Open Access Article
Open Access ArticleA class of novel luminescent layered double hydroxide nanotubes†
Dimy Nanclares ,
Alysson F. Morais
,
Alysson F. Morais *,
Thainá Calaça
*,
Thainá Calaça ,
Ivan G. N. Silva
,
Ivan G. N. Silva and
Danilo Mustafa
and
Danilo Mustafa
Instituto de Física, Universidade de São Paulo, São Paulo/SP, Brazil. E-mail: alyssonfmorais@gmail.com; Tel: +55 11 3091 6879
First published on 15th July 2021
Abstract
Herein, we report a class of novel lanthanide-doped self-supported layered double hydroxide (LDH) nanotubes featuring a combination of micro- and mesoporosity. The synthesis of the nanotubes has been achieved by a soft-templating strategy. Incorporation of La3+, Pr3+, Nd3+, Sm3+, Eu3+, Gd3+ or Tb3+ in the LDHs assisted the self-assembly of the double hydroxide layers onto the surface of Pluronic P-123 worm-like micelles, enabling the formation of the nanotubes. Removal of the micellar template provides accessibility to the mesopores, yielding a network of hollow cylindrical nanotubes with internal diameter of about 10 nm. An antenna molecule (benzene-1,3,5-tricarboxylate, BTC) is hosted in their 1-nanometre-wide micropores. Upon UV excitation, the nanotubes emit light in a set of wavelengths ranging from the ultraviolet to the infrared.
Introduction
To address the high demand for new functional materials from fields such as catalysis, separation science, chromatography, gas storage and sensing, major efforts have been invested in the development of new hierarchically porous materials with tunable composition, pore size and chemical functionality.1–5 Layered double hydroxides (LDHs) are intrinsically microporous anion exchange matrices related to the sheet mineral brucite – Mg(OH)2. Equimolar substitution of the divalent metal cations (MII) in MII(OH)2 brucite-like layers by trivalent elements (MIII) yields positively charged [MII1−zMIIIz(OH)2]z+ lamellae. Neutral LDH solids are formed by the stacking of these hydroxide sheets with hydrated anions (An−·yH2O), leading to the general chemical formula [MII1−zMIIIz(OH)2]z+[An−]z/n·yH2O. A wide set of polyvalent metals and anions can be introduced, respectively, in the hydroxide layers and in the interlayer gallery of LDHs, making the physico-chemical properties of these synthetic minerals tunable towards the desired application. Owing to this flexibility, LDHs have been successfully applied as solid electrolytes, catalysts, adsorbents and drug delivery vectors.6–13In regard to the porous structure of LDHs, they are typically microporous solids that exhibit low specific surface area. Nanostructuring is a promising route to increase the specific surface area of LDHs.14 In addition, the combination of micro- and mesoporosity in hierarchical 3D morphologies should lead to LDHs with improved diffusional and adsorptive properties. Development of novel hierarchical LDH nanoparticles featuring large and accessible mesopores could provide interesting opportunities in catalysis and nanomedicine.15,16
Carbon nanotubes, NiCo2O4 nanowires and Fe3O4 microspheres have successfully been used to produce hard template-supported LDH nanoparticles.17–20 While these formulations successfully yielded 3D nanostructures, the presence of the support blocks mesopore accessibility. To date, only a few self-supported hierarchical LDHs structures have been reported, with the production of nanospheres, microspheres, nanocones and nanoscrolls.21–24 Production of self-supported LDH nanoparticles with accessible mesopores could be within reach by using soft templating methods to induce the self-assembly of the double hydroxides layers onto the soft-template surface. A mesoporous structure would be obtained after removal of the structure directing agent. However, employing soft templating