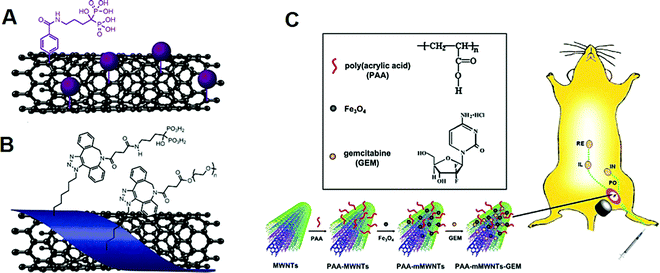 | ||
| Fig. 5 Chemical structures of the prepared (A) covalently functionalized and (B) non-covalently functionalized SWCNTs. Reproduced with permission from ref. 153 copyright 2020 American Chemical Society. (C) MWCNTs for magnetic lymphatic gemcitabine drug delivery. PO: popliteal lymph node; IN: inguinal lymph node; IL: para-iliac lymph node; RE: renal hilar lymph nodes. Reproduced with permission from ref. 146 Copyright 2011 Elsevier. | ||
The treated C6 glioma cells with DNA wrapped SWCNTs (5 μg mL−1) were exposed to bi-phasic electric pulses (6.6 V m−1, 200 Hz, pulse duration 1 ms).154 The low-frequency and low-strength electric field stimulation of glioma cells exposed to SWCNTs could lead to enhanced CNTs accumulation inside the cells.154 In another study, the coupled SWCNTs with hyaluronic acid and chlorin e6 were explored against colon cancer using photodynamic therapy.155 The nanosystem improved the efficacy of photodynamic therapy and triggered the death in colon cancerous cells.155 For evaluating the tumor detectability and fluorescence image-guided surgery effects on post-operative survivals, an innovative custom-built reflectance/fluorescence imaging nanosystem was introduced.156 The composed contrast agent from SWCNTs was an intraperitoneal injectable nano-molecular probe coupled to an M13 bacteriophage delivering a modified peptide attached to the extracellular protein overexpressed in ovarian cancer, SPARC protein. This imaging nanosystem could detect second near-infrared window fluorescence with real-time video imagery potentials for better controlling the intra-operative tumor surgical removal; the microscopic detection of tumors with high quality and resolution has been reported.156
5. Cytotoxicity and biocompatibility
Although CNTs can be desirable candidates in medicine and particularly for biomedical applications, there is not a unified consensus among the scientific community in terms of their toxic effects on cells.9 An undeniable fact is that there are no standard guidelines in scientific publications to calibrate and compare the result of one study with another. The most important and challenging issue is the ability to prepare CNTs of uniform length and diameter, because only after this accomplishment that the biological functional and cytotoxicity of CNTs can be improved. This hurdle gets even more critical while deploying SWCNTs, as chirality plays a key role in displaying the proper bio-function of the CNTs. Only if all these criteria are met, can the applications of CNTs in general be taken into green chemistry arena. Thus, it is imperative that all the CNTs should be the same length/diameter/chirality so that the toxicity profile of each one of the CNTs can be considered the same, thus enabling the full comprehension of the toxicological effects of all CNTs. However, it appears that this message is not well-understood and conveyed even in scientific studies nowadays.If the physical properties (including aspect ratio known as the ratio of length to diameter, and area), dose, time of exposure, purity, and chemical agents for functionalization of CNTs are not well-engineered, then they can have fatal consequences because the CNTs can accumulate in different tissues/organs (such as brain, spleen, heart, kidney, lung, liver, etc.), produce reactive oxygen species (ROS) and eventually damage the healthy cells.12,157,158 This sequence of events happens through a process called phagocytosis (Fig. 6). Indeed, by exposing or entering inappropriate CNTs into the living body, the immune system recognizes them as foreign agents and attempts to phagocytes them by immune cells (macrophages) (Fig. 6). The effect of two different structural type of CNTs, short/tangled MWCNTs and long/rigid MWCNTs, have been studied on phagocytosis by macrophages and their following cleaning from the living tissues, to establish the safety profile of CNTs, in vivo.98 The results revealed that low aspect ratio MWCNTs can be engulfed by macrophages before their clearance by draining lymph vessels, while high aspect ratio MWCNTs cannot be cleared and therefore gets accumulated in tissues, which in turn promote carcinogenesis (Fig. 6a).159 The efficiency of phagocytosis for varied geometries of CNTs was also mapped (Fig. 6b). As depicted in Fig. 6b, an incomplete phagocytosis occurs for large aspect ratio MWCNTs (upper left corner, long and thick CNTs, Mph < 1), while an effective phagocytosis was discerned for low aspect ratio of MWCNTs (lower left corner, short and small CNTs, Mph > 1).160 Other factors that can influence the safety of CNTs in vivo includes the increased solubility of CNTs which in turn can prevent their aggregation and facilitate their clearance from the body and consequently less accumulation in the tissues/organs (Fig. 6c).159 To overcome the aforementioned challenges, significant advances have been made in slicing both SWCNTs and MWCNTs to specific lengths with adherence to green chemistry tenets, for example, deploying continuous flow thin film microfluidics to ensure that the processing is scalable (Fig. 6d).161
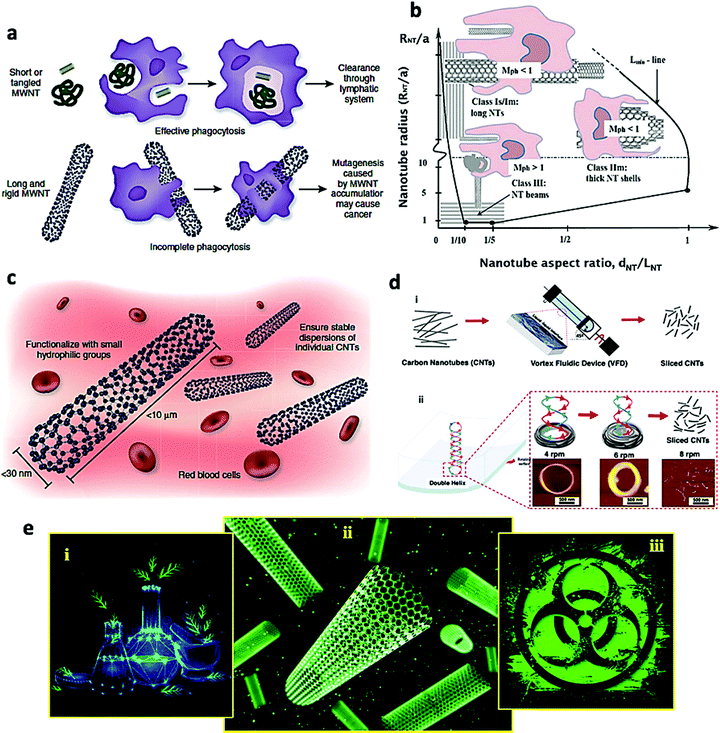 | ||
| Fig. 6 Some important factors affecting the safety of CNTs in vivo: (a and b) the effect of CNTs structure on the efficiency of phagocytosis by macrophages and clearing from tissues, in vivo. Reproduced with permission from ref. 159 Copyright 1969, Nature Publishing Group and ref. 160 Copyright 2017 Elsevier. (c) Other factors such as the solubility and aggregation of CNTs influence the safety profile of CNTs in vivo. Reproduced with permission from ref. 159 Copyright 1969, Nature Publishing Group (d) (i) schematic illustration of the process for slicing both SWCNTs and MWCNTs via a vortex fluidic device (VFD) technique, and (ii) double helical topological fluid flow coiling at 4k and 6k rpm, with CNTs slicing at 8k rpm. Reproduced with permission from ref. 161 Copyright 2021 American Chemical Society (e) CNTs produced via greener processes could be a promising, cost-effective, eco-friendly, and leading to improve the biocompatibility and biodistribution of the CNTs, while exploiting the use of natural, renewable and sustainable resources instead of toxic chemicals. | ||
The surface area, retention time, fibre dimensions, (bio)persistency, and reactivity/inherent toxicity can affect the pathogenicity, biocompatibility, and toxicity of CNTs.162 In the context of medical and biomedical use, precise cellular analyses and specific histopathological assessments need to be performed to evaluate the biocompatibility and pathogenicity of these nanostructures;112 though the chemically-functionalized CNTs displayed low toxicity, the possible scepticism due to non-biodegradability should be considered. It was reported that MWCNT-altered polyvinylchloride surfaces induced direct platelet activation/aggregation in vitro, and could also produce massive thrombosis in an in vivo rabbit model.163 Pulmonary biocompatibility evaluations of administered CNTs in mice demonstrated severe decrease in antioxidants such as catalase, superoxide dismutase, and glutathione, and could induce the oxidants such as oxidative stress, lipid peroxidation, and myeloperoxidase (MPO).164
The lack of stimulating acute adverse reactions (e.g., cell detachment, tissue necrosis, and apoptosis), non-acute inflammatory reactions, non-toxicity of chemical components of metabolism, and non-toxic or without longstanding tissue bioaccumulation are some of the important features, which should be considered for further applications of CNTs.165 In one study, results obtained from cellular tests indicated the high level of cellular viability in contact with CNTs and the minor collagen generation enhancement, as well as the absence of pro-inflammatory interleukin 6 cytokine and the lack of free radicals induction from the prepared CNTs.166 The evaluation of a single-chirality DNA-encapsulated SWCNTs complex upon intravenous administration exhibited suitable short- and long-term biocompatibility. After histopathological analyses, no alterations upon administration of CNTs were detected; also, the route of administration, purity, types, and functionalization affected the biocompatibility and biodistribution of these nanostructures.167
Biocompatible polymeric materials for the functionalization of CNTs can help to improve their biocompatibility, preventing their possible toxicity towards living organisms. For instance, biocompatible glycopolymer decorated onto MWCNTs could successfully improve their biocompatibility and anticancer delivery potentials.152 For preventing low dispersity and possible cytotoxicity, a new technique without utilizing any artificial chemical agents was reported for the production of biocompatible CNT ink with high stability up to months; these CNTs were stabilized by sustainable silk sericin protein, and the hybrid sericin–CNTs structures were formed via non-covalent interactions.168 Some organic materials like tannic acid could effectively improve the dispersibility of CNTs and decrease their cytotoxicity, thus improving their biocompatibility.169 The attachment of biocompatible polymers such as polyethylene-glycol (PEG) on CNTs was another strategy for improving the biocompatibility and dispersity, which are vital for their future clinical and biomedical applications; but this can cause the degradation, the formation of anti-PEG antibodies, and low cellular uptake.170 Accordingly, Jain et al.170 reported the surface functionalization of MWCNTs by applying the bovine-milk-derived protein succinylated β-lactoglobuline instead of PEG, providing superior biocompatibility, dispersion stability, and in vitro cell uptake. The succinylated β-lactoglobuline-functionalized MWCNTs demonstrated enhanced aqueous colloidal stability and half-maximal inhibitory concentration (IC50) values (Fig. 7).170
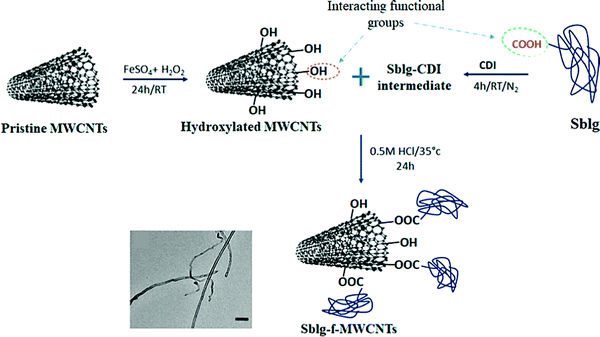 | ||