to produce mesoporous LDH nanoparticles involves some challenges. Within the hydroxide layers of LDHs, the metal cations are typically octahedrally coordinated. The close packing of these octahedral units yields a flat 2D layer with high rigidity with respect to transverse distortions and curvature.25 Thus, without the introduction of defects in the hydroxide layers, template methods aiming at inducing a 3D hierarchical morphology on LDHs tend to fail. On the other hand, it is well known that in layered rare earth hydroxides (LREH), which are layered materials related to the LDHs, a sinusoidal wave-like topology develops from the non-octahedral coordination of the rare earth elements.26,27 Inspired on this, we addressed the challenge of imposing a 3D hierarchical morphology on LDHs by introducing triply charged lanthanide ions (Ln3+) in the hydroxide layers of LDHs (ZnAlLnX%–BTC–P123, X denoting the substitution fraction Ln/(Ln + Al)). Inclusion of this non-octahedral site in the LDH structure enabled the curvature of the LDH sheets, unlocking the possibility of using a soft-templating strategy to produce a class of novel hierarchical LDH nanotubes.28–31 Pluronic® P-123 worm-like micelles were used as structure directing agents. Among the lanthanides, La3+, Pr3+, Nd3+, Sm3+, Eu3+, Gd3+ and Tb3+ were observed to induce the formation of the nanotubes. Contrastingly, inclusion of the smaller lanthanides Dy3+, Ho3+, Er3+, Tm3+, Yb3+ and Lu3+ in the LDH structure still produced platy-like LDH crystals. In the interlayer gallery, the LDH nanotubes host an antenna molecule (benzene-1,3,5-tricarboxylate, BTC), responsible to absorb UV radiation and transfer to the lanthanides.32 Upon UV excitation, the nanotubes emit light in a set of wavelengths ranging from the ultraviolet to the infrared.
In a typical synthetic procedure, ZnAlLnX%–BTC–P123 hollow nanotubes singly doped with a lanthanide ion (Ln3+) were produced as follows. Pluronic P-123 worm-like micelles were synthesized by dissolving BTC (11.5 × 10−3 mol L−1) and P-123 (0.15 wt%) in deionized water (200 mL) at 60 °C. After cooling down the solution to room temperature, a second heating cycle to 60 °C was done in order to optimize micellization. The LDHs were prepared by slowly dosing (10 mL h−1) a 10 millilitres-solution containing Zn(NO3)2·6H2O, Al(NO3)3·9H2O and Ln(NO3)3·6H2O in the stoichiometric ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–X
1–X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) X and at a total metal concentration of 1 mol L−1 into the solution containing the micelles. The pH of the mother solution was stated to pH 8 by a Metrohm 848 Titrino plus automatic titrator. After the metal solution has been completely dosed, the resulting slurry was recovered by centrifugation. Removal of the polymeric structure-directing agent was accomplished by liquid extraction with methanol in ultrasonically agitated bath (confirmed by thermogravimetric analysis, Fig. S1†). The detailed synthetic and characterization procedures are provided in the ESI.†
X and at a total metal concentration of 1 mol L−1 into the solution containing the micelles. The pH of the mother solution was stated to pH 8 by a Metrohm 848 Titrino plus automatic titrator. After the metal solution has been completely dosed, the resulting slurry was recovered by centrifugation. Removal of the polymeric structure-directing agent was accomplished by liquid extraction with methanol in ultrasonically agitated bath (confirmed by thermogravimetric analysis, Fig. S1†). The detailed synthetic and characterization procedures are provided in the ESI.†
Results and discussion