| Fig. 7 The preparation process of succinylated β-lactoglobuline-functionalized MWCNTs. CDI: N,N′-carbonyldiimidazole, Sblg: succinylated β-lactoglobuline. Reproduced with permission from ref. 170 Copyright 2019 American Chemical Society. | ||
6. Concluding remarks and future perspectives
CNTs have been explored for various biological and biomedical applications owing to their attractive physicochemical features such as high thermo-electrical conductivity, large surface areas, multifunctionality, and significant mechanical strength. However, preparing them on a large scale is either expensive or sometimes ecologically unfriendly. In this context, plenty of studies have been conducted to discover innovative methods to fabricate CNTs in an eco-friendly and inexpensive manner. A benign and sustainable alternative to address the above-mentioned concerns would be to consider plant extracts and agriculture wastes as renewable, natural, and biodegradable raw materials, which can reduce or eliminate the greenhouse gases, and exploitation of unsafe/toxic agents to help grow sustainable and greener technologies.Various types of functionalized CNTs can be prepared via environmentally benign and sustainable techniques utilizing inexpensive, renewable, and natural resources as catalysts and abundant hydrocarbon precursors; these natural precursors and waste carbon have efficiently produced CNTs with desired stability and size/morphology. However, after the production of CNTs, suitable functionalization and tailoring of their surface chemistry are crucial for improving their toxicity, biocompatibility, and physical features; comprehensive in vivo and in vitro analyses are also vital for their future clinical and biomedical applications. These CNTs may suffer from some intrinsic problems, including poor solubility in aqueous solutions or organic solvents. The covalently functionalized CNTs exhibited approved pharmacokinetics and biodistribution properties, which are vital for the clinical and biomedical applications. The toxicity of CNTs depends on the type of nanotubes, administration methods, dose, and targeted tissue types, and it can be minimized by using greener surface functionalization or synthesis techniques; biodistribution, toxicological, and biosafety assessments are essential for future clinical and biomedical applications of these CNT-based nanosystems.
Conflicts of interest
The author(s) declare no competing interest.Acknowledgements
E. M. would like to acknowledge the support from the National Institute of Biomedical Imaging and Bioengineering (5T32EB009035).References
- Z. Chen, A. Zhang, X. Wang, J. Zhu, Y. Fan, H. Yu and Z. Yang, J. Nanomater., 2017, 2017, DOI:10.1155/2017/3418932.
- E. Mostafavi, P. Soltantabar and T. J. Webster, Biomaterials in translational medicine, Elsevier, 2019, pp. 191–212 Search PubMed.
- S. Iravani, Green Chem., 2011, 13, 2638–2650 RSC.
- S. Vivekanandhan, M. Schreiber, S. Muthuramkumar, M. Misra and A. K. Mohanty, J. Appl. Polym. Sci., 2017, 134, 1–15 CrossRef.
- S. Iravani and R. S. Varma, Green Chem., 2020, 22, 2643–2661 RSC.
- S. Iravani and R. S. Varma, Green Chem., 2020, 22, 612–636 RSC.
- S. Iravani and R. S. Varma, Environ. Chem. Lett., 2020, 18, 703–727 CrossRef CAS PubMed.
- M. Zarghami Dehaghani, F. Yousefi, S. M. Sajadi, M. Tajammal Munir, O. Abida, S. Habibzadeh, A. H. Mashhadzadeh, N. Rabiee, E. Mostafavi and M. R. Saeb, Molecules, 2021, 26, 4920 CrossRef CAS PubMed.
- H. Zare, S. Ahmadi, A. Ghasemi, M. Ghanbari, N. Rabiee, M. Bagherzadeh, M. Karimi, T. J. Webster, M. R. Hamblin and E. Mostafavi, Int. J. Nanomed., 2021, 16, 1681–1706 CrossRef PubMed.
- T. Saliev, J. Carbon Res., 2019, 5, 1–22 Search PubMed.
- R. Shoukat and M. Imran Khan, Microsyst. Technol., 2022, 28, 885–901 CrossRef CAS.
- S. Rathinavel, K. Priyadharshini and D. Panda, Mater. Sci. Eng., B, 2021, 268, 115095 CrossRef CAS.
- B. F. Dizaji, S. Khoshbakht, A. Farboudi, M. H. Azarbaijan and M. Irani, Life Sci., 2020, 257, 118059 CrossRef PubMed.
- P. Utreja, S. Jain and A. K. Tiwary, Curr. Drug Delivery, 2010, 7, 152–161 CrossRef CAS PubMed.
- R. Singh and S. V. Torti, Adv. Drug Delivery Rev., 2013, 65, 2045–2060 CrossRef CAS PubMed.
- S. Marchesan, K. Kostarelos, A. Bianco and M. Prato, Mater. Today, 2015, 18, 12–19 CrossRef CAS.
- M. Sheikhpour, M. Naghinejad, A. Kasaeian, A. Lohrasbi, S. S. Shahraeini and S. Zomorodbakhsh, Int. J. Nanomed., 2020, 15, 7063–7078 CrossRef CAS PubMed.
- D. Chen, C. A. Dougherty, K. Zhu and H. Hong, J. Controlled Release, 2015, 210, 230–245 CrossRef CAS PubMed.
- K. Kostarelos, L. Lacerda, G. Pastorin, W. Wu, S. Wieckowski, J. Luangsivilay, S. Godefroy, D. Pantarotto, J.-P. Briand, S. Muller, M. Prato and A. Bianco, Nat. Nanotechnol., 2007, 2, 108–113 CrossRef CAS PubMed.
- A. Sanginario, B. Miccoli and D. Demarchi, Biosensors, 2017, 7, 9 CrossRef PubMed.
- I. Kościk, D. Jankowski and A. Jagusiak, J. Carbon Res., 2022, 8, 1–16 Search PubMed.
- M. A. Deshmukh, J. Y. Jeon and T. J. Ha, Biosens. Bioelectron., 2020, 150, 111919 CrossRef CAS PubMed.
- L. Tang, Q. Xiao, Y. Mei, S. He, Z. Zhang, R. Wang and W. Wang, J. Nanobiotechnol., 2021, 19, 423 CrossRef CAS PubMed.
- S. Hossen, M. K. Hossain, M. K. Basher, M. N.-H. Mia, M. T. Rahman and M. J. Uddin, J. Adv. Res., 2019, 15, 1–18 CrossRef CAS PubMed.
- Z. Liao, S. W. Wong, H. L. Yeo and Y. Zhao, NanoImpact, 2020, 20, 100253 CrossRef.
- S. Niyogi, M. A. Hamon, H. Hu, B. Zhao, P. Bhowmik, R. Sen, M. E. Itkis and R. C. Haddon, Acc. Chem. Res., 2002, 35, 1105–1113 CrossRef CAS PubMed.
- G. Jamalipour Soufi, P. Iravani, A. Hekmatnia, E. Mostafavi, M. Khatami and S. Iravani, Comments Inorg. Chem., 2021 DOI:10.1080/02603594.2021.1990890.
- G. Jamalipour Soufi and S. Iravani, Green Chem., 2020, 22, 2662–2687 RSC.
- V. Negri, J. Pacheco-Torres, D. Calle and P. López-Larrubia, Top. Curr. Chem., 2020, 378, 15 CrossRef CAS PubMed.
- Z. Chen, A. Zhang, X. Wang, J. Zhu, Y. Fan, H. Yu and Z. Yang, J. Nanomater., 2017, 2017, 1–13 Search PubMed.
- J. Simon, E. Flahaut and M. Golzio, Materials, 2019, 12, 624 CrossRef CAS PubMed.
- A. B. Suriani, A. A. Azira, S. F. Nik, R. Md Nor and M. Rusop, Mater. Lett., 2009, 63, 2704–2706 CrossRef CAS.
- L. Deng, Q. Xu and H. Wu, Proc. Environ. Sci., 2016, 31, 662–667 CrossRef.
- B. M. Chufa, H. C. Ananda Murthy, B. A. Gonfa and T. Y. Anshebo, Green Chem. Lett. Rev., 2021, 14, 640–657 CAS.
- M. Ukai, H. Kameya, H. Nakamura and Y. Shimoyama, Spectrochim. Acta, Part A, 2008, 69, 1417–1422 CrossRef PubMed.
- J. Zhang, A. Tahmasebi, J. E. Omoriyekomwan and J. Yu, J. Anal. Appl. Pyrolysis, 2018, 130, 142–148 CrossRef CAS.
- J. E. Omoriyekomwan, A. Tahmasebi, J. Dou, R. Wang and J. Yu, Fuel Process. Technol., 2021, 214, 106686 CrossRef CAS.
- Y. Liu, J. He, N. Zhang, W. Zhang, Y. Zhou and K. Huang, J. Mater. Sci., 2021, 56, 12559–12583 CrossRef CAS.
- R. Kumar, R. K. Singh and D. P. Singh, Renewable Sustainable Energy Rev., 2016, 58, 976–1006 CrossRef CAS.
- B. Makgabutlane, L. N. Nthunya, M. S. Maubane-Nkadimeng and S. D. Mhlanga, J. Environ. Chem. Eng., 2021, 9, 104736 CrossRef CAS.
- E. A. Burakova, T. P. Dyachkova, A. V. Rukhov, E. N. Tugolukov, E. V. Galunin, A. G. Tkachev, A. A. Basheer and I. Ali, J. Mol. Liq., 2018, 253, 340–346 CrossRef CAS.
- M. Chen, C. Chen, S. Shi and C. Chen, Jpn. J. Appl. Phys., 2003, 42, 614–619 CrossRef CAS.
- J. Y. Lee and B. S. Lee, Thin Solid Films, 2002, 418, 85–88 CrossRef CAS.
- A. Hirata and N. Yoshioka, Tribol. Int., 2004, 37, 893–898 CrossRef CAS.
- N. M. Mubarak, J. N. Sahu, E. C. Abdullah, N. S. Jayakumar and P. Ganesan, Res. Chem. Intermed., 2016, 42, 3257–3281 CrossRef CAS.
- J. A. Menéndez, A. Arenillas, B. Fidalgo, Y. Fernández, L. Zubizarreta, E. G. Calvo and J. M. Bermúdez, Fuel Process. Technol., 2010, 91, 1–8 CrossRef.
- S. Guo, Q. Dai, Z. Wang and H. Yao, Composites, Part B, 2017, 124, 134–143 CrossRef CAS.
- J. Zhang, A. Tahmasebi, J. E. Omoriyekomwan and J. Yu, Diamond Relat. Mater., 2019, 91, 98–106 CrossRef CAS.
- K. Shi, J. Yan, E. Lester and T. Wu, Ind. Eng. Chem. Res., 2014, 53, 15012–15019 CrossRef CAS.
- Z. Wang, H. Ogata, S. Morimoto, J. Ortiz-Medina, M. Fujishige, K. Takeuchi, H. Muramatsu, T. Hayashi, M. Terrones, Y. Hashimoto and M. Endo, Carbon, 2015, 94, 479–484 CrossRef CAS.
- P. Hildago-Oporto, R. Navia, R. Hunter, G. Coronado and M. E. Gonzalez, J. Environ. Manag., 2019, 244, 83–91 CrossRef CAS PubMed.
- B. Debalina, R. B. Reddy and R. Vinu, J. Anal. Appl. Pyrolysis, 2017, 124, 310–318 CrossRef CAS.
- J. E. Omoriyekomwan, A. Tahmasebi, J. Zhang and J. Yu, Energy Convers. Manag., 2019, 192, 88–99 CrossRef CAS.
- J. Li, J. Dai, G. Liu, H. Zhang, Z. Gao, J. Fu, Y. He and Y. Huang, Biomass Bioenergy, 2016, 94, 228–244 CrossRef CAS.