The PXRD patterns of the ZnAlLn5%–BTC–P123 LDHs are displayed in Fig. 1 (for La and from Nd to Er) and Fig. S2A† (for Pr and from Ho to Lu). The Bragg reflections were indexed based on a 3-layer hexagonal unit cell. The (003) basal peak at around 6.7° 2θ directly relates to the basal spacing of the LDHs (see Table S1†). Discounting the typical thickness of the hydroxide layers (∼0.48 nm) the average interlayer distance can be calculated to be around 0.84 nm. This is compatible with the longitudinal size of BTC and indicates the presence of this anion in the interlayer gallery with an orientation transverse to the hydroxide layers.32,33 In the region from 58° to 62° 2θ, the Ln3+-substituted LDHs present a broad diffraction peak formed by the (110) and (113) Bragg reflections. From the position of this broad feature, the average metal–metal distance (dMM) within the LDH sheets can be estimated as: dMM = 2d(110) ∼0.304 nm (see Table S1†).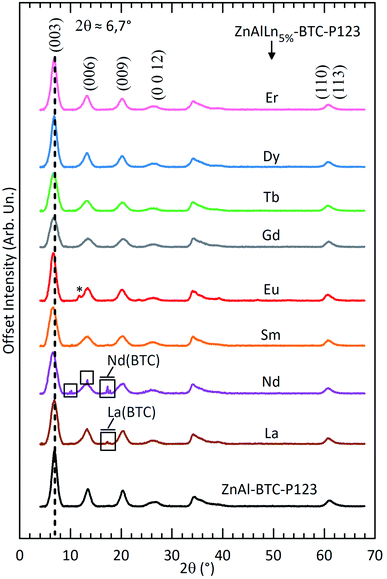 | ||
| Fig. 1 Powder X-ray diffraction patterns of ZnAl–BTC–P123 and ZnAlLn5%–BTC–P123 (Ln = La and from Nd to Er). The Bragg peaks inside the rectangles are attributed to the formation of the respective Ln(BTC) complex.35–37 The starred peak are ascribed to a carbonate-intercalated LDH phase formed in the Eu3+-containing solid. | ||
Compared to Ln3+-free ZnAl–BTC–P123 LDHs, the basal reflections for ZnAlLn5%–BTC–P123 are broadened (see Table S1†). This broadening accounts to the distortions caused in the hydroxide layers by the heteromorphic substitution of Al3+ and Zn2+ by the lanthanides. Also contributing to this broadening is the decrease in crystallite size caused by the introduction of lanthanides in the LDH sheets, as has been already observed in previous investigations.34
While the formation constant of the Ln–benzenecarboxylate complexes is kept almost unchanged over the lanthanides, the solubility constant of their hydroxide phases decreases along the lanthanide series.38,39 The combination of these two features explains the here observed trend that the limit Ln3+ concentration for which only a pure LDH phase is formed increases along the lanthanide series, as shown by PXRD. In fact, lanthanum hydroxide has the highest solubility constant over the lanthanides. Formation of the La(BTC) complex is observed already with a 3% substitution fraction (Fig. S2B†). On the other hand, no complex formation was observed in the Lu3+-containing solids up to X = 15% (Fig. S2C†).
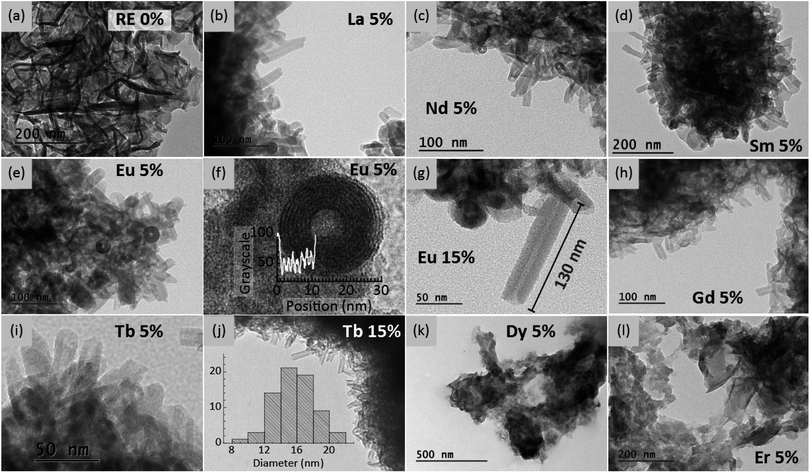
The morphology of the Ln-containing LDH particles was characterized by transmission electron microscopy (Fig. 2 and S3†). Without incorporation of lanthanides (ZnAl–BTC–P123, Fig. 2a) only flake-like LDH crystals are formed. For Tb3+ and the other lanthanides with larger ionic radius (La to Tb), the combination of Ln3+, BTC and P-123 worm-like micelles produced well defined LDH nanotubes with typical external diameter of 16 nm (inset of Fig. 2j). Contrastingly, introduction of the smaller lanthanides Dy3+, Ho3+, Er3+, Tm3+, Yb3+ and Lu3+ only yielded plate-like LDHs. Interestingly, a similar feature has been observed for LREH, for which a high coordination number for the metal sites is needed to stabilize the sinusoidal topology of these layered structures.27,40 On this basis, the decrease in coordination capacity along the lanthanide series hinders the formation of these materials. Here, the same explanation can be used to speculate on the role of the lanthanides in the self-assembly of the nanotubes: one can tentatively explain the formation of these self-supported 3D structures by the increased stability of a bent LDH sheet containing non-octahedral metal sites with high coordination number. Within this hypothesis, the high coordination capacity of the larger Ln3+ ions enables the hydroxide layers to accommodate the curvature necessary for the formation of the nanotubes.