- A. S. Rossi, M. S. Pereira, J. M. dos Santos, I. Petri Jr and C. H. Ataíde, Mater. Sci. Forum, 2017, 899, 528–533 Search PubMed.
- P. Ingole, A. Ranveer, S. Deshmukh and K. Deshmukh, Int. J. Adv. Technol. Eng. Sci., 2016, 4, 78–84 Search PubMed.
- A. K.-M. M. Haque, G. S. Oh, T. Kim, J. Kim, J. Noh, S. Huh, H. Chung and H. Jeong, Mater. Res. Bull., 2016, 73, 247–255 CrossRef.
- J. Zheng, R. Bao, J. Yi and P. Yang, Diamond Relat. Mater., 2016, 68, 93–101 CrossRef CAS.
- V. Gomez, S. Irusta, O. B. Lawal, W. Adams, R. H. Hauge, C. W. Dunnill and A. R. Barron, RSC Adv., 2016, 6, 11895–11902 RSC.
- Y. Ling and A. Deokar, in Carbon Nanotubes Applications on Electron Devices, ed. J. M. Marulanda, IntechOpen, UK (London), 2010, pp. 128–141 Search PubMed.
- S. P. Economopoulos, N. Karousis, G. Rotas, G. Pagona and N. Tagmatarchis, Curr. Org. Chem., 2011, 15, 1121–1132 CrossRef CAS.
- J. Luo, Y. Liu, H. Wei, B. Wang, K.-H. Wu, B. Zhang and D. S. Su, Green Chem., 2017, 19, 1052–1062 RSC.
- M. Jung, S.-g Hong and J. Moon, Mater. Des., 2020, 193, 108813 CrossRef CAS.
- X. T. Cao, M. Prakash Patil, Q. T. Phan, C. M.-Q. Le, B.-H. Ahn, G.-D. Kim and K. T. Lim, J. Ind. Eng. Chem., 2020, 83, 173–180 CrossRef CAS.
- S. Mallakpour, A. Abdolmaleki and F. Azimi, Ultrason. Sonochem., 2017, 39, 589–596 CrossRef CAS PubMed.
- C. M.-Q. Le, X. T. Cao and K. T. Lim, Ultrason. Sonochem., 2017, 39, 321–329 CrossRef CAS PubMed.
- C. P. Li, B. K. Teo, X. H. Sun, N. B. Wong and S. T. Lee, Chem. Mater., 2005, 17 Search PubMed.
- S.-H. Jeong, J.-H. Ko, J.-B. Park and W. Park, J. Am. Chem. Soc., 2004, 126, 15982–15983 CrossRef CAS PubMed.
- S. E. Skrabalak, Phys. Chem. Chem. Phys., 2009, 11, 4930–4942 RSC.
- A. D. Goswami, D. H. Trivedi, N. L. Jadhav and D. V. Pinjari, J. Environ. Chem. Eng., 2021, 9, 106118 CrossRef CAS.
- V. Romanovicz, B. A. Berns, S. D. Carpenter and D. Carpenter, Int. J. Energy Power Eng., 2013, 7, 665–668 Search PubMed.
- M. Kumar and Y. Ando, J. Phys.: Conf. Ser., 2007, 61, 129 CrossRef.
- T. U. Wani, R. Mohi-Ud-Din, T. A. Wani, R. H. Mir, A. M. Itoo, F. A. Sheikh, N. A. Khan and F. H. Pottoo, Curr. Pharm. Biotechnol., 2021, 22, 793–807 CAS.
- S. K. Verma, A. K. Das, S. Gantait, V. Kumar and E. Gurel, Sci. Total Environ., 2019, 667, 485–499 CrossRef CAS PubMed.
- M. Endo, K. Takeuchi, Y. A. Kim, K. C. Park, T. Ichiki, T. Hayashi, T. Fukuyo, S. Iinou, D. S. Su, M. Terrones and M. S. Dresselhaus, ChemSusChem, 2008, 1, 820–822 CrossRef CAS PubMed.
- Q. Zhang, M.-Q. Zhao, J.-Q. Huang, Y. Liu, Y. Wang, W.-Z. Qian and F. Wei, Carbon, 2009, 47, 2600–2610 CrossRef CAS.
- W.-D. Zhang, I. Y. Phang and T. X. Liu, Adv. Mater., 2006, 18, 73–77 CrossRef CAS.
- A. Kumar, Y. Kostikov, M. Zanatta, G. D. Sorarù, B. Orberger, G. D. Nessim and G. Mariotto, Diamond Relat. Mater., 2019, 97, 107433 CrossRef CAS.
- S. Malik, Polyhedron, 2018, 152, 90–93 CrossRef CAS.
- N. Tripathi, V. Pavelyev and S. S. Islam, Appl. Nanosci., 2017, 7, 557–566 CrossRef CAS.
- D. Janas, Sustainability, 2020, 12, 4115 CrossRef CAS.
- J. Qu, C. Luo, Q. Cong and X. Yuan, J. Mater. Cycles Waste Manag., 2014, 16, 162–166 CrossRef CAS.
- S. Paul and S. K. Samdarshi, New Carbon Mater., 2011, 26, 85–88 CrossRef CAS.
- Z. Abdel Hamid, A. Abdul Azim, F. Abdel Mouez and S. S. Abdel Rehim, J. Anal. Appl. Pyrolysis, 2017, 126, 218–229 CrossRef CAS.
- D. K. Patel, H.-B. Kim, S. D. Dutta, K. Ganguly and K.-T. Lim, Materials, 2020, 13, 1679 CrossRef CAS PubMed.
- P. Ghosh, R. A. Afre, T. Soga and T. Jimbo, Mater. Lett., 2007, 61, 3768–3770 CrossRef CAS.
- J. Zhu, J. Jia, F. L. Kwong, D. H. Leung Ng and S. C. Tjong, Biomass Bioenergy, 2012, 36, 12–19 CrossRef CAS.
- V. F. Lotfy, N. A. Fathy and A. H. Basta, J. Environ. Chem. Eng., 2018, 6, 6263–6274 CrossRef CAS.