Aside to assisting the mesostructuring of the LDHs, doping different lanthanides into the hydroxide layers renders these materials photoluminescent.41 Most remarkable are the pinksh, reddish, greenish and bluish PL of Sm3+, Eu3+, Tb3+ and Dy3+, respectively (see Fig. 3). The emission and excitation spectra of the LDH samples are shown in Fig. S4.†
The pinkish emission of the Sm3+-substituted LDHs nanotubes arises from the combination of the characteristic orange emission of Sm3+ with a relevant contribution from the violaceous fluorescence of BTC. The Sm3+ 4f–4f emission bands (Fig. S4A†) are mainly located in the visible region and correspond to the (J + 1/2) Stark components of the 4G5/2 → 6HJ/2 (J = 5, 7, 9 or 11) intraconfigurational electronic transitions with wavelengths centered at 563.8 nm, 599.4 nm, 645.8 nm and 706.8 nm, respectively. In the excitation spectrum of the Sm3+-containing nanotubes, the excitation bands are assigned to transitions from the 6H5/2 ground state to the following excited levels of Sm3+ (barycentre in cm−1): 4H9/2 (29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 085), 4D3/2 (27
085), 4D3/2 (27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 599), 6P7/2 (26
599), 6P7/2 (26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 801), 6P3/2, 4L13/2 (24
801), 6P3/2, 4L13/2 (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850), 6P5/2 (23
850), 6P5/2 (23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 964), 4I13/2 (21
964), 4I13/2 (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 632). Additionally, the broad and most intense band below 380 nm is ascribed to the S0(π) → Sn(1π*) singlet–singlet absorption of BTC with posterior energy transfer to Sm3+. The high intensity of this ligand-to-metal excitation band as compared to the direct excitation bands of Samarium shows that BTC works as an efficient antenna molecule in this system.
632). Additionally, the broad and most intense band below 380 nm is ascribed to the S0(π) → Sn(1π*) singlet–singlet absorption of BTC with posterior energy transfer to Sm3+. The high intensity of this ligand-to-metal excitation band as compared to the direct excitation bands of Samarium shows that BTC works as an efficient antenna molecule in this system.
The photoluminescence and absorption spectra of the Eu3+-containing nanotubes are displayed on Fig. S4B.† The excitation spectrum obtained by monitoring the (Eu3+)5D0 → 7F2 hypersensitive transition at 614 nm features a broad and intense band from 240 to 350 nm, ascribed to the S0(π) → Sn(1π*) transition of BTC. The sharp bands arising from direct excitation of the (Eu3+)7F0,1 → 5L6 and (Eu3+)7F0,1 → 5D2 4f–4f intraconfigurational transitions of Eu3+ are also visible at 394.3 and 464.7 nm, respectively. In the emission spectrum, the (Eu3+)5D0 → 7F0–6 emission bands are visible. The Eu3+-containing LDH nanotubes feature a characteristic red PL under UV excitation which is dominated by the (Eu3+)D0 → 7F2 hypersensitive transition. As the (Eu3+)D0 → 7F2 and (Eu3+)D0 → 7F4 emissions are much more intense than the (Eu3+)D0 → 7F1, it is mostly likely that the lanthanide ions in the hydroxide layers are hosted in a site with no inversion symmetry.42 This clearly rules out the octahedral symmetry commonly observed for the metal sites in LDHs and further indicate that the inclusion of the lanthanides in these materials distorts the hydroxide layers.