- K. Awasthi, R. Kumar, R. S. Tiwari and O. N. Srivastava, J. Exp. Nanosci., 2010, 5, 498–508 CrossRef CAS.
- R. Kumar, R. K. Singh and R. S. Tiwari, Mater. Des., 2016, 94, 166–175 CrossRef CAS.
- R. Kumar, R. S. Tiwari and O. N. Srivastava, Nanoscale Res. Lett., 2011, 6, 92 CrossRef PubMed.
- S. Augustine, J. Singh, M. Srivastava, M. Sharma, A. Das and B. D. Malhotra, Biomater. Sci., 2017, 5, 901–952 RSC.
- M. Derakhshi, S. Daemi, P. Shahini, A. Habibzadeh, E. Mostafavi and A. A. Ashkarran, J. Funct. Biomater., 2022, 13, 27 CrossRef CAS PubMed.
- M. Ashrafizadeh, H. Saebfar, M. H. Gholami, K. Hushmandi, A. Zabolian, P. Bikarannejad, M. Hashemi, S. Daneshi, S. Mirzaei, E. Sharifi, A. P. Kumar, H. Khan, H. H. Sheikh Hossein, M. Vosough, N. Rabiee, V. K. Thakur, P. Makvandi, Y. K. Mishra, F. R. Tay, Y. Wang, A. Zarrabi, G. Orive and E. Mostafavi, Expert Opin. Drug Delivery, 2022, 19, 355–382 CrossRef CAS PubMed.
- A. Vasilescu, A. Hayat, S. Gáspár and J.-L. Marty, Electroanalysis, 2018, 30, 2–19 CrossRef CAS.
- J. Saleem, L. Wang and C. Chen, Adv. Healthcare Mater., 2018, 7, 1800525 CrossRef PubMed.
- K. Park, ACS Nano, 2013, 7, 7442–7447 CrossRef CAS PubMed.
- C. A. Poland, R. Duffin, I. Kinloch, A. Maynard, W. A. Wallace, A. Seaton, V. Stone, S. Brown, W. MacNee and K. Donaldson, Nat. Nanotechnol., 2008, 3, 423–428 CrossRef CAS PubMed.
- Y. Grosse, D. Loomis, K. Z. Guyton, B. Lauby-Secretan, F. E. Ghissassi, V. Bouvard, L. Benbrahim-Tallaa, N. Guha, C. Scoccianti, H. Mattock and K. Straif, Lancet Oncol., 2014, 15, 1427–1428 CrossRef CAS PubMed.
- D. Chen, C. A. Dougherty, K. Zhu and H. Hong, J. Controlled Release, 2015, 210, 230–245 CrossRef CAS PubMed.
- F. M. Tonelli, V. A. Goulart, K. N. Gomes, M. S. Ladeira, A. K. Santos, E. Lorençon, L. O. Ladeira and R. R. Resende, Nanomedicine, 2015, 10, 2423–2450 CrossRef CAS PubMed.
- K. Bhattacharya, S. P. Mukherjee, A. Gallud, S. C. Burkert, S. Bistarelli, S. Bellucci, M. Bottini, A. Star and B. Fadeel, Nanomedicine, 2016, 12, 333–351 CrossRef CAS PubMed.
- A. V.-V. V. Ravi Kiran, G. Kusuma Kumari and P. T. Krishnamurthy, J. Drug Delivery Sci. Technol., 2020, 59, 101892 CrossRef CAS.
- S. Raj, S. Khurana, R. Choudhari, K. K. Kesari, M. A. Kamal, N. Garg, J. Ruokolainen, B. C. Das and D. Kumar, Semin. Cancer Biol., 2021, 69, 166–177 CrossRef CAS PubMed.
- C. C. Carrion, M. Nasrollahzadeh, M. Sajjadi, B. Jaleh, G. Jamalipour Soufi and S. Iravani, Int. J. Biol. Macromol., 2021, 178, 193–228 CrossRef CAS PubMed.
- M. Nasrollahzadeh, M. Sajjadi, S. Iravani and R. S. Varma, Nanomaterials, 2020, 10, 1784, DOI:10.3390/nano10091784.
- M. Nasrollahzadeh, M. Sajjadi, S. Iravani and R. S. Varma, Chemosphere, 2021, 263, 128005 CrossRef CAS PubMed.
- S. Iravani and R. S. Varma, Mater. Adv., 2021, 2, 2906–2917 RSC.
- S. Iravani and R. S. Varma, ACS Biomater. Sci. Eng., 2021, 7, 1900–1913 CrossRef CAS PubMed.
- N. Badea, M. M. Craciun, A. S. Dragomir, M. Balas, A. Dinischiotu, C. Nistor, C. Gavan and D. Ionita, Mater. Chem. Phys., 2020, 241, 122435 CrossRef CAS.
- A. A.-G. El-Shahawy, N. Elnagar, M. Zohery, M. S. Abd Elhafeez and S. I. El-Dek, Int. J. Polym. Mater. Polym. Biomater., 2021 DOI:10.1080/00914037.2021.1925277.
- K. Aoki and N. Saito, Nanomaterials, 2020, 10, 264 CrossRef CAS PubMed.
- M. A. Saleemi, Y. L. Kong, P. V.-C. Yong and E. H. Wong, J. Drug Delivery Sci. Technol., 2020, 59, 101855 CrossRef CAS.
- M. Eskandari, S. H. Hosseini, M. Adeli and A. Pourjavadi, Iran. Polym. J., 2014, 23, 387–403 CrossRef CAS.
- Y. Zhang, Y. Bai and B. Yan, Drug Discovery Today, 2010, 15, 428–435 CrossRef CAS PubMed.
- M. R. Berber, H. Elkhenany, I. H. Hafez, A. El-Badawy, M. Essawy and N. El-Badri, Nanomedicine, 2020, 15, 793–808, DOI:10.2217/nnm-2019-0445.
- N. J. Singhai, R. Maheshwari and S. Ramteke, Colloids Interface Sci. Commun., 2020, 35, 100235 CrossRef CAS.
- Y.-J. Gu, J. Cheng, J. Jin, S. H. Cheng and W.-T. Wong, Int. J. Nanomed., 2011, 6, 2889–2898 CAS.
- Y. Wang, C. Wang, Y. Jia, X. Cheng, Q. Lin, M. Zhu, Y. Lu, L. Ding, Z. Weng and K. Wu, PLoS One, 2014, 9, e104209 CrossRef PubMed.
- B. Koh, S. B. Park, E. Yoon, H. M. Yoo, D. Lee, J. N. Heo and S. Ahn, J. Pharm. Sci., 2019, 108, 3704–3712 CrossRef CAS PubMed.
- H. Li, N. Zhang, Y. Hao, Y. Wang, S. Jia and H. Zhang, Drug Delivery, 2019, 26, 1017–1026 CrossRef CAS PubMed.
- G. Bartholomeusz, P. Cherukuri, J. Kingston, L. Cognet, R. Lemos, T. K. Leeuw, L. Gumbiner-Russo, R. B. Weisman and G. Powis, Nano Res., 2009, 2, 279–291 CrossRef CAS PubMed.
- X. Wang, J. Ren and X. Qu, ChemMedChem, 2008, 3, 940–945 CrossRef CAS PubMed.
- A. Karmakar, S. M. Bratton, E. Dervishi, A. Ghosh, M. Mahmood, Y. Xu, L. Mohammed Saeed, T. Mustafa, D. Casciano, A. Radominska-Pandya and A. S. Biris, Int. J. Nanomed., 2011, 6, 1045–1055 CAS.
- G. Cirillo, O. Vittorio, D. Kunhardt, E. Valli, A. Farfalla, M. Curcio, U. G. Spizzirri and S. Hampel, Materials, 2019, 12, 2889 CrossRef CAS PubMed.
- S. K. Prajapati, A. Jain, C. Shrivastava and A. K. Jain, Int. J. Biol. Macromol., 2019, 123, 691–703 CrossRef CAS PubMed.
- D. Salas-Treviño, O. Saucedo-Cárdenas, M. D. Loera-Arias, H. Rodríguez-Rocha, A. García-García, R. Montes-de-Oca-Luna, E. I. Piña-Mendoza, F. F. Contreras-Torres, G. García-Rivas and A. Soto-Domínguez, Nanomaterials, 2019, 9, 1572 CrossRef PubMed.
- X. Weng, M. Wang, J. Ge, S. Yu, B. Liu, J. Zhong and J. Kong, Mol. BioSyst., 2009, 5, 1224–1231 RSC.
- Y. Zhu, Q. Sun, Y. Liu, T. Ma, L. Su, S. Liu, X. Shi, D. Han and F. Liang, R. Soc. Open Sci., 2018, 5, 180159 CrossRef PubMed.
- B. Zhang, H. Wang, S. Shen, X. She, W. Shi, J. Chen, Q. Zhang, Y. Hu, Z. Pang and X. Jiang, Biomaterials, 2016, 79, 46–55 CrossRef CAS PubMed.
- P. Zhang, W. Yi, J. Hou, S. Yoo, W. Jin and Q. Yang, Int. J. Nanomed., 2018, 13, 3069 CrossRef CAS PubMed.
- W. Yi, P. Zhang, J. Hou, W. Chen, L. Bai, S. Yoo, A. Khalid and X. Hou, Int. J. Biol. Macromol., 2018, 120, 1525–1532 CrossRef CAS PubMed.
- X. Zhang, J. Chen, Z. Weng, Q. Li, L. Zhao, N. Yu, L. Deng, W. Xu, Y. Yang, Z. Zhu and H. Huang, Mol. Immunol., 2020, 119, 48–58 CrossRef CAS PubMed.
- P. S.-O. Ozgen, S. Atasoy, B. Z. Kurt, Z. Durmus, G. Yigite and A. Dag, J. Mater. Chem. B, 2020, 8, 3123–3137 RSC.
- A. Battigelli, J. T.-W. Wang, J. Russier, T. Da Ros, K. Kostarelos, K. T. Al-Jamal, M. Prato and A. J.-S. Bianco, Small, 2013, 9, 3610–3619 CrossRef CAS PubMed.
- P. Singh, C. Samorì, F. M. Toma, C. Bussy, A. Nunes, K. T. Al-Jamal, C. Ménard-Moyon, M. Prato, K. Kostarelos and A. Bianco, J. Mater. Chem., 2011, 21, 4850–4860 RSC.
- M. S. Hasnain and A. K. Nayak, Carbon Nanotubes for Targeted Drug Delivery, Springer, 2019, pp. 1–9 DOI:10.1007/978-981-15-0910-0_1.
- R. Maleki, H. H. Afrouzi, M. Hosseini, D. Toghraie, A. Piranfar and S. Rostami, Comput. Methods Prog. Biomed., 2020, 186, 105210 CrossRef PubMed.
- N. Suo, M. Wang, Y. Jin, J. Ding, X. Gao, X. Sun, H. Zhang, M. Cui, J. Zheng, N. Li, X. Jin and S. Jiang, Int. J. Nanomed., 2019, 14, 1241–1254 CrossRef CAS PubMed.
- R. P. Morais, G. B. Novais, L. S. Sangenito, A. L.-S. Santos, R. Priefer, M. Morsink, M. C. Mendonça, E. B. Souto, P. Severino and J. C. Cardoso, Int. J. Mol. Sci., 2020, 21, 4557 CrossRef CAS PubMed.
- R. P. Singh, G. Sharma Sonali, S. Singh, S. Bharti, B. L. Pandey, B. Koch and M. S. Muthu, Mater. Sci. Eng., C, 2017, 77, 446–458 CrossRef CAS PubMed.