The photoluminescence (PL) of the Tb-containing LDH nanotubes (Fig. S4C†) is dominated by the (Tb3+)5D4 → 7F5 emission, which confers the greenish PL of this material. All (Tb3+)5D4 → 7FJ (J = 0–6) emissions are visible, respectively at 488.9, 542.5, 584.4, 622.2, 647.8, 671.0 and 681.6 nm. Given the very high ligand-to-metal excitation band observed in the excitation spectrum of ZnAlTb5%–BTC–P123, the excitation bands of the Tb3+ activators are not discernible, which shows that BTC works as an excellent antenna in the Tb-containing nanotubes, giving rise to samples with very bright PL under UV excitation (Fig. 3). In fact, the energy of the Tb3+ emitting level (5D4, ∼20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 cm−1) is resonant with the T1(3π*) band of BTC (∼ from 18
440 cm−1) is resonant with the T1(3π*) band of BTC (∼ from 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 to 25
600 to 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cm−1), which facilitates intersystem energy transfer.
000 cm−1), which facilitates intersystem energy transfer.
The emission spectrum of platy-like LDHs ZnAlDy5%–BTC–P123 is given in Fig. S4D.† The (Dy3+)4F9/2 → 6HJ (J = 15, 13, 11, 9 or 7) intraconfigurational transitions are observed at, respectively, 480.8, 574.4, 663.8, 751.7 and 835.0 nm. The ligand-to-metal energy transfer band with promotion of the S0(π) → Sn(1π*) singlet–singlet transition of BTC dominates the excitation spectrum indicating a efficient sensitization of Dy3+, which features a bright bluish PL under UV excitation.
A violaceous PL is observed for the La-, Nd-, Gd- and Er-containing LDHs, attributed to the fluorescence of BTC in the UV/violet region (Fig. S5†). BTC fluorescence is observable either due to the lack of an efficient ligand-to-metal energy transfer route (the case for the La- and Gd-containing materials) or due to luminescence quenching in systems containing the narrow band gap lanthanides Nd3+ and Er3+. In fact, for La3+, an element with an empty 4f shell, no BTC-to-La energy transfer can occur, making light emission from BTC more likely to occur. Also, Gd3+ is a half-full 4f shell metal featuring a wide energy band gap (∼32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 cm−1) between the 8S7/2 ground state and the 6P7/2 first excited level.43 The (Gd3+)6P7/2 level is far above the triplet levels of BTC, making that no ligand-to-metal energy transfer can occur.44
200 cm−1) between the 8S7/2 ground state and the 6P7/2 first excited level.43 The (Gd3+)6P7/2 level is far above the triplet levels of BTC, making that no ligand-to-metal energy transfer can occur.44
Conclusions
Introduction of large lanthanide sites in LDHs assisted the self-assembly of self-supported LDH nanotubes featuring a combination of micro- and mesoporosity. Synthesis of nanotubes containing La3+, Pr3+, Nd3+, Sm3+, Eu3+, Gd3+ or Tb3+ was accomplished by a soft templating strategy with the use of Pluronic P-123 worm-like micelles as structure directing agents. Not all the elements in the lanthanide series yielded nanotubes, with the inclusion of the smaller cations Dy3+, Ho3+, Er3+, Tm3+, Yb3+ and Lu3+ in the double hydroxide layers still producing platy-like LDH crystals. This indicates that the inclusion of sites with high coordination number in the LDHs is key to unlock the possibility of using soft templating strategies to produce novel 3D LDH morphologies.Author contributions
DN: investigation, data curation, formal analysis and writing – original draft. AFM: conceptualization, methodology, formal analysis, data validation, writing – original draft and writing – review & editing. TC: investigation (La3+, Dy3+ and Tm3+-containing samples). IGNS: conceptualization, supervision and writing – review and editing. DM: conceptualization, project administration, funding acquisition and supervision. All authors discussed the results and revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the Laboratory of Crystallography (IFUSP, São Paulo) for assistance with the PXRD measurements and Alfredo Duarte (Central Analítica – IQUSP, São Paulo) for assistance with the TEM measurements. This research was funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, 2015/19210-0 and 2018/13837-0), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, 1723707, Finance Code 001) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, 142127/2014-0, 403055/2016-4 and 140196/2017-0).References
- X.-Y. Yang, L.-H. Chen, Y. Li, J. C. Rooke, C. Sanchez and B.-L. Su, Chem. Soc. Rev., 2017, 46, 481–558 RSC.
- M.-H. Sun, S.-Z. Huang, L.-H. Chen, Y. Li, X.-Y. Yang, Z.-Y. Yuan and B.-L. Su, Chem. Soc. Rev., 2016, 45, 3479–3563 RSC.
- Y. Zhong, G. Chen, X. Liu, D. Zhang, N. Zhang, J. Li, S. Liang, R. Ma and G. Qiu, Nanoscale, 2017, 9, 8185–8191 RSC.
- H. Wang, Z. Sun, X. Zou, J. Ren and C. Zhang, Nanoscale, 2021, 13, 6241–6247 RSC.
- C. Perego and R. Millini, Chem. Soc. Rev., 2014, 42, 3956–3976 RSC.
- A. I. Khan and D. O'Hare, J. Mater. Chem., 2002, 12, 3191–3198 RSC.
- G. Fan, F. Li, D. G. Evans and X. Duan, Chem. Soc. Rev., 2014, 43, 7040–7066 RSC.
- R. Santos, J. Tronto, V. Briois and C. Santilli, J. Mater. Chem. A, 2017, 5, 9998–10009 RSC.
- S. Zhang, F. Yao, L. Yang, F. Zhang and S. Xu, Carbon, 2015, 93, 143–150 CrossRef CAS.
- L. Mohapatra and K. Parida, J. Mater. Chem. A, 2016, 4, 10744–10766 RSC.
- A. Khenifi, Z. Derriche, C. Mousty, V. Prévot and C. Forano, Appl. Clay Sci., 2010, 47, 362–371 CrossRef CAS.
- K. Ladewig, Z. P. Xu and G. Q. M. Lu, Expert Opin. Drug Delivery, 2009, 6, 907–922 CrossRef CAS PubMed.
- X. Bi, H. Zhang and L. Dou, Pharmaceutics, 2014, 6, 298–332 CrossRef CAS PubMed.
- Y. Zhao, G. Chen, T. Bian, C. Zhou, G. I. N. Waterhouse, L. Wu, C. Tung, L. J. Smith, D. O'Hare and T. Zhang, Adv. Mater., 2015, 27, 7824–7831 CrossRef CAS PubMed.
- Y. Li, D. Liu, H. Ai, Q. Chang, D. Liu, Y. Xia, S. Liu, N. Peng, Z. Xi and X. Yang, Nanotechnology, 2010, 21, 105101 CrossRef PubMed.
- S. Li, J. Li, C. J. Wang, Q. Wang, M. Z. Cader, J. Lu, D. G. Evans, X. Duan and D. O'Hare, J. Mater. Chem. B, 2013, 1, 61–68 RSC.
- X. Chen, F. Mi, H. Zhang and H. Zhang, Mater. Lett., 2012, 69, 48–51 CrossRef CAS.
- L. Li, Y. Feng, Y. Li, W. Zhao and J. Shi, Angew. Chem., Int. Ed., 2009, 48, 5888–5892 CrossRef CAS PubMed.
- L. Huang, D. Chen, Y. Ding, S. Feng, Z. L. Wang and M. Liu, Nano Lett., 2013, 13, 3135–3139 CrossRef CAS PubMed.
- J. Zhao, J. Chen, S. Xu, M. Shao, Q. Zhang, F. Wei, J. Ma, M. Wei, D. G. Evans and X. Duan, Adv. Funct. Mater., 2014, 24, 2938–2946 CrossRef CAS.
- X. Liu, R. Ma, Y. Bando and T. Sasaki, Adv. Mater., 2012, 24, 2148–2153 CrossRef CAS.
- L. Li, R. Ma, N. Iyi, Y. Ebina, K. Takada and T. Sasaki, Chem. Commun., 2006, 3125–3127 RSC.
- M. A. Rocha, P. A. Petersen, E. Teixeira-Neto, H. M. Petrilli, F. Leroux, C. Taviot-Gueho and V. R. Constantino, RSC Adv., 2016, 6, 16419–16436 RSC.
- M. Shao, F. Ning, Y. Zhao, J. Zhao, M. Wei, D. G. Evans and X. Duan, Chem. Mater., 2012, 24, 1192–1197 CrossRef CAS.
- M. Thorpe and S. Solin, Access in Nanoporous Materials, Springer, 2002, pp. 59–71 Search PubMed.
- R. Ma and T. Sasaki, Adv. Mater., 2010, 22, 5082–5104 CrossRef CAS.
- J. Liang, R. Ma and T. Sasaki, Dalton Trans., 2014, 43, 10355–10364 RSC.
- A. F. Morais, I. G. Silva, S. P. Sree, F. M. de Melo, G. Brabants, H. F. Brito, J. A. Martens, H. E. Toma, C. E. Kirschhock and E. Breynaert, et al., Chem. Commun., 2017, 53, 7341–7344 RSC.
- I. G. N. Silva, A. F. Morais, B. C. Lima, F. A. Garcia and D. Mustafa, Appl. Clay Sci., 2020, 199, 105861 CrossRef CAS.
- A. F. Morais, I. G. N. Silva, B. C. Lima, F. A. Garcia and D. Mustafa, ACS Omega, 2020, 5, 23778–23785 CrossRef CAS PubMed.
- A. F. Morais, D. Nanclares, I. G. N. Silva, A. Duarte, F. A. Garcia, E. Breynaert and D. Mustafa, Nanoscale, 2021 10.1039/D1NR02477A.
- A. F. Morais, F. O. Machado, A. C. Teixeira, I. G. N. Silva, E. Breynaert and D. Mustafa, J. Alloys Compd., 2019, 771, 578–583 CrossRef CAS.
- B. Shao, P. Feng, X. Wang, F. Cui and X. Yang, J. Phys. Chem. C, 2019, 123, 7467–7474 CrossRef CAS.
- A. W. Musumeci, Z. P. Xu, S. V. Smith, R. F. Minchin and D. J. Martin, J. Nanopart. Res., 2010, 12, 111–120 CrossRef CAS.
- I. G. Silva, D. Mustafa, B. Andreoli, M. C. Felinto, O. L. Malta and H. F. Brito, J. Lumin., 2016, 170, 364–368 CrossRef CAS.
- C. Daiguebonne, O. Guilloa, Y. Gérault, A. Lecerf and K. Boubekeur, Inorg. Chim. Acta, 1999, 284, 139–145 CrossRef CAS.
- C. Serre, F. Millange, C. Thouvenot, N. Gardant, F. Pellé and G. Férey, J. Mater. Chem., 2004, 14, 1540–1543 RSC.
- G. R. Choppin, P. A. Bertrand, Y. Hasegawa and E. N. Rizkalla, Inorg. Chem., 1982, 21, 3722–3724 CrossRef CAS.
- P. L. Brown and C. Ekberg, Hydrolysis of Metal Ions - Chap 6: Scandium, Yttrium and the Lanthanide Metals, Wiley, 2016 Search PubMed.
- B. Shao, P. Feng, X. Wang, F. Cui and X. Yang, J. Phys. Chem. C, 2019, 123, 7467–7474 CrossRef CAS.
- A. W. Musumeci, Z. P. Xu, S. V. Smith, R. F. Minchin and D. J. Martin, J. Nanopart. Res., 2010, 12, 111–120 CrossRef CAS.
- K. Binnemans, Coord. Chem. Rev., 2015, 295, 1–45 CrossRef CAS.
- P. S. Peijzel, P. Vermeulen, W. J. M. Schrama, A. Meijerink, M. F. Reid and G. W. Burdick, Phys. Rev. B, 2005, 71, 125126 CrossRef.
- E. R. Souza, I. G. Silva, E. E. Teotonio, M. C. Felinto and H. F. Brito, J. Lumin., 2010, 130, 283–291 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of new compounds, X-ray diffraction, electron micrographs, photoluminescence spectroscopy and thermogravimetric curves. See DOI: 10.1039/d1ra03948b |
| This journal is © The Royal Society of Chemistry 2021 |