- Z. Ji, G. Lin, Q. Lu, L. Meng, X. Shen, L. Dong, C. Fu and X. Zhang, J. Colloid Interface Sci., 2012, 365, 143–149 CrossRef CAS PubMed.
- Y. J. Lu, K. C. Wei, C. C.-M. Ma, S. Y. Yang and J. P. Chen, Colloids Surf., B, 2012, 89, 1–9 CrossRef CAS PubMed.
- H. Liu, H. Xu, Y. Wang, Z. He and S. Li, Drug Dev. Ind. Pharm., 2012, 38, 1031–1038 CrossRef CAS PubMed.
- M. Adeli, F. Hakimpoor, M. Ashiri, R. Kabiri and M. Bavadi, Soft Matter, 2011, 7, 4062–4070 RSC.
- F. Yang, C. Jin, D. Yang, Y. Jiang, J. Li, Y. Di, J. Hu, C. Wang, Q. Ni and D. Fu, Eur. J. Cancer, 2011, 47, 1873–1882 CrossRef CAS PubMed.
- M. Mohammadi, Z. Salmasi, M. Hashemi, F. Mosaffa, K. Abnous and M. Ramezani, Int. J. Pharm., 2015, 485, 50–60 CrossRef CAS PubMed.
- M. R. McDevitt, D. Chattopadhyay, B. J. Kappel, J. S. Jaggi, S. R. Schiffman, C. Antczak, J. T. Njardarson, R. Brentjens and D. A. Scheinberg, J. Nucl. Med., 2007, 48, 1180–1189 CrossRef CAS PubMed.
- X. Dong, Z. Sun, X. Wang and X. Leng, Nanomedicine, 2017, 13, 2271–2280 CrossRef CAS PubMed.
- J. Chen, S. Chen, X. Zhao, L. V. Kuznetsova, S. S. Wong and I. Ojima, J. Am. Chem. Soc., 2008, 130, 16778–16785 CrossRef CAS PubMed.
- Z. Zhang, X. Yang, Y. Zhang, B. Zeng, S. Wang, T. Zhu, R. B.-S. Roden, Y. Chen and R. Yang, Clin. Cancer Res., 2006, 12, 4933–4939 CrossRef CAS PubMed.
- P. S.-O. Ozgen, S. Atasoy, B. Z. Kurt, Z. Durmus, G. Yigit and A. Dag, J. Mater. Chem. B, 2020, 8, 3123–3137 RSC.
- A. R. Genady, D. Fong, S. R. Slikboer, M. E. El-Zaria, R. Swann, N. Janzen, A. Faraday, S. A. McNelles, M. Rezvani, S. Sadeghi, A. Adronov and J. F. Valliant, ACS Appl. Nano Mater., 2020, 3, 11819–11824 CrossRef CAS.
- L. Golubewa, T. Kulahava, Y. Kunitskaya, P. Bulai, M. Shuba and R. Karpicz, Biochem. Biophys. Res. Commun., 2020, 529, 647–651 CrossRef CAS PubMed.
- P. Sundaram and H. Abrahamse, Int. J. Mol. Sci., 2020, 21, 4745 CrossRef CAS PubMed.
- L. Ceppi, N. M. Bardhan, Y. Na, A. Siegel, N. Rajan, R. Fruscio, M. G. D. Carmen, A. M. Belcher and M. J. Birrer, ACS Nano, 2019, 13, 5356–5365 CrossRef CAS PubMed.
- A. M. Díez-Pascual, Macromolecules, 2021, 1, 64–83 Search PubMed.
- R. Dubey, D. Dutta, A. Sarkar and P. Chattopadhyay, Nanoscale Adv., 2021, 3, 5722–5744 RSC.
- K. Kostarelos, Nat. Biotechnol., 2008, 26, 774–776 CrossRef CAS PubMed.
- V. M. Harik, Toxicol. Lett., 2017, 273, 69–85 CrossRef CAS PubMed.
- T. M. Alharbi, Q. Li and C. L. Raston, ACS Sustainable Chem. Eng., 2021, 9, 16044–16051 CrossRef CAS.
- S. K. Smart, A. I. Cassady, G. Q. Lu and D. J. Martin, Carbon, 2006, 44, 1034–1047 CrossRef CAS.
- A. M. Gaffney, M. J. Santos-Martinez, A. Satti, T. C. Major, K. J. Wynne, Y. K. Gun'ko, G. M. Annich, G. Elia and M. W. Radomski, Nanomedicine, 2015, 11, 39–46 CrossRef CAS PubMed.
- P. Ravichandran, S. Baluchamy, R. Gopikrishnan, S. Biradar, V. Ramesh, V. Goornavar, R. Thomas, B. L. Wilson, R. Jeffers, J. C. Hall and G. T. Ramesh, J. Biol. Chem., 2011, 286, 29725–29733 CrossRef CAS PubMed.
- A. Bianco, K. Kostarelos and M. Prato, Chem. Commun., 2011, 47, 10182–10188 RSC.
- J. Chłopek, B. Czajkowska, B. Szaraniec, E. Frackowiak, K. Szostak and F. Béguin, Carbon, 2006, 44, 1106–1111 CrossRef.
- T. V. Galassi, M. Antman-Passig, Z. Yaari, J. Jessurun, R. E. Schwartz and D. A. Heller, PLoS One, 2020, 15, e0226791 CrossRef CAS PubMed.
- X. Liang, H. Li, J. Dou, Q. Wang, W. He, C. Wang, D. Li, J.-M. Lin and Y. Zhang, Adv. Mater., 2020, 32, 2000165 CrossRef CAS PubMed.
- X. Zhang, M. Liu, X. Zhang, F. Deng, C. Zhou, J. Hui, W. Liu and Y. Wei, Toxicol. Res., 2015, 4, 160–168 CrossRef.
- S. Jain, S. M. Dongave, T. Date, V. Kushwah, R. R. Mahajan, N. Pujara, T. Kumeria and A. Popat, ACS Biomater. Sci. Eng., 2019, 5, 3361–3372 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |