Open Access Article
Open Access ArticleChallenges in application of Raman spectroscopy to biology and materials
Nikki Kuhar†
a,
Sanchita Sil†
b,
Taru Verma†
c and
Siva Umapathy
 *ad
*ad
aDepartment of Inorganic & Physical Chemistry, Indian Institute of Science, Bangalore, India-560012. E-mail: umapathy@iisc.ac.in
bDefence Bioengineering & Electromedical Laboratory, DRDO, C V Raman Nagar, Bangalore, India-560093
cCentre for Biosystems Science and Engineering, Indian Institute of Science, Bangalore, India-560012
dDepartment of Instrumentation & Applied Physics, Indian Institute of Science, Bangalore, India-560012
First published on 20th July 2018
Abstract
Raman spectroscopy has become an essential tool for chemists, physicists, biologists and materials scientists. In this article, we present the challenges in unravelling the molecule-specific Raman spectral signatures of different biomolecules like proteins, nucleic acids, lipids and carbohydrates based on the review of our work and the current trends in these areas. We also show how Raman spectroscopy can be used to probe the secondary and tertiary structural changes occurring during thermal denaturation of protein and lysozyme as well as more complex biological systems like bacteria. Complex biological systems like tissues, cells, blood serum etc. are also made up of such biomolecules. Using mice liver and blood serum, it is shown that different tissues yield their unique signature Raman spectra, owing to a difference in the relative composition of the biomolecules. Additionally, recent progress in Raman spectroscopy for diagnosing a multitude of diseases ranging from cancer to infection is also presented. The second part of this article focuses on applications of Raman spectroscopy to materials. As a first example, Raman spectroscopy of a melt cast explosives formulation was carried out to monitor the changes in the peaks which indicates the potential of this technique for remote process monitoring. The second example presents various modern methods of Raman spectroscopy such as spatially offset Raman spectroscopy (SORS), reflection, transmission and universal multiple angle Raman spectroscopy (UMARS) to study layered materials. Studies on chemicals/layered materials hidden in non-metallic containers using the above variants are presented. Using suitable examples, it is shown how a specific excitation or collection geometry can yield different information about the location of materials. Additionally, it is shown that UMARS imaging can also be used as an effective tool to obtain layer specific information of materials located at depths beyond a few centimeters.
Introduction
‘VidyaViniyogaVikasah’ (a Sanskrit phrase) means progress comes from proper application of knowledge. This statement is true for the research and development in the field of Raman spectroscopy, which is considered as an evolving technology towards a direct impact to society. Although the discovery happened in 1928, the number of publications remained low until the 1960s; the web of science data indicates that there was an upsurge in the number of publications in the decade following the invention of lasers followed by a steady growth in the number of publications. The 90's saw a revival in Raman research both in theory as well as on instrumentation. Its potential applications were understood immediately as this is a straightforward technique without much need for sample preparation and almost any material, in any state under extreme conditions, can be probed. In addition, due to its non-destructive nature, it can be used as a technique for in situ measurements. Moreover, Raman microscopy has made it possible to probe very small quantity of materials with high spatial resolution. With the advent of techniques like Scanning Near-Field Microscopy (SNOM) and Tip-Enhanced Raman Spectroscopy (TERS), resolution of the order of few nanometres can also be achieved. This advantage makes it an essential and unique technique in the field of biology and materials science to explore the molecular basis of structure and function.1–4Progress in the field of Raman spectroscopy can be largely attributed to the recent advances made in the areas of instrumentation.5 Historically, analytical applications involving Raman spectroscopy was thought to be difficult, however, developments in the sources such as diode lasers, charge-coupled devices (CCDs), small spectrometers, optics along with interfacing optical fibres with Raman have made it a novel method for analytical applications. Confluence of nanotechnology and Raman spectroscopy which led to the discovery of Surface Enhanced Raman Spectroscopy (SERS) has opened new vistas for detection of analytes at very low concentrations. It is possible to obtain Raman signals of biomolecules, chemicals, explosives, drugs etc. at parts per billion (even at single molecule level) with great sensitivity and specificity6–17
Raman spectroscopy owing to its molecular specificity can be a boon, however, in the case of biological samples, it complicates the analyses. Firstly, all biological systems are composed of biochemicals such as lipids, proteins, nucleic acids, carbohydrates etc. Therefore, vibrations from all the modes comprising the chemicals shall be manifested in the Raman spectra. To an untrained eye, spectra of a cell, tissue and a bacterium may appear very similar. Therefore, analyses of such convoluted complex spectra and assignments can be a challenging endeavour. Despite this, these complex spectra can yield a treasure of information pertaining to the structure and dynamics of the sample under evaluation. Once the assignments are made, the whole world of biochemical processes, such as metabolic pathways or dynamics can be unearthed. The second challenge towards obtaining Raman spectra of biomolecules is the occurrence of peaks from the matrix. For instance, paraffin fixed tissue may show a similar peak to a C–H stretch.18,19 Differentiating the actual spectra from the matrix therefore, becomes an equally important part before analyses. Thirdly, understanding the system under study and making an informed judgement based on the experiments and correlating it with the available data is crucial. Finally, signal processing of a large number of data set can be a contributing factor towards understanding of the system.
The objective of this article is based on the review of our work to demonstrate the challenges and potential of Raman spectroscopy for applications in biology and materials. Emphasis has largely been placed on the challenges of using Raman spectroscopic techniques for studying biomolecules and non-invasive, depth sensitive identification of materials. This laboratory has been working on various facets of Raman spectroscopy for the last three decades. Only a few results pertaining towards research on biological systems and materials have been included in this review which is organized in the following manner:
(1) Discussion on the challenges of probing biomolecules using Raman spectroscopy. Various examples on Raman spectroscopic study of biomolecules such as proteins, lipids etc. have been discussed. Furthermore, examples of Raman spectroscopy for disease diagnosis have been presented. In particular, identifying and assigning Raman spectral bands to specific molecular structures in relation to multitude of complex biomolecules present in a system.
(2) Discussion on depth sensitive Raman measurements such as spatially offset Raman spectroscopy, universal multiple angle Raman spectroscopy towards depth sensitive detection of chemicals (explosives) and transmission Raman spectroscopy. Recent developments and the relative advantages have also been presented.
Results and discussions
(I) Raman spectroscopy towards biological applications
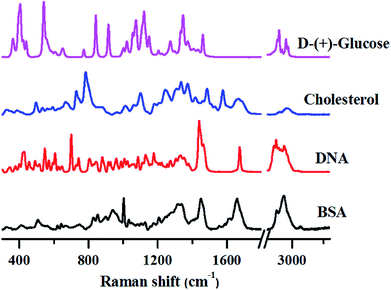
The following sections review how fundamental studies on different types of biomolecules can be carried out and also the complications involved in Raman band assignments using Raman spectroscopy. We also discuss some of the very recent applications of Raman spectroscopy in translational research, citing examples of how this technique can help in understanding and diagnosing different diseases like cancer, malaria, dengue and others.To demonstrate this aspect, we have recorded Raman spectra of pure biomolecules belonging to different classes-protein (Bovine Serum Albumin (BSA)), DNA (calf-thymus DNA), lipids (cholesterol) and carbohydrates (glucose), as shown in Fig. 1.
Although the Raman spectra shown above are quite complex, several unique marker bands can be identified for each of these biomolecules as explained below.
Proteins. Proteins have numerous functions like catalyzing many reactions, guiding the flow of electrons in reactions, transmitting information between specific cells, controlling the passage of molecules around the cell membrane or providing the filamentous architecture within cells etc. They are characterized by their unique three-dimensional architecture.22 A small change in the conformation can make them biologically inactive and may even be toxic. Various life-threatening diseases have been associated with such denaturation of proteins for e.g., Alzheimer's disease, Parkinson's disease etc.23,24 Raman spectroscopy is an excellent tool to look at the protein structure. Several vibrational modes can be used for the analysis of peptide structure. Most characteristic bands are associated with the CONH group, referred to as amide A (NH stretching, about 3500 cm−1), amide B (NH stretching, about 3100 cm−1) and Amide I to VII.25 Out of all, Amide I & III are frequently used to estimate the secondary structure of proteins. Amide I mode which ranges from 1580 cm−1 to 1700 cm−1 arises from stretching vibration of C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.26 It is very sensitive to the backbone conformation and not affected by the side chains. Amide I band can be deconvoluted with various sub-bands which directly correlate with various secondary structures. Similarly, Amide III band (1200 cm−1–1300 cm−1) which arises from C–N stretching coupled with N–H bending vibrations can also give complementary information about the protein backbone conformations.27,28 Asher's group suggested an empirical relation between Amide III frequency and dihedral angle Φ, which paves the way to analyse conformation of proteins using Amide III band of Raman spectra.29,30
O.26 It is very sensitive to the backbone conformation and not affected by the side chains. Amide I band can be deconvoluted with various sub-bands which directly correlate with various secondary structures. Similarly, Amide III band (1200 cm−1–1300 cm−1) which arises from C–N stretching coupled with N–H bending vibrations can also give complementary information about the protein backbone conformations.27,28 Asher's group suggested an empirical relation between Amide III frequency and dihedral angle Φ, which paves the way to analyse conformation of proteins using Amide III band of Raman spectra.29,30Apart from the secondary structure, characteristic Raman bands have also been identified for various side chains and disulphide regions as well. George J. Thomas resolved the signature for each of the eight cysteines per 666-residue subunit in the trimeric tall spike of an icosahedral bacteriophage P22.31 They demonstrated that a broad range of S–H⋯X interactions is accessible to the protein. The integration of microscope in Raman spectrometer has enabled studying of protein crystals, which led to genesis of a new technique termed as Raman crystallography.32 Raman crystallography can identify and follow the conformations of reaction intermediates in single crystals. Helfand et al. monitored the reactions between three clinically relevant inhibitors, tazobactam, sulbactam, and clavulanic acid, and SHV beta-lactamase (EC 3.5.2.6) in single crystals using a Raman microscope and observed acyl-enzyme formed between Ser70 and the lactam ring's C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group as a key intermediate in the reaction pathway.33 Various characteristic Raman bands for proteins are summarized in Table 1.
O group as a key intermediate in the reaction pathway.33 Various characteristic Raman bands for proteins are summarized in Table 1.
| Contributing group | Frequency (cm−1) | Assignment |
|---|---|---|
| a α: alpha helices, β: beta sheets, GGG: gauche–gauche–gauche, GGT: gauche–gauche–trans, GTG: gauche–trans–gauche, TGT: trans–gauche–trans. | ||
| Backbone | 1670–1680 | β turn, random structure, Amide I26 |
| 1660–1670 | β sheet, Amide I34 | |
| 1650–1655 | α helices, Amide I35 | |
| 1630–1635 | β sheet, Amide I36,37 | |
| 1550 | Amide II25 | |
| 1270–1300 | α helices, Amide III38 | |
| 1240–1250 | Random structure, Amide III39 | |
| 1230–1240 | β sheet, Amide III39 | |
| Disulphide | 505–515 | GGG conformation40,41 |
| 520–530 | GGT, GTG conformation40,41 | |
| 540–545 | TGT conformation40,41 | |
| Side chain | 1615, 830, 643 | Tyrosine42 |
| 850/830 | Hydrogen bonding state of tyrosine;43 | |
| 1550, 1010, 750 | Tryptophan44 | |
| 880/1361 | Ring environment of indole ring of tryptophan45 | |
| 1360/1340 | Hydrophobicity marker for tryptophan44 | |
| 1609, 1205, 1003 | Phenylalanine46 | |
Nucleic acids. Richard C. Lord and co-workers recorded the Raman spectra of various nucleic acid constituents for the first time in 1960.47 Further improvements in the instrumentation have enabled studying of various problems concerning the structure and interaction in nucleic acids, nucleic acid hydration, interaction with metal ions, DNA melting, DNA damage etc.48–51 Raman spectra of nucleic acids contain a large number of bands originating from various moieties like sugar, phosphate, bases etc. which provide very useful information about backbone conformation, base pairing etc.
Very strong Raman peaks are observed by ring breathing modes of various bases in the region of 600 cm−1 to 800 cm−1.20 Therefore, this region can be used to differentiate various bases. Out of all the bases, thymine has a very distinctive peak at around 1671 cm−1 originating from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching which makes it easily distinguishable from rest of the bases.20 Different conformations of backbone (A-DNA, B-DNA and Z-DNA) can be identified using Raman spectra. J. M. Benevides and co-workers have studied different backbone conformation at various salt conformations. They could qualitatively identify the A, B and Z-DNA using phosphodiester Raman band lying in the region of 750 to 800 cm−1.52 Apart from the above, many important group vibrations have also been assigned for nucleic acids in vibrational spectra, for example, region from 1800–1500 cm−1 has been assigned for nucleobase vibrations appear which are extremely sensitive to base pairing interactions and 1500–1250 cm−1 region have been assigned for vibrational coupling between the base–sugar entities, stacking ligation etc.53 Okotrub et al. (2014) quantified DNA in the cell the nucleus using intensity of phosphate mode at 1096 cm−1 without extraction or dye labelling using Raman spectroscopy.54 Wood's group (2013) probed DNA double-strand breaks (DSBs) with TERS coupled with Atomic Force Microscopy (AFM) and suggested that the O–C bond in the DNA backbone is most sensitive to UVC radiation and its cleavage causes DSBs most of the time.55 Deckert's group recorded the TERS spectra from an artificial DNA single strand with alternating blocks of adenine and cytosine. They could resolve adenine and cytosine bases placed at a distance of 2.1 nm by unique Raman spectral features proving that TERS can offer resolutions upto single nucleobase level.56,57 Various characteristic Raman bands for nucleic acid are summarized in Table 2.
O stretching which makes it easily distinguishable from rest of the bases.20 Different conformations of backbone (A-DNA, B-DNA and Z-DNA) can be identified using Raman spectra. J. M. Benevides and co-workers have studied different backbone conformation at various salt conformations. They could qualitatively identify the A, B and Z-DNA using phosphodiester Raman band lying in the region of 750 to 800 cm−1.52 Apart from the above, many important group vibrations have also been assigned for nucleic acids in vibrational spectra, for example, region from 1800–1500 cm−1 has been assigned for nucleobase vibrations appear which are extremely sensitive to base pairing interactions and 1500–1250 cm−1 region have been assigned for vibrational coupling between the base–sugar entities, stacking ligation etc.53 Okotrub et al. (2014) quantified DNA in the cell the nucleus using intensity of phosphate mode at 1096 cm−1 without extraction or dye labelling using Raman spectroscopy.54 Wood's group (2013) probed DNA double-strand breaks (DSBs) with TERS coupled with Atomic Force Microscopy (AFM) and suggested that the O–C bond in the DNA backbone is most sensitive to UVC radiation and its cleavage causes DSBs most of the time.55 Deckert's group recorded the TERS spectra from an artificial DNA single strand with alternating blocks of adenine and cytosine. They could resolve adenine and cytosine bases placed at a distance of 2.1 nm by unique Raman spectral features proving that TERS can offer resolutions upto single nucleobase level.56,57 Various characteristic Raman bands for nucleic acid are summarized in Table 2.
| Contributing group | Frequency (cm−1) | Assignment |
|---|---|---|
| a ν: stretching (s: symmetric, as: asymmetric), b: bending, t: twisting, df: deformation, A: adenine, T: thymine, C: cytosine, G: guanine, U: uracil, A-DNA: A-form of DNA, B-DNA: B form of DNA, Z-DNA: Z form of DNA. | ||
| Base | 726 | Ring breathing of A58 |
| 668, 746, 785 | Ring breathing of T58 | |
| 668 | Ring breathing of G58 | |
| 781, 785 | Ring breathing of C58 | |
| 785 | Ring breathing of U58 | |
| 1257 | Ring mode (C, T)58 | |
| 1336 | Ring mode (A, G); A form of DNA59 | |
| 1485 | Ring stretching (G, A) A form of DNA59 | |
| 1576 | Ring stretching (A, G); A form of DNA59 | |
| Backbone | 807 | νas(O–P–O), νs(O–P–O); A-DNA52 |
| 1099 | νs(PO2−); A-DNA52 | |
| 1415 | df(CH2); A-DNA52 | |
| 784 | νs(O–P–O); B-DNA52 | |
| 830 | νas(O–P–O); B-DNA52 | |
| 1090 | νs(PO2−); B-DNA52 | |
| 1420 | df(CH2); B-DNA52 | |
| 748 | νs(O–P–O); Z-DNA | |
| 792 | νas(O–P–O); Z-DNA | |
| 810 | νas(O–P–O); Z-DNA | |
| 1095 | νs(PO2−); Z-DNA | |
| 1425 | df(CH2); Z-DNA | |
Lipids. Lipids, also called fats, are one of the important classes of biomolecules. They have important role in energy storage, cellular signalling, digestion and absorption of food, building of cellular membranes and various tissues, maintaining body temperature etc. Raman spectroscopy provides an invaluable tool to estimate various structural properties of lipids like the degree of unsaturation, level of E, Z unsaturation etc.60 The stretching vibrations from C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in lipids are observed at around 1749 cm−1 which are quite distinct and far from that of proteins. Similarly, many other markers bands for lipids like Raman bands at 1430 cm−1 (CH2 scissoring), 1443 cm−1 (CH2 and CH3 deformation of lipids and triglycerides), 1453 cm−1 (C–H bending of lipids) have been used to quantify lipids in oral cancer tissues.61 Nieva et al. (2012) characterized the lipid phenotype of breast cancer cells using Raman spectroscopy.62 Raman spectra analysed in the range of 2820–3030 cm−1, where total fatty acid content (TFA) and total unsaturated fatty acids (TUFA) (3015 cm−1) bands were quantified using bands at 2845 cm−1 and 3015 cm−1 respectively. Popp's group (2015) analysed fungal lipids inside living hyphae using Raman spectroscopy.63 Raman spectra from single vesicles were collected and analysed for peak intensity ratio I (1270 cm−1)/I (1445 cm−1) from the signals of
O in lipids are observed at around 1749 cm−1 which are quite distinct and far from that of proteins. Similarly, many other markers bands for lipids like Raman bands at 1430 cm−1 (CH2 scissoring), 1443 cm−1 (CH2 and CH3 deformation of lipids and triglycerides), 1453 cm−1 (C–H bending of lipids) have been used to quantify lipids in oral cancer tissues.61 Nieva et al. (2012) characterized the lipid phenotype of breast cancer cells using Raman spectroscopy.62 Raman spectra analysed in the range of 2820–3030 cm−1, where total fatty acid content (TFA) and total unsaturated fatty acids (TUFA) (3015 cm−1) bands were quantified using bands at 2845 cm−1 and 3015 cm−1 respectively. Popp's group (2015) analysed fungal lipids inside living hyphae using Raman spectroscopy.63 Raman spectra from single vesicles were collected and analysed for peak intensity ratio I (1270 cm−1)/I (1445 cm−1) from the signals of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH and –CH2/–CH3 groups, respectively. This ratio is linked to the iodine values of lipids. Raman spectra from lipids can be characterized in seven regions as explained in Table 3 (all assignments have been taken from Czamara et al.60).
CH and –CH2/–CH3 groups, respectively. This ratio is linked to the iodine values of lipids. Raman spectra from lipids can be characterized in seven regions as explained in Table 3 (all assignments have been taken from Czamara et al.60).
| Frequency (cm−1) | Assignment | |
|---|---|---|
| a ν: stretching (s: symmetric, as: asymmetric), b: bending, sc: scissoring, t: twisting, df: deformation, skel: skeletal. | ||
| Region I | 2800–3100 | ν(CH), νas(CH3), νs(CH3), νas(CH2), νs(CH2) |
| Region II | 1600–1800 | ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), ν(C C), ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
| Region III | 1400–1500 | b(CH2/CH3), sc(CH2/CH3), b(CH2) |
| Region IV | 1200–1300 | t(CH2), df(CH) |
| Region V | 1050–1200 | ν(C–C), ν(P–O) |
| Region VI | 800–1050 | b(CH), skeletal(C–O–O), νasN+(CH3)3 |
| Region VII | 500–700 | νsN+(CH3)3, cholesterol ring df, df(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–O), b(CH2) ring O–O), b(CH2) ring |
Carbohydrates. Carbohydrates are naturally occurring compounds and are essential constituents for living organisms. They have significant functions, like, main source of energy, major constituent of cell wall of plants and microorganisms, molecular agents in cellular recognition etc. Carbohydrates can be classified as monosaccharides, oligosaccharides and polysaccharides. Nonetheless, structural investigations on different carbohydrates are difficult as the basic monomeric unit in the polymeric chains is very similar. Despite this, different nature of H-bonding or the ring configuration gives rise to several marker peaks for carbohydrates. Wiercigroch et al. (2017) have compiled Raman and IR features of 14 carbohydrates in the solid state.64 Arboleda et al. recorded Raman spectra of nine anomerically stable monosaccharides in solutions with concentrations as low as 10 mM and volumes as small as 15 μL. It is observed that Raman spectra are sensitive to the configuration of the carbon centres and unique spectra are obtained of all nine monosaccharides.65 Stuart et al. (2006) demonstrated in vivo quantification of glucose in animal model for the first time using Surface-Enhanced Raman Spectroscopy (SERS).66 Raman spectra of carbohydrates can be categorised in five main regions as given in Table 4. All assignments have been taken from Wiercigroch et al.64
| Frequency (cm−1) | Assignment | |
|---|---|---|
| a ν: stretching (s: symmetric, as: asymmetric), b: bending, sc: scissoring, t: twisting, df: deformation. | ||
| Region I | 3100–3600 | ν(OH) |
| Region II | 2800–3100 | ν(CH), νas(CH2), νs(CH2) |
| Region III | 1200–1500 | df(CH/CH2) |
| Region IV | 800–1200 | ν(C–O), ν(C–C) |
| Region V | 100–800 | df(CCO) |
The above work provides extensive evidence that different biomolecules yield distinct Raman spectra. While understanding the absolute structure of any biomolecule can be a confounding task, monitoring changes in the chemical structure in response to some perturbation can be efficiently analysed using Raman spectra. Consequently, Raman spectroscopy has been used to study changes in the structure of various biomolecules with external perturbation, like protein folding, protein–protein interaction, DNA-drug interaction, DNA melting etc.67
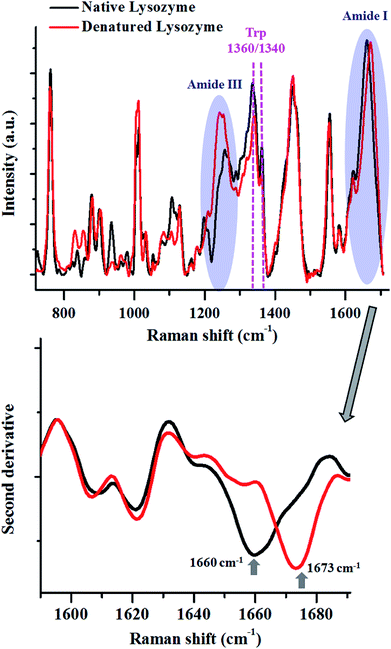
To elucidate further, we performed thermal denaturation studies on the protein, lysozyme and compared the heat-induced changes in the Raman spectra with the native form (Fig. 2).
The Amide I peak in a Raman spectrum is a broad band which ranges from 1580–1720 cm−1. It can be deconvoluted into various sub-bands corresponding to different secondary structures like helices, β-sheets, random structures etc. The band at around 1655 cm−1 is usually assigned for helices, 1665 cm−1 for β-sheets and 1675 cm−1 for random structures. Lysozyme in its native state, has 41% helices and 10% β-sheets,68 therefore, Amide I for the native state of this protein had a peak centre around 1660 cm−1. After heat denaturation, the content of β-sheets and random structure increased at the expense of helices. Hence, the intensity of the band corresponding to the helices (∼1655 cm−1) decreased and the intensity of the bands for β-sheets (∼1665 cm−1) and random structures (∼1675 cm−1) increased. This resulted in a change in the band shape of Amide I and a consequent shift in the peak maxima. This shift in the peak maxima was better understood by taking the second derivative of the spectra.
The changes in the Amide III band (∼1200–1300 cm−1) also corroborated this fact. There was a decrease in the intensity of the Amide III band around 1300 cm−1, assigned for alpha-helices. This was coupled with an increase in the intensity of the band between 1200–1260 cm−1, which has contributions from β-sheets and random coils.
The modulations in the Amide I and III bands demonstrated how one can probe the changes in the secondary structure of proteins.
The tertiary structure of the protein can also be probed using various Raman marker bands for side-chains. For instance, the ratio of 1360 cm−1 and 1340 cm−1 serves as a hydrophobicity marker for tryptophan. As a consequence of thermal denaturation of lysozyme, there was a decrease in this ratio, indicating a change in the environment of buried tryptophan residues from hydrophobic to hydrophilic. Similarly, there are several other bands assigned for side chains of proteins which can be analysed using Raman spectroscopy (see Table 1).
This highlights how Raman spectroscopy has proved to be a valuable technique in analysing conformational changes in the proteins as result of different perturbations.
However, it can become a very daunting task when one wants to analyse complex biological systems like cells, tissues, body fluids etc. While biomolecules are ubiquitously present in all biological systems, their relative concentrations can be vastly different. This becomes very evident when the Raman spectra of different tissues and body fluids are compared, since a differential concentration of the biomolecules results in a characteristic Raman spectrum.
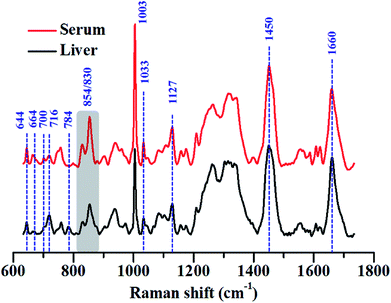
We have highlighted this fact by comparing the Raman spectra of liver tissue and blood serum isolated from healthy mice. Raman spectra were recorded from 50 μm thin sections of liver tissue and ∼2 μL of blood serum isolated from BALB/c mice using 785 nm laser excitation (Fig. 3). The region, 600–1725 cm−1 was analysed since this constitutes the fingerprint region for most biological samples. Intense generic bands with contributions from proteins and lipids, like 1003, 1451 and 1660 cm−1, were observed in the Raman spectra of liver and serum.
The serum is predominantly composed of different kinds of proteins. It contains up to 80 mg mL−1 of protein along with other constituents like amino acids, salts, lipids and sugars like glucose.69 The bulk of the protein content of serum comes from albumins, globulins and lipoproteins. This could also be seen in the Raman spectra of serum which was heavily dominated by Raman bands characteristic of proteins, for example, 644, 830, 854, 1003 and 1033, 1127, 1200–1300 cm−1 (Amide III) and 1660 cm−1 (Amide I) respectively. All assignments have been listed in the preceding tables.
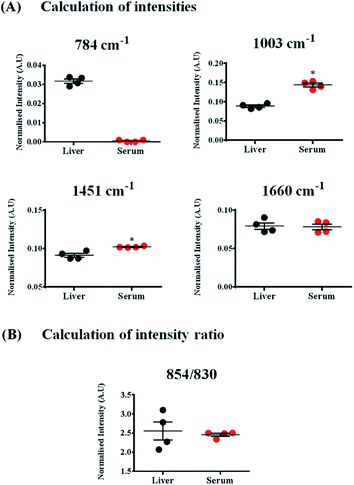
While these bands were also present in the Raman spectra of liver, their relative intensities and the ratios varied across both the samples, indicating differences in the relative distribution of biomolecules (Fig. 4(A) and (B)).
The Raman band for DNA at 784 cm−1 was observed only in the spectra of liver and was completely absent in the serum indicating negligible amounts of DNA present in the serum (Fig. 4(A)). Liver, being a tissue is made up of a large number of cells containing nucleic acids like DNA and RNA. On the contrary, blood serum is largely cell-free and thus devoid of any quantifiable amounts of DNA under normal conditions.
Other peaks corresponding to biomolecules such as cholesterol (700 cm−1) and phosphatidylcholine (716 cm−1) could also be observed in the Raman spectra of both the samples. The Raman bands for glycogen could be seen at 854 and 937 cm−1.70,71 These bands were more intense in the spectra of liver, which is quite expected, since liver is the main organ that stores glycogen.
Quantifying ratios of Raman bands can be very advantageous as they are least affected by background fluctuations and pre-processing methods.72 The ratio of two Raman bands at 830 and 854 cm−1 has been shown in Fig. 4(B). These bands have been famously called as the tyrosine Fermi doublet, originating from proteins. The ratio has extensive significance and can be a very good marker for the H-bonding status of the phenolic OH of tyrosine.69 In serum, these two bands can be assigned to tyrosine since serum is a rich source of proteins. However, in case of liver, the assignment of the band at 854 cm−1 can be ambiguous. In liver, the main contribution to this band comes from glycogen, since it is this organ where glycogen is primarily stored. This point brings to one's notice that most Raman bands can have overlapping contributions from several biomolecules and therefore, understanding the system under study is essential before band assignments are made.
As already mentioned that although the Raman spectra of such biological entities are quite complex, certain marker Raman bands can still be assigned for different classes of biomolecules in such systems.51,73,74 This can help us monitor the changes induced as a result of any stress or change in the native environment. This point can be proven through an example by monitoring the biomolecular changes induced in bacteria as a consequence of heat treatment.
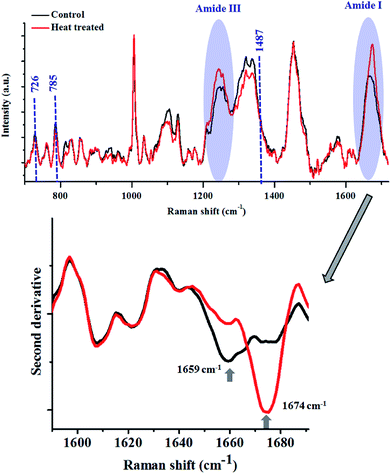
Heat treatment of the bacterial cells induced several changes in the Raman spectra (Fig. 5), as highlighted in the figure above. The major changes observed were in the Amide I and III bands, similar to what was noted in the lysozyme-denaturation experiment shown in Fig. 2. This emphasises that these Raman bands can be unambiguously assigned to proteins, even in complex systems like bacteria.
After heat treatment, a prominent increase in the intensity of the Raman band at 1674 cm−1 was observed, which indicated the increase in the content of β-sheets/random coils. This was also highlighted by the second derivative spectra. Complementary information was obtained from the analysis of the Amide III band. Accumulation of β-sheet/random coils is highlighted by the increase in intensity of Raman bands in the range of 1200–1260 cm−1 at the expense of helical structure (1300 cm−1). Another biomolecule which showed changes as a result of heat treatment was DNA. All the major Raman bands for DNA (726, 785, 1487 cm−1) showed a marginal decrease in the heat treated bacterial cells.
These changes showcase how complex systems can also be studied using Raman spectroscopy by analysing the unique features assigned for different biomolecules. In the field of microbiology such information is deemed useful for classification of different bacterial species or understanding biochemical changes in bacteria due to external perturbations in the environment.73,75 This paves the way for monitoring other perturbations like diseases in more complex living systems like human beings.76–78 Any disease can also be considered as a perturbation to the native composition of these biomolecules in different tissues and body fluids.
Every disease is associated with a change in biochemistry. This principle is the central doctrine for modern medicine. The biochemical changes can either be a cause for disease manifestation or may be a consequence of the disease itself. Since Raman spectroscopy probes bond vibrations, the spectra generated are very molecule-specific. Any change in the concentration or the conformation of these biomolecules as a result of a disease, however subtle, is reflected in a Raman spectrum. This principle has formed the basis for a Raman-based diagnostic tool. Though Raman scattering is a weak phenomenon, technological advancements over the past few decades have led to shorter acquisition times and improved signal-to noise ratio, making the use of Raman spectrometers in a clinical setting easier. The clinical practicality has also been enhanced by the advent of fibre-optic probes for delivery of light as well as collection of Raman scattered photons. The use of advanced chemometric methods for handling Raman data sets has also made the diagnosis and classification process user-friendly.79
Different methods are available that help the user in deriving meaningful information from a complex spectral dataset. Principal component analysis (PCA) is one such technique that reduces the spectra into a defined number of principal components (PCs) that account for spectral variance. A detailed analysis of cluster analysis and PCA in spectral data analysis is demonstrated by Bonnier and Byrne.80 PCA is an unsupervised method and hence cannot be used for classification and prediction purposes. Hence, supervised methods like Support Vector Machines, Artificial Neural Network, Linear Discriminant Analysis etc. are employed. A concise description of different chemometric methods used for Raman spectral data analysis has been given in the review by Butler et al.81
In the following sections, we discuss examples of how Raman spectroscopy has been used to understand some pathological conditions of the human body like cancer, infection and inflammation.
Cancer. Of the several diseases and pathological conditions that have been studied using Raman spectroscopy, cancer tops the list. In 2015, around 8.8 million deaths were due to cancer. As is true for any disease, early and rapid diagnosis of cancer is of utmost need. Traditionally, cancer diagnosis occurs through histopathology which requires a biopsy to be taken from the suspected area. This method of detection is highly subjective and requires the availability of a trained pathologist. Other methods of diagnosis include imaging based modalities like Positron Emission Tomography (PET) scan, Magnetic Resonance Imaging (MRI), Computed Tomography (CT) etc. However, these methods are difficult to be included in intra-operative procedures as most of these techniques require extensive labelling. Cancer, like most other diseases, is accompanied by distinct modulations in biochemistry which are expected to be reflected in the Raman spectrum. Consequently, Raman spectroscopy has been used for the detection of various types of cancers. Kopec et al. used a combination of Raman and AFM imaging to determine the biochemical composition as well as the mechanical properties of blood vessels in a tumour mass of human breast tissue. They found an increase in glycogen and lactic acid in breast cancer patients coupled with an increase in the collagen–fibroblast–glycocalyx network.82 An insight into how Raman spectroscopy can function as a tool for personalised radiation therapy was given by Van Nest et al. They used human breast adenocarcinoma xenografts after radiation therapy and found spectral changes linked to different biomolecules like proteins, lipids and nucleic acids on a temporal scale, highlighting the potential of Raman spectroscopy for assessing radiation-responses of tumours.83 The use of cancerous cell lines as a platform to differentiate invasive vs. non-invasive breast cancer on the basis of Raman spectral markers was done by Chaturvedi et al. The Raman bands at 1447 cm−1 (CH2 bending from lipids), 1003 cm−1 (phenylalanine) and 876 cm−1 (tryptophan) were found to be higher in invasive cell lines vis-à-vis the non-invasive ones. Their work is in agreement with other reports that have correlated increased lipid levels to more aggressive and metastatic cancers and, therefore, a poor prognosis.84 The work of Kim S. et al., shows how cancer detection can be improved by coupling Raman spectroscopy to other methods like pH sensing. It is known that pH levels in cancerous tissues are much lower than that of normal tissues. They exploited the chemical information revealed by Raman spectroscopy as well as the metabolic information obtained by the pH sensor for improving the specificity of cancer diagnosis. Their study was done on human breast tissue samples and both the Raman and pH sensors were fibre-optic probes.85 A recent study undertaken by Winnard et al., has beautifully captured the potential of Raman spectroscopy to characterise organ-specific metastatic lesions at a molecular level, to give insights into metastatic progression. A combinatorial approach of Raman spectroscopy and metabolomics was employed in their study.86 In another study, stromal adaptations occurring in pre-metastatic lungs that were primed by breast cancer were analysed using Raman spectroscopy. This work was done in mouse models where the mice were xenografted with human breast cancer cells of varying metastatic potentials. Changes in the extra-cellular matrix of pre-metastatic lungs, such as increase in collagen (859 cm−1) and proteoglycan (1061 cm−1) were identified and this was a direct correlation of the metastatic potential of the breast cancer cells used.74
Desroches et al., utilized the higher wavenumber Raman signatures for designing, developing and testing of an in situ intraoperative system for brain cancer detection. They engineered this Raman-based system into a commercially available biopsy system for analysis before the tissue was extracted. Using this approach, they could achieve sensitivity and specificity of brain cancer detection in a swine model system, upto 80 and 90% respectively.87 The reprogramming of lipid biosynthesis and proteins in high-grade medulloblastoma was demonstrated by Abramczyk and Imiela very recently. Medulloblastoma is a term given to high grade cancers of the central nervous system. Such samples showed a higher content of β-sheets and lower levels of α-helix when compared to normal tissues. The ratio of the Raman intensities at 2930 and 2845 cm−1 was also indicative of cancer progression. For all the cancerous samples, this ratio had a value of 1.99 in comparison to the value of 1.45 for healthy tissue.88
Murali Krishna's group demonstrated the use of Raman spectroscopy for identification of sites in the oral cavity that have a higher propensity to become cancerous even before the manifestation of clinical symptoms of oral cancer recurrence. This study was performed on 99 patients of oral cancer and spectra were recorded from the cancerous sites as well as contralateral normal mucosa.89 Nasopharyngeal cancer has also been diagnosed using Raman spectroscopy by Ming et al. in 2017. They demonstrated the use of a fibre-optic Raman probe for in vivo surveillance of patients suffering from nasopharyngeal cancer. This form of cancer is often very difficult to diagnose owing to the deep anatomical location. This study had grouped patients under three categories – normal, cancerous and post-radiation. All experiments were performed using a 1.8 mm probe, which is currently the smallest probe used for Raman-based diagnosis. A specificity of ∼96% was observed in distinguishing normal, cancerous and post-radiation patients.90
Almond et al. utilised Raman spectroscopy and translated it into an endoscopic tool for the early detection of dysplasia in patients suffering from Barret's oesophagus. A high-grade dysplasia (HGD) often results into oesophageal malignancy. Tissues from a cohort of 62 patients were taken for the analysis and sensitivity and specificity of 86 and 88% respectively were obtained for detecting HGD and adenocarcinoma.91
Another useful application of Raman spectroscopy in cancer research was highlighted by the work of Moradi et al., where they tested the potential of this method to discriminate between human ovarian cancer cells that were sensitive or resistant to cisplatin. An increase in the relative concentration of proteins and glutathione was observed in the resistant cells compared to the sensitive ones.92 The ability of Raman spectroscopy-based tool to serve an intra-operative device for detecting distant invasive grade 2–4 gliomas was shown by Jermyn et al. in 2016. They found that Raman spectroscopy could detect as few as 6 cancer cells per 0.0625 mm2, which was much better than traditionally used methods like MRI. All the Raman measurements were performed during brain tumour resections.93 A detailed review on intra-operative Raman spectroscopy has been given by Brusatori et al.94
The use of body fluids like blood plasma/serum, urine, tears etc. can pave way for a diagnostic method that is minimally invasive. Body fluids can be obtained easily and therefore repeated samplings can be done. Unlike tissues and cells, biofluids can be used for routine monitoring as well as under intra-operative conditions. They contain several biomolecules and therefore can be very good indicators of the health status of a person or the underlying disease. The major limitation in using bio fluids for Raman spectroscopy is the relatively low concentrations of biomolecules when compared to tissues and cells. This problem can be easily circumvented by the use of Drop Coated Deposition Raman Spectroscopy (DCDRS) where the biomolecules are concentrated.95–97 Harvey et al. in 2008 demonstrated the use of urine for the detection of prostate cancer where increased proteins and nucleic acids were associated with the cancerous cells.98 The work of Corsetti et al. demonstrated the efficacy of Raman spectroscopy in distinguishing hormone (androgen)-resistant prostate cancer cells. Bands specific to amino acids like arginine were found to be diminished in the cancerous cells whereas those with contributions from phenylalanine and tyrosine were found to be increased. The androgen-resistant cancer cells also had increased DNA and Amide III bands when compared to the sensitive cells.99 O'Malley et al., in their study, have highlighted Raman-based lipid quantification to be of diagnostic value for prostate cancer. Increased lipid amounts are directly correlated to the tumour stage.100
The use for serum-based cancer diagnosis has also been explored for different types of cancers. Ovarian cancer was studied using a combined approach of ATR-FTIR and Raman spectroscopy on human ovarian cancer patients by Owens et al. in 2014.101 The basis for differentiation using Raman spectroscopy was the spectral shifts in the Raman bands at 711 cm−1 (DNA) and 913 cm−1 (sphingolipids).
Infectious, inflammatory and other diseases. While a plethora of information can be found for Raman-based cancer diagnosis, infectious and inflammatory diseases also require significant attention since they constitute a large percentage of the global burden on the health care system.
Infection with the dengue virus claims several lives especially in the developing countries. Current methods for diagnosis include measuring serum IgG and IgM levels and the Ns1 antigen. Mahmood et al., demonstrated the use of Raman spectroscopy for profiling dengue-associated biochemical changes occurring in the blood plasma of 17 infected patients. Changes were seen mostly in the Raman bands associated with lipids (608 cm−1, 875 cm−1, 1297 cm−1, 1719 cm−1 and 1736 cm−1) which increased with infection. The peak intensity of the Amide III band at 1239 cm−1 was also higher in the infected samples. An increase in the Raman band at 1409 cm−1 was also observed which can be assigned to IgG. The method showed specificity and sensitivity of 95 and 98% respectively.102 Dengue-associated spectral changes were also studied by Bilal et al. and they found the peaks for lactate at 750, 830 and 1450 cm−1 to be higher in intensity in all 50 dengue patients when compared to 20 healthy controls. They also spiked the serum of healthy controls with lactate in a controlled manner and found that indeed the abovementioned peaks were due to lactate.103 Ahmed's group from Pakistan has tried to develop a Raman-based diagnostic method for dengue using several multivariate approaches like Support Vector Machines (SVM), random forest method, etc.104
The same group has also conducted Raman studies on human malarial samples and found 10 distinct spectral signatures that could differentiate malarial samples from healthy controls as well as dengue samples and patients suffering from non-malarial fever. A multivariate model based on partial least square (PLS) regression was used and the accuracy of detection was 86%.105
Malaria-induced changes in the spleen of mice were evaluated using Raman spectroscopy by Frame et al. and they found an increase in the heme-associated Raman bands. This directly correlated to the hemozoin detection with peaks at ∼1370, 1529, 1588, and 1628 cm−1 which matched closely with the reference spectra.106 A comprehensive review on the potential of vibrational spectroscopy to diagnose and study malaria and the malarial parasite was written very recently by Perez-Guaita et al.107
One of the major causes of death in developing countries is tuberculosis (TB). In India alone, around 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 people die annually because of TB infections. While tuberculosis is mainly pulmonary in origin, extra-pulmonary TB also exists and is more difficult to diagnose. Tuberculous meningitis is one of the forms of extra-pulmonary TB and requires culturing of Mycobacterium for a precise diagnosis. Satyavathi et al., used Raman spectroscopy on cerebrospinal fluid of patients suffering from tuberculous meningitis and found that the silicate Raman signature from M. tb positive cases was a very useful indicator of the underlying infection.108
000 people die annually because of TB infections. While tuberculosis is mainly pulmonary in origin, extra-pulmonary TB also exists and is more difficult to diagnose. Tuberculous meningitis is one of the forms of extra-pulmonary TB and requires culturing of Mycobacterium for a precise diagnosis. Satyavathi et al., used Raman spectroscopy on cerebrospinal fluid of patients suffering from tuberculous meningitis and found that the silicate Raman signature from M. tb positive cases was a very useful indicator of the underlying infection.108
Another leading cause of mortality in the Intensive Care Units of hospitals is sepsis. Sepsis can be triggered by infection of any kind and results in an uncontrolled immune response known as the Systemic Inflammatory Response Syndrome (SIRS). SIRS, however, can be a manifestation of other conditions like burns, trauma etc. Presently, no reliable marker exists that can efficiently distinguish SIRS from sepsis. In order to achieve this, Raman spectroscopy combined with multivariate approaches was used by Popp's group and they found that SIRS could be distinguished from sepsis with a predictive accuracy of 80%.109
Different inflammatory conditions like ulcerative colitis have also been studied using Raman spectroscopy. Ding et al. investigated the colon mucosal composition in vivo using an endoscope-coupled Raman probe in patients suffering from ulcerative colitis and age and BMI-matched healthy controls. A decrease in the phosphatidylcholine (720 cm−1) and total lipids (1303 cm−1) was observed in the inflamed colon tissue. The degree of lipid unsaturation, determined by calculating the ratio between Raman bands at 1657 and 1447 cm−1, was found to be high in the patient group. These differences were also seen when active colitis patients were compared with the quiescent ones.110 Their results were corroborated by the work of Addis et al., which also showed reduction in phospholipids to be of diagnostic value for ulcerative colitis.111
Inflammatory bowel disease (IBD) was also diagnosed by Mahadevan-Jansen's group where a colonoscopy-coupled fibre optic Raman probe was used. IBD includes ulcerative colitis (UC) and Crohn's disease (CD) and the motive in this study was to determine the spectral signatures of both UC and CD.112
Inflammation in the oral cavity as a result of periodontal disease was also assessed using Raman spectroscopy by Camerlingo et al. They used gingival crevicular fluid from patients with periodontal disease and found an increase in the carotene content to be diagnostic of the condition (1537 cm−1). They also studied the chemical and structural changes occurring in the periodontal ligament after orthodontic force was applied and found changes in the secondary structures of proteins (Amide I band) to change with application of the force.113
In addition to the above mentioned inflammatory diseases, Raman spectroscopy has been extensively used to study and diagnose neurodegenerative diseases like Alzheimer's disease (AD). Till date, there is no cure for such diseases primarily due to their delayed diagnosis which occurs when the extent of brain damage is already quite pronounced. In fact, a definitive diagnosis can only be established post-mortem. It is now known that changes in the brain of such individuals begin much earlier than the appearance of the first symptoms. This can pave way for an early diagnosis which may make therapeutic interventions more successful. Protein misfolding and aggregation are central to most neurodegenerative diseases. Raman spectroscopy is an excellent tool to study protein conformation and therefore, has significant potential in diagnosing such diseases. This has been efficiently shown in the work of Paraskevaidi et al., where blood plasma was used to successfully discriminate Alzheimer's disease from healthy controls as well as from patients suffering from Dementia with Lewy Bodies (DLB). It is important to highlight that patients suffering from DLB are often misdiagnosed as AD and hence the choice of the patient cohorts is well justified. The same study also showed that patients suffering from early and late stages of AD could be differentiated from healthy controls with a sensitivity of 84%.114
In another study, Ryzhikova et al., demonstrated the use of Raman microspectroscopy on blood serum for diagnosis of AD using a cohort of 20 AD patients, 18 from other neurodegenerative diseases and 10 healthy controls with specificity and sensitivity of ∼95%.115
(II) Raman spectroscopic studies on materials
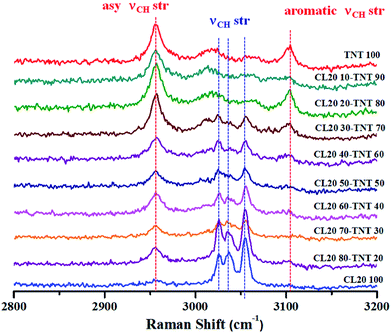
Preceding discussions revolved around the use of Raman spectroscopy in the field of biology. It should be emphasized that biomolecules have a low Raman cross-section and as a consequence show weak Raman signals. Probing such weak scatterers has been made possible due to the introduction of Raman microscope in the early 70s.7–9 The other advantages which Raman spectroscopy offers over other analytical tools are its non-invasive and non-destructive nature. This has resulted in its prolific use in the field of materials such as studies on carbon allotropes, explosives, semiconductors, pharmaceuticals, art, painting and archaeology. Raman spectroscopy has been an indispensable tool for characterization of different allotropes of carbon. Ranging from 3D diamond or graphite to 2D materials such as graphene or 1D materials like carbon nanotubes (CNT), Raman spectroscopy provides information pertaining to lattice vibrations, defects, orientations etc.116,117 For instance, single walled and multi walled CNTs can be distinguished using this technique. The radial breathing modes which are unique to single walled CNT are absent in multi walled CNT.118 Additionally, various sp2 carbon can also be differentiated.119 One of the more recent interests on carbon allotropes has been in graphene research. Here, Raman spectroscopy has proved to be an integral tool to determine number of layers of graphene along with orientational information, edges, strains, defects etc.120,121 Enhancement of Raman signals of analytes using carbon materials has also been explored.122–125 In the field of explosives detection, surface enhanced Raman spectroscopy has been employed for trace level detection.126,127 Furthermore, studies on feasibility of Raman spectroscopy for stand-off detection have also been explored.128,129 The subsequent sections provide a different aspect of this technique to demonstrate the usefulness of Raman spectroscopy towards studies on explosives. The first example shows how Raman spectroscopy can be used to monitor melt cast explosives formulations. In the second case, detection of concealed materials in non-metallic containers using depth sensitive Raman spectroscopy such as spatially offset Raman spectroscopy (SORS), transmission Raman and universal multiple angle Raman spectroscopy (UMARS) has been demonstrated.High explosives are used in military applications to achieve the terminal effect i.e. destruction of tactical and strategic targets. Typical examples of industrial explosives are blasting gelatine and ammonium nitrate slurries. However, high explosives are useful in civilian applications as advanced demolition devices (ADDs) for demolition of structures with precision and least collateral damage. Most of the ADDs comprise of composition-B (RDX/TNT 60-40) or similar compositions. These are usually manufactured using melt cast technology.138 TNT forms the work horse for such formulation owing to its low melting point (∼80 °C) which allows the ease of mixing and homogenization of other explosive ingredients. Therefore, the entire process requires constant monitoring to ensure thorough mixing and formation of a uniform melt. Explosives are aliphatic, aromatic or nitramine based organic molecules which are rich in Raman signatures. Since melt cast compositions are physical mixtures, the structures of the molecules are unaltered (in most of the cases). Therefore, Raman spectroscopy can be a potential tool for in situ monitoring of such processes. Fig. 6 depicts an example of an explosives formulation comprising of CL-20 (nitramine class) and TNT (nitroaromatic class) at different weight ratios. Raman spectra were acquired for different compositions at higher wavenumber regions as these molecules have unique spectral features which can be attributed to C–H stretches.139–141 Fibre optics based Raman spectroscopy can be employed for online monitoring of such processes which least human intervention. Although a less explored area, Raman spectroscopy can be a useful tool in the area of explosives research.
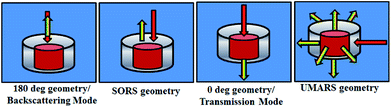
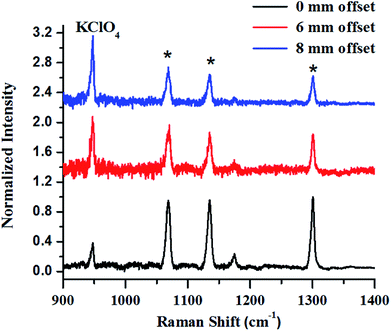
The first example describes SORS for detection of KClO4, an oxidizer used towards making explosive formulations (Fig. 8). In this study, KClO4 was taken inside a 3 mm thick high-density polyethylene (HDPE) container. Experiments revealed that a certain offset from the excitation point was necessary to discriminate the Raman signals of the content from that of the container. It may be noted that at no offset, the signal from the container was pronounced (peaks with asterisk). As the offset was increased, the Raman signal intensity of KClO4 improved with respect to the container, while that of the container did not show any appreciable increase.
In the next set of studies, experiments were performed using transmission and backscattering geometry on the same material by using a focused beam of incident light (Fig. 9). We noted, however, that the transmission geometry yielded better content signal quality for the same container wall thickness (3 mm), as shown in the figure, and the signal from the container was remarkably suppressed. In this case the signal was collected at the back of the 25 mm thick square container, opposite to the point of excitation. This is possible in transmission geometry because of the long migration paths of Raman photons in non-absorbing powder media.147
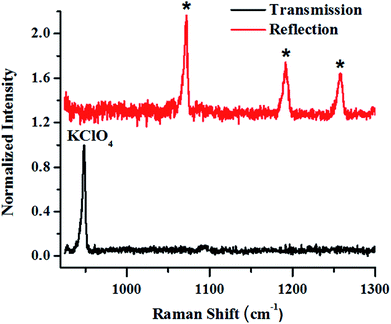 | ||
| Fig. 9 Raman signals of layers of KClO4 explosive in a 3 mm thick HDPE container obtained in reflection (backscattering) and right transmission mode. | ||
From the above-mentioned geometry-specific Raman experiments it is clear that backscattering mode yielded appreciable Raman signals of the top layer. This was overcome by using spatial offsets. However, transmission geometry provided Raman signals with even less interference from the top layer signals. It should be noted that transmission geometry is limited by the path length of the system due to the fact that after a certain depth, the photons would attenuate and low Raman signals can be acquired beyond that. In the cases where the material thickness exceeds a few centimetres, retrieving Raman signatures from material located at a depth will be a challenge by utilizing the above-mentioned geometries. It is worth mentioning that direct evidence of the extra pathlength for Raman photons from deeper layer can be obtained via time resolved Raman spectroscopic techniques.142 For instance, Petterson et al., have reported detection of explosives concealed behind opaque, diffusely scattering materials using picoseconds time resolved Raman spectroscopy.157
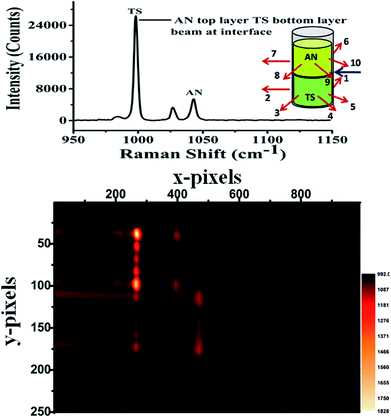
Our laboratory has demonstrated UMARS for the first time where we have obtained Raman signals in strongly scattering samples beyond a few centimetres.125,158–162 As a demonstration, imaging experiments using the UMARS technique were conducted for bilayered solid compounds; in this case, ammonium nitrate (AN) was taken as a top layer and t-stilbene (TS) as a bottom layer. The excitation fibre was placed at the interface of the two layers, collection fibres 1–5 were placed around the bottom layer, TS, while fibres 6–10 were placed around the top layer, i.e., AN. The experimental arrangement is shown schematically in the inset of Fig. 10. Before recording the image, Raman spectra were recorded for the separate components. Peaks centred at 992 cm−1 and 1044 cm−1 correspond to TS & AN, respectively. TS, being a very strong Raman scatterer, showed a higher peak intensity as compared to AN. When the CCD was used in imaging mode, different collection fibres produced separate Raman spectra at different heights of the CCD. Y axis of the image shows the projection of 10 separate collection fibres on different pixel rows. Spectrum at the top of the figure corresponds to the sum of all y pixels, i.e., a full vertical binning of the signal. Since fibres 1–5 were placed around TS, those spots appeared on the top section of the CCD, while the bottom section contained mostly spots of AN. It was observed that in addition to the AN signal, fibres 6–10 also collected some signals from TS. This indicates that TS photons migrated to the top layer and contributed to the Raman signal. These results show that the spatial locations of the species present in the layers could be identified based on the position (image) of the fibres. In this setup, spectra of the individual fibres are collected simultaneously and can be evaluated separately. This is an important attribute of UMARS, as spatial information will be very important in the case of medical diagnostics. As expected, Rayleigh scattering was strongest in fibres 1 and 5 and fibres 6 and 9; closest to the point of excitation. The Raman signal of the bilayer clearly shows the TS and AN peaks.
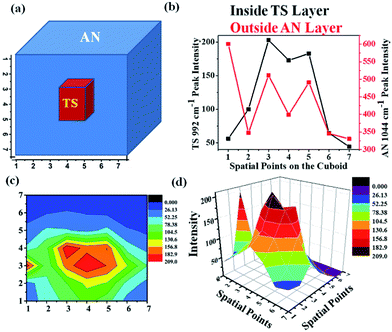
In order to ascertain the location of the inner layer, a special experiment was designed. An HDPE cuboid container was taken and it was filled with AN as an outer layer and TS as an inner layer as shown in Fig. 11(a). The cuboid was divided into a 7 × 7 grid. The excitation fibre was placed at each point in the grid, while the collection fibres were placed around the periphery maintaining the same plane as the input fibre. UMARS signals were collected from all the 49 points and the obtained spectra were analysed to retrieve depth specific molecular information. At first, the Raman spectral intensity variation of TS & AN were recorded by keeping the y-axis constant (y = 4) i.e., at the centre of the cuboid and by varying the x at the y level, i.e., from x = 1 to x = 7 (Fig. 11b). It was observed that the Raman intensity of AN was strongest at x = 1 while in the case of TS, the signal was weak at x = 1, followed by an increase in Raman intensity to reach a plateau and finally decreased. It was also noted from the spatial points 3 to 5, the intensity of TS remained more or less same. This implied that TS was present in higher amounts close to those spatial points as compared to other points. To get a clear picture, a contour and a 3D surface map was plotted for the TS Raman intensity (992 cm−1 peak) for the 7 × 7 matrix (49 spectral points) (Fig. 11c and d). This confirmed the presence of TS at the center of the cuboid. This example provides sufficient evidence of the capability of UMARS as a non-invasive, depth sensitive technique. Further experiments on phantoms and biological samples are being conducted in our laboratory to understand the potential of UMARS towards 3D imaging. We anticipate that UMARS would pave way towards Raman tomography for disease diagnosis in the future.
Experimental
Biomolecules
Bovine Serum Albumin (BSA), calf thymus DNA, D-(+)-glucose and cholesterol were purchased from Sigma Aldrich and used without further purification. Spectra were recorded from all the samples in powdered form using 785 nm laser and 50× L objective (N. A = 0.5) using Renishaw InVia Raman Microscope.Thermal denaturation of lysozyme
Hen egg white lysozyme (lysozyme) was purchased as lyophilized powder from Sigma and was dissolved in sterile MilliQ water to obtain a working concentration of 30 mg mL−1. For thermal denaturation studies, the protein solution was heated to a temperature of 85 °C for 8 hours. Spectra were recorded from the native and denatured protein solution using 785 nm laser and 50× L objective using Renishaw InVia Raman Microscope at room temperature.Liver and serum studies
6–8 weeks old healthy BALB/c mice were sacrificed and liver and blood were harvested. For collection of serum, blood was allowed to clot at 37 °C for 4 hours, followed by centrifugation at 4000 rpm for 5 min. For recording Raman spectra of liver, ∼50 μm thin cryo-sections were used. All Raman experiments were conducted using Renishaw in Via system using a 785 laser and 50× objective (N. A = 0.75).Bacterial heat treatment
E. coli MG1655 K-12 strain was cultured in a shaking condition at 37 °C and 160 rpm for 8 hours. 1 mL of the culture was subjected to heat shock at a temperature of 85 °C for 15 minutes. The control and the heated samples were then washed with sterile water three times to remove any residual media components; ∼3 μL of the bacterial sample was drop cast onto a magnesium fluoride coverslip before collecting Raman spectra. All Raman experiments were performed at room temperature using Renishaw in Via system using a 785 laser and 100× objective (N. A = 0.85).Data analysis
All spectra were processed using Wire 4.2, Origin 8.5 and MATLAB softwares. Statistical analysis (Mann–Whitney test) was performed using GraphPad Prism 6.Raman spectroscopic studies on materials
CL-20 and TNT were provided by DRDO laboratory for conducting the Raman experiments. The CL-20-TNT melt cast formulations were prepared in milligram quantities in the laboratory. Required quantity of TNT was first melted followed by addition of appropriate amount of CL-20 and homogenized using a wooden pestle. All chemicals used in SORS, reflection, transmission and UMARS experiments except ammonium nitrate (Nice Chemicals Pvt. Ltd.), were procured from Sigma Aldrich. The solid powder samples used in the experiments were either filled in commercial high-density polyethylene (HDPE) containers, quartz cuvettes or glass vials of assorted sizes.The Raman experiments on explosives formulation was conducted using Renishaw in Via system using a 514.5 nm Ar+ laser. The power at the sample was 2.5 mW and the spectra were acquired for 10 seconds.
The SORS, reflection and transmission Raman experiments were carried out using the 830 nm laser diode. The spectrometer was a high resolution imaging spectrometer (Horiba Jobin Yvon, iHR320) fitted with a LN2-cooled CCD camera. Grating with a groove density of 1200 lines per mm was used for the experiments. In the case of UMARS experiments, the laser was delivered to the sample through an optical fibre of core diameter 400 μm and a numerical aperture of 0.22 and the Raman signals were collected using 10 fibres of the same core diameter and NA placed on the sample container sides at various angles. The ten collection fibres terminated in a vertical linear array, placed in front of a telescopic arrangement fixed to the entrance slit of the spectrometer. The power of the laser was maintained between 250–450 mW. The acquisition time for the experiments varied from 10 s to a few minutes.
The acquired data were processed using Wire 4.2 and Labspec softwares and were plotted using Origin 8.5 and Origin 9.1.
Conclusion
Raman spectroscopy has proved to be a versatile tool in studying different diseases occurring because of the altered biochemistry in the body. Various advancements have been made in the field of Raman spectroscopy with respect to instrumentation and data handling methods. In this review, we have attempted to provide a glimpse of some of the research activities which are being pursued in our laboratory in the area of biophotonics, explosives detection and development of novel Raman techniques. We started by showing Raman spectra of pure molecules which comprise of the building blocks of cells and tissues. Examples of Raman spectroscopy of tissues are presented to understand the unique spectral features along with the complexity of the Raman signals. The second part of the review deals with Raman spectroscopic applications towards materials by taking explosives as a representative example. The last part of the review discusses the variants of Raman spectroscopy like SORS, transmission and UMARS for depth sensitive detection of layered materials. Finally, it is shown that UMARS imaging is possible and that chemical as well as spatial information of the materials lying underneath other materials can be clearly obtained using UMARS when the fibres are separately imaged on the CCD. The day is not far when Raman spectroscopy-based diagnostic methods will be routinely prescribed in clinics and public health care systems. One of the most anticipated uses of Raman spectroscopy is in clinical diagnostics. Most of the research on Raman is focused on biological research and imaging. It remains to be seen whether Raman spectroscopy would take off as portable equipment for point of care application. In his concluding remarks of his review paper, Prof Kiefer wrote, “Sometimes I am asking myself what cannot be done with Raman spectroscopy – a wonderful analytical tool for science and technology which was discovered by Sir Chandrashekhar Venkata Raman and Krishnan already eight decades ago at the Indian Association for the Cultivation of Science in Calcutta, India. I am looking forward for many more years of significant advancements in this research field.163”Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge Department of Science & Technology, Defence Research & Development Organization, Council of Scientific & Industrial Research, Indian Institute of Science, Bangalore for their financial support. S. U. acknowledges the J. C. Bose fellowship from DST.References
- R. L. McCreery, Raman Spectroscopy for Chemical Analysis, Wiley, 2005 Search PubMed
.
- R. S. Das and Y. K. Agrawal, Raman spectroscopy: Recent advancements, techniques and applications, Vib. Spectrosc., 2011, 57, 163–176 CrossRef
.
- D. A. Long, Raman Spectroscopy, McGraw-Hill, New York, 1977 Search PubMed
.
- L. A. Lyon, C. D. Keating, A. P. Fox, B. E. Baker, L. He and S. R. Nicewarner, et al., Raman spectroscopy, Anal. Chem., 1998, 70, 341R–61R CrossRef PubMed
.
- P. Vandenabeele, H. G. M. Edwards and L. Moens, A decade of Raman spectroscopy in art and archaeology, Chem. Rev., 2007, 107, 675–686 CrossRef PubMed
.
- H. J. Butler, S. W. Fogarty, J. G. Kerns, P. L. Martin-Hirsch, N. J. Fullwood and F. L. Martin, Gold nanoparticles as a substrate in bio-analytical near-infrared surface-enhanced Raman spectroscopy, Analyst, 2015, 140, 3090–3097 RSC
.
- J. Barbillat, P. Dhamelincourt, M. Delhaye and E. Dasilva, Raman Confocal Microprobing, Imaging and Fiberoptic Remote-Sensing – a Further Step in Molecular Analysis, J. Raman Spectrosc., 1994, 25, 3–11 CrossRef
.
- M. Delhaye and P. Dhamelincourt, Raman Microprobe and Microscope with Laser Excitation, J. Raman Spectrosc., 1975, 3, 33–43 CrossRef
.
- R. L. Mccreery, M. Fleischmann and P. Hendra, Fiber Optic Probe for Remote Raman Spectrometry, Anal. Chem., 1983, 55, 146–148 CrossRef
.
- M. G. Albrecht and J. A. Creighton, Anomalously Intense Raman-Spectra of Pyridine at a Silver Electrode, J. Am. Chem. Soc., 1977, 99, 5215–5217 CrossRef
.
- A. Campion and P. Kambhampati, Surface-enhanced Raman scattering, Chem. Soc. Rev., 1998, 27, 241–250 RSC
.
- M. Fleischmann, P. J. Hendra and A. J. Mcquillan, Raman-Spectra of Pyridine Adsorbed at a Silver Electrode, Chem. Phys. Lett., 1974, 26, 163–166 CrossRef
.
- D. L. Jeanmaire and R. P. Vanduyne, Surface Raman Spectroelectrochemistry 1 Heterocyclic, Aromatic, and Aliphatic-Amines Adsorbed on Anodized Silver Electrode, J. Electroanal. Chem., 1977, 84, 1–20 CrossRef
.
- K. Kneipp, Y. Wang, H. Kneipp, L. T. Perelman, I. Itzkan and R. Dasari, et al., Single molecule detection using surface-enhanced Raman scattering (SERS), Phys. Rev. Lett., 1997, 78, 1667–1670 CrossRef
.
- E. C. Le Ru and P. G. Etchegoin, Single-Molecule Surface-Enhanced Raman Spectroscopy, Annu. Rev. Phys. Chem., 2012, 63, 65–87 CrossRef PubMed
.
- S. M. Nie and S. R. Emery, Probing single molecules and single nanoparticles by surface-enhanced Raman scattering, Science, 1997, 275, 1102–1106 CrossRef PubMed
.
- W. Xie and S. Schlucker, Medical applications of surface-enhanced Raman scattering, Phys. Chem. Chem. Phys., 2013, 15, 5329–5344 RSC
.
- O. Ibrahim, A. Maguire, A. D. Meade, S. Flint, M. Toner and H. J. Byrne, et al., Improved protocols for pre-processing Raman spectra of formalin fixed paraffin preserved tissue sections, Anal. Methods, 2017, 9, 4709–4717 RSC
.
- F. Downey, N. Cade, R. Cook, R. Springall, C. Gillet and D. Richards, et al., Use of Raman Spectroscopy in Characterizing Formalin-Fixed, Paraffin-Embedded Breast Tumor Samples (abstract), AIP Conf. Proc., 2009, vol. 1119, p. 211 Search PubMed
.
- J. De Gelder, K. De Gussem, P. Vandenabeele and L. Moens, Reference database of Raman spectra of biological molecules, J. Raman Spectrosc., 2007, 38, 1133–1147 CrossRef
.
- P. R. Carey, Biochemical applications of Raman and resonance Raman spectroscopies, Academic Press, New York, 1982 Search PubMed
.
- R. H. Schirmer and G. E. Sa, Principles of Protein Structure, Springer-Verlag, Berlin-Heidelberg-New York, 1979 Search PubMed
.
- T. K. Chaudhuri and S. Paul, Protein-misfolding diseases and chaperone-based therapeutic approaches, FEBS J., 2006, 273, 1331–1349 CrossRef PubMed
.
- F. U. Hartl, Protein Misfolding Diseases, Annu. Rev. Biochem., 2017, 86, 21–26 CrossRef PubMed
.
- A. Rygula, K. Majzner, K. M. Marzec, A. Kaczor, M. Pilarczyk and M. Baranska, Raman spectroscopy of proteins: a review, J. Raman Spectrosc., 2013, 44, 1061–1076 CrossRef
.
- N. C. Maiti, M. M. Apetri, M. G. Zagorski, P. R. Carey and V. E. Anderson, Raman spectroscopic characterization of secondary structure in natively unfolded proteins: alpha-synuclein, J. Am. Chem. Soc., 2004, 126, 2399–2408 CrossRef PubMed
.
- J. L. Lippert, D. Tyminski and P. J. Desmeules, Determination of the secondary structure of proteins by laser Raman spectroscopy, J. Am. Chem. Soc., 1976, 98, 7075–7080 CrossRef PubMed
.
- R. W. Williams, Protein Secondary Structure-Analysis Using Raman Amide-I and Amide-III Spectra, Methods Enzymol., 1986, 130, 311–331 Search PubMed
.
- A. Ianoul, M. N. Boyden and S. A. Asher, Dependence of the peptide Amide III vibration on the phi dihedral angle, J. Am. Chem. Soc., 2001, 123, 7433–7434 CrossRef PubMed
.
- A. V. Mikhonin, S. V. Bykov, N. S. Myshakina and S. A. Asher, Peptide secondary structure folding reaction coordinate: correlation between uv raman Amide III frequency, Psi Ramachandran angle, and hydrogen bonding, J. Phys. Chem. B, 2006, 110, 1928–1943 CrossRef PubMed
.
- G. J. Thomas, New structural insights from Raman Spectroscopy of proteins and their assemblies, Biopolymers, 2002, 67, 214–225 CrossRef PubMed
.
- P. R. Carey, Raman crystallography and other biochemical applications of Raman microscopy, Annu. Rev. Phys. Chem., 2006, 57, 527–554 CrossRef PubMed
.
- M. S. Helfand, M. A. Totir, M. P. Carey, A. M. Hujer, R. A. Bonomo and P. R. Carey, Following the reactions of mechanism-based inhibitors with beta-lactamase by Raman crystallography, Biochemistry, 2003, 42, 13386–13392 CrossRef PubMed
.
- S. J. Hamodrakas, S. A. Asher, G. D. Mazur, J. C. Regier and F. C. Kafatos, Laser Raman studies of protein conformation in the silkmoth chorion, Biochim. Biophys. Acta, 1982, 703, 216–222 CrossRef
.
- S. Mangialardo, F. Piccirilli, A. Perucchi, P. Dore and P. Postorino, Raman analysis of insulin denaturation induced by high-pressure and thermal treatments, J. Raman Spectrosc., 2012, 43, 692–700 CrossRef
.
- R. W. Williams and A. K. Dunker, Determination of the secondary structure of proteins from the Amide I band of the laser Raman spectrum, J. Mol. Biol., 1981, 152, 783–813 CrossRef PubMed
.
- R. Tuma, Raman spectroscopy of proteins: from peptides to large assemblies, J. Raman Spectrosc., 2005, 36, 307–319 CrossRef
.
- J. Bandekar, Amide Modes and Protein Conformation, Biochim. Biophys. Acta, 1992, 1120, 123–143 CrossRef
.
- S. Cai and B. R. Singh, Identification of beta-turn and random coil Amide III infrared bands for secondary structure estimation of proteins, Biophys. Chem., 1999, 80, 7–20 CrossRef PubMed
.
- K. Nakamura, S. Era, Y. Ozaki, M. Sogami, T. Hayashi and M. Murakami, Conformational changes in seventeen cystine disulfide bridges of bovine serum albumin proved by Raman spectroscopy, FEBS Lett., 1997, 417, 375–378 CrossRef PubMed
.
- A. Dong, P. Huang and W. S. Caughey, Protein secondary structures in water from second-derivative Amide I infrared spectra, Biochemistry, 1990, 29, 3303–3308 CrossRef PubMed
.
- B. Hernandez, Y. M. Coic, F. Pfluger, S. G. Kruglik and M. Ghomi, All characteristic Raman markers of tyrosine and tyrosinate originate from phenol ring fundamental vibrations, J. Raman Spectrosc., 2016, 47, 210–220 CrossRef
.
- M. N. Siamwiza, R. C. Lord, M. C. Chen, T. Takamatsu, I. Harada and H. Matsuura, et al., Interpretation of the doublet at 850 and 830 cm−1 in the Raman spectra of tyrosyl residues in proteins and certain model compounds, Biochemistry, 1975, 14, 4870–4876 CrossRef PubMed
.
- H. Takeuchi, Raman structural markers of tryptophan and histidine side chains in proteins, Biopolymers, 2003, 72, 305–317 CrossRef PubMed
.
- T. Miura, H. Takeuchi and I. Harada, Characterization of Individual Tryptophan Side-Chains in Proteins Using Raman-Spectroscopy and Hydrogen-Deuterium Exchange Kinetics, Biochemistry, 1988, 27, 88–94 CrossRef PubMed
.
- B. Hernandez, F. Pfluger, S. G. Kruglik and M. Ghomi, Characteristic Raman lines of phenylalanine analyzed by a multiconformational approach, J. Raman Spectrosc., 2013, 44, 827–833 CrossRef
.
- R. C. Lord and G. J. Thomas Jr, Raman studies of nucleic acids II Aqueous purine and pyrimidine mixtures, Biochim. Biophys. Acta, 1967, 142, 1–11 CrossRef
.
- P. Piredda, M. Berning, P. Boukamp and A. Volkmer, Subcellular Raman Microspectroscopy Imaging of Nucleic Acids and Tryptophan for Distinction of Normal Human Skin Cells and Tumorigenic Keratinocytes, Anal. Chem., 2015, 87, 6778–6785 CrossRef PubMed
.
- A. C. S. Talari, C. A. Evans, I. Holen, R. E. Coleman and I. U. Rehman, Raman spectroscopic analysis differentiates between breast cancer cell lines, J. Raman Spectrosc., 2015, 46, 421–427 CrossRef
.
- J. L. Denbigh, D. Perez-Guaita, R. R. Vernooij, M. J. Tobin, K. R. Bambery and Y. Xu, et al., Probing the action of a novel anti-leukaemic drug therapy at the single cell level using modern vibrational spectroscopy techniques, Sci. Rep., 2017, 7, 2649 CrossRef PubMed
.
- A. Dutta, R. Gautam, S. Chatterjee, F. Ariese, S. K. Sikdar and S. Umapathy, Ascorbate protects neurons against oxidative stress: a Raman microspectroscopic study, ACS Chem. Neurosci., 2015, 6, 1794–1801 CrossRef PubMed
.
- J. M. Benevides and G. J. Thomas, Characterization of DNA Structures by Raman-Spectroscopy – High-Salt and Low-Salt Forms of Double Helical Poly(Dg-Dc) in H2O and D2O Solutions and Application to B-DNA, Z-DNA and A-DNA, Nucleic Acids Res., 1983, 11, 5747–5761 CrossRef PubMed
.
- J. Duguid, V. A. Bloomfield, J. Benevides and G. J. Thomas, Raman-Spectroscopy of DNA-Metal Complexes. 1. Interactions and Conformational Effects of the Divalent-Cations – Mg, Ca, Sr, Ba, Mn, Co, Ni, Cu, Pd, and Cd, Biophys. J., 1993, 65, 1916–1928 CrossRef PubMed
.
- K. A. Okotrub, N. V. Surovtsev, V. F. Semeshin and L. V. Omelyanchuk, Raman Spectroscopy for DNA Quantification in Cell Nucleus, Cytometry, Part A, 2015, 87A, 68–73 CrossRef PubMed
.
- E. Lipiec, R. Sekine, J. Bielecki, W. M. Kwiatek and B. R. Wood, Molecular Characterization of DNA Double Strand Breaks with Tip-Enhanced Raman Scattering, Angew. Chem., Int. Ed., 2014, 53, 169–172 CrossRef PubMed
.
- E. Bailo and V. Deckert, Tip-enhanced Raman spectroscopy of single RNA strands: Towards a novel direct-sequencing method, Angew. Chem., Int. Ed., 2008, 47, 1658–1661 CrossRef PubMed
.
- R. Treffer, R. Bohme, T. Deckert-Gaudig, K. Lau, S. Tiede and X. Lin, et al., Advances in TERS (tip-enhanced Raman scattering) for biochemical applications, Biochem. Soc. Trans., 2012, 40, 609–614 CrossRef PubMed
.
- M. M. Hlaing, B. Wood, D. McNaughton, D. Y. Ying and M. A. Augustin, Raman spectroscopic analysis of Lactobacillus rhamnosus GG in response to dehydration reveals DNA conformation changes, J. Biophotonics, 2017, 10, 589–597 CrossRef PubMed
.
- J. M. Benevides, S. A. Overman and G. J. Thomas, Raman, polarized Raman and ultraviolet resonance Raman spectroscopy of nucleic acids and their complexes, J. Raman Spectrosc., 2005, 36, 279–299 CrossRef
.
- K. Czamara, K. Majzner, M. Z. Pacia, K. Kochan, A. Kaczor and M. Baranska, Raman spectroscopy of lipids: a review, J. Raman Spectrosc., 2015, 46, 4–20 CrossRef
.
- S. P. Singh and C. M. Krishna, Raman spectroscopic studies of oral cancers: correlation of spectral and biochemical markers, Anal. Methods, 2014, 6, 8613–8620 RSC
.
- C. Nieva, M. Marro, N. Santana-Codina, S. Rao, D. Petrov and A. Sierra, The lipid phenotype of breast cancer cells characterized by Raman microspectroscopy: towards a stratification of malignancy, PLoS One, 2012, 7, e46456 CrossRef PubMed
.
- U. Munchberg, L. Wagner, C. Rohrer, K. Voigt, P. Rosch and G. Jahreis, et al., Quantitative assessment of the degree of lipid unsaturation in intact Mortierella by Raman microspectroscopy, Anal. Bioanal. Chem., 2015, 407, 3303–3311 CrossRef PubMed
.
- E. Wiercigroch, E. Szafraniec, K. Czamara, M. Z. Pacia, K. Majzner and K. Kochan, et al., Raman and infrared spectroscopy of carbohydrates: A review, Spectrochim. Acta, Part A, 2017, 185, 317–335 CrossRef PubMed
.
- P. H. Arboleda and G. R. Loppnow, Raman spectroscopy as a discovery tool in carbohydrate chemistry, Anal. Chem., 2000, 72, 2093–2098 CrossRef PubMed
.
- D. A. Stuart, J. M. Yuen, N. Shah, O. Lyandres, C. R. Yonzon and M. R. Glucksberg, et al., In vivo glucose measurement by surface-enhanced Raman spectroscopy, Anal. Chem., 2006, 78, 7211–7215 CrossRef PubMed
.
- R. S. Jakubek, J. Handen, S. E. White, S. A. Asher and I. K. Lednev, TrAC, Trends Anal. Chem., 2018, 103, 223–229 CrossRef
.
- P. J. Artymiuk, C. C. F. Blake, D. W. Rice and K. S. Wilson, The structures of the monoclinic and orthorhombic forms of hen egg-white lysozyme at 6 A resolution, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1982, 38, 778–783 CrossRef
.
- J. A. Jaros, P. C. Guest, S. Bahn and D. Martins-de-Souza, Affinity depletion of plasma and serum for mass spectrometry-based proteome analysis, Methods Mol. Biol., 2013, 1002, 1–11 CrossRef PubMed
.
- R. Gautam, S. Vanga, A. Madan, N. Gayathri, U. Nongthomba and S. Umapathy, Raman spectroscopic studies on screening of myopathies, Anal. Chem., 2015, 87, 2187–2194 CrossRef PubMed
.
- Z. Movasaghi, S. Rehman and I. U. Rehman, Raman spectroscopy of biological tissues, Appl. Spectrosc. Rev., 2007, 42, 493–541 CrossRef
.
- S. Kumar, T. Verma, R. Mukherjee, F. Ariese, K. Somasundaram and S. Umapathy, Raman and infra-red microspectroscopy: towards quantitative evaluation for clinical research by ratiometric analysis, Chem. Soc. Rev., 2016, 45, 1879–1900 RSC
.
- S. Kumar, N. Matange, S. Umapathy and S. S. Visweswariah, Linking carbon metabolism to carotenoid production in mycobacteria using Raman spectroscopy, FEMS Microbiol. Lett., 2015, 362, 1–6 CrossRef PubMed
.
- S. K. Paidi, A. Rizwan, C. Zheng, M. Cheng, K. Glunde and I. Barman, Label-Free Raman Spectroscopy Detects Stromal Adaptations in Premetastatic Lungs Primed by Breast Cancer, Cancer Res., 2017, 77, 247–256 CrossRef PubMed
.
- S. Sil, R. Mukherjee, N. S. Kumar, S. Aravind, J. K. Kingston. and U. Singh, Detection and classification of Bacteria using Raman Spectroscopy Combined with Multivariate Analysis, Def. Life Sci. J., 2017, 2, 435–441 CrossRef
.
- S. Sil, R. Gautam and S. Umapathy, Applications of Raman and Infrared Microscopy to Materials and Biology, Molecular and Laser Spectroscopy, Gupta V. P., Elsevier, 2018, ch. 6, pp. 117–146 Search PubMed
.
- B. Singh, R. Gautam, S. Kumar, B. N. V. Kumar, U. Nongthomba and D. Nandi, et al., Application of vibrational microspectroscopy to biology and medicine, Curr. Sci., 2012, 102, 232–244 Search PubMed
.
- R. Gautam, A. Samuel, S. Sil, D. Chaturvedi, A. Dutta and F. Ariese, et al., Raman and mid-infrared spectroscopic imaging: applications and advancements, Curr. Sci., 2015, 108, 341–356 Search PubMed
.
- R. Gautam, S. Vanga, F. Ariese and S. Umapathy, Review of multidimensional data processing approaches for Raman and infrared spectroscopy, EPJ Techniques and Instrumentation, 2015, 2, 8 CrossRef
.
- F. Bonnier and H. J. Byrne, Understanding the molecular information contained in principal component analysis of vibrational spectra of biological systems, Analyst, 2012, 137, 322–332 RSC
.
- H. J. Butler, L. Ashton, B. Bird, G. Cinque, K. Curtis and J. Dorney, et al., Using Raman spectroscopy to characterize biological materials, Nat. Protoc., 2016, 11, 664–687 CrossRef PubMed
.
- M. Kopec and H. Abramczyk, Angiogenesis – a crucial step in breast cancer growth, progression and dissemination by Raman imaging, Spectrochim. Acta, Part A, 2018, 198, 338–345 CrossRef PubMed
.
- S. J. Van Nest, L. M. Nicholson, L. DeVorkin, A. G. Brolo, J. J. Lum and A. Jirasek, Raman Spectroscopic Signatures Reveal Distinct Biochemical and Temporal Changes in Irradiated Human Breast Adenocarcinoma Xenografts, Radiat. Res., 2018, 189, 497–504 CrossRef PubMed
.
- D. Chaturvedi, S. A. Balaji, V. K. Bn, F. Ariese, S. Umapathy and A. Rangarajan, Different Phases of Breast Cancer Cells: Raman Study of Immortalized, Transformed, and Invasive Cells, Biosensors, 2016, 6, 57 CrossRef PubMed
.
- S. Kim, S. H. Lee, S. Y. Min, K. M. Byun and S. Y. Lee, Dual-modal cancer detection based on optical pH sensing and Raman spectroscopy, J. Biomed. Opt., 2017, 22, 1–6 Search PubMed
.
- P. T. Winnard Jr, C. Zhang, F. Vesuna, J. W. Kang, J. Garry and R. R. Dasari, et al., Organ-specific isogenic metastatic breast cancer cell lines exhibit distinct Raman spectral signatures and metabolomes, Oncotarget, 2017, 8, 20266–20287 CrossRef PubMed
.
- J. Desroches, M. Jermyn, M. Pinto, F. Picot, M. A. Tremblay and S. Obaid, et al., A new method using Raman spectroscopy for in vivo targeted brain cancer tissue biopsy, Sci. Rep., 2018, 8, 1792 CrossRef PubMed
.
- H. Abramczyk and A. Imiela, The biochemical, nanomechanical and chemometric signatures of brain cancer, Spectrochim. Acta, Part A, 2018, 188, 8–19 CrossRef PubMed
.
- A. Malik, A. Sahu, S. P. Singh, A. Deshmukh, P. Chaturvedi and D. Nair, et al., In vivo Raman spectroscopy-assisted early identification of potential second primary/recurrences in oral cancers: An exploratory study, Head Neck, 2017, 39, 2216–2223 CrossRef PubMed
.
- L. C. Ming, N. R. Gangodu, T. Loh, W. Zheng, J. Wang and K. Lin, et al., Real time near-infrared Raman spectroscopy for the diagnosis of nasopharyngeal cancer, Oncotarget, 2017, 8, 49443–49450 CrossRef PubMed
.
- L. M. Almond, J. Hutchings, G. Lloyd, H. Barr, N. Shepherd and J. Day, et al., Endoscopic Raman spectroscopy enables objective diagnosis of dysplasia in Barrett's esophagus, Gastrointest. Endosc., 2014, 79, 37–45 CrossRef PubMed
.
- H. Moradi, A. Ahmad, D. Shepherdson, N. H. Vuong, G. Niedbala and L. Eapen, et al., Raman micro-spectroscopy applied to treatment resistant and sensitive human ovarian cancer cells, J. Biophotonics, 2017, 10, 1327–1334 CrossRef PubMed
.
- M. Jermyn, J. Desroches, J. Mercier, K. St-Arnaud, M. C. Guiot and F. Leblond, et al., Raman spectroscopy detects distant invasive brain cancer cells centimeters beyond MRI capability in humans, Biomed. Opt. Express, 2016, 7, 5129–5137 CrossRef PubMed
.
- M. Brusatori, G. Auner, T. Noh, L. Scarpace, B. Broadbent and S. N. Kalkanis, Intraoperative Raman Spectroscopy, Neurosurg. Clin. N. Am., 2017, 28, 633–652 CrossRef PubMed
.
- J. Klener, K. Hofbauerova, A. Bartos, J. Ricny, D. Ripova and V. Kopecky, Instability of cerebrospinal fluid after delayed storage and repeated freezing: a holistic study by drop coating deposition Raman spectroscopy, Clin. Chem. Lab. Med., 2014, 52, 657–664 Search PubMed
.
- E. Kocisova, A. Antalik and M. Prochazka, Drop coating deposition Raman spectroscopy of liposomes: role of cholesterol, Chem. Phys. Lipids, 2013, 172–173, 1–5 CrossRef PubMed
.
- E. Kocisova, M. Prochazka and L. Vaculciakova, Drop-Coating Deposition Raman (DCDR) Spectroscopy as a Tool for Membrane Interaction Studies: Liposome-Porphyrin Complex, Appl. Spectrosc., 2015, 69, 939–945 CrossRef PubMed
.
- T. J. Harvey, E. C. Faria, A. Henderson, E. Gazi, A. D. Ward and N. W. Clarke, et al., Spectral discrimination of live prostate and bladder cancer cell lines using Raman optical tweezers, J. Biomed. Opt., 2008, 13, 064004 CrossRef PubMed
.
- S. Corsetti, T. Rabl, D. McGloin and G. Nabi, Raman spectroscopy for accurately characterizing biomolecular changes in androgen-independent prostate cancer cells, J. Biophotonics, 2018, 11, e201700166 CrossRef PubMed
.
- J. O'Malley, R. Kumar, A. N. Kuzmin, A. Pliss, N. Yadav and S. Balachandar, et al., Lipid quantification by Raman microspectroscopy as a potential biomarker in prostate cancer, Cancer Lett., 2017, 397, 52–60 CrossRef PubMed
.
- G. L. Owens, K. Gajjar, J. Trevisan, S. W. Fogarty, S. E. Taylor and B. Da Gama-Rose, et al., Vibrational biospectroscopy coupled with multivariate analysis extracts potentially diagnostic features in blood plasma/serum of ovarian cancer patients, J. Biophotonics, 2014, 7, 200–209 CrossRef PubMed
.
- T. Mahmood, H. Nawaz, A. Ditta, M. I. Majeed, M. A. Hanif and N. Rashid, et al., Raman spectral analysis for rapid screening of dengue infection, Spectrochim. Acta, Part A, 2018, 200, 136–142 CrossRef PubMed
.
- M. Bilal, R. Ullah, S. Khan, H. Ali, M. Saleem and M. Ahmed, Lactate based optical screening of dengue virus infection in human sera using Raman spectroscopy, Biomed. Opt. Express, 2017, 8, 1250–1256 CrossRef PubMed
.
- S. Khan, R. Ullah, A. Khan, A. Sohail, N. Wahab and M. Bilal, et al., Random Forest-Based Evaluation of Raman Spectroscopy for Dengue Fever Analysis, Appl. Spectrosc., 2017, 71, 2111–2117 CrossRef PubMed
.
- M. Bilal, M. Saleem, S. T. Amanat, H. A. Shakoor, R. Rashid and A. Mahmood, et al., Optical diagnosis of malaria infection in human plasma using Raman spectroscopy, J. Biomed. Opt., 2015, 20, 017002 CrossRef PubMed
.
- L. Frame, J. Brewer, R. Lee, K. Faulds and D. Graham, Development of a label-free Raman imaging technique for differentiation of malaria parasite infected from non-infected tissue, Analyst, 2017, 143, 157–163 RSC
.
- D. Perez-Guaita, K. M. Marzec, A. Hudson, C. Evans, T. Chernenko and C. Matthaus, et al., Parasites under the Spotlight: Applications of Vibrational Spectroscopy to Malaria Research, Chem. Rev., 2018, 118, 5330–5358 CrossRef PubMed
.
- R. Sathyavathi, N. C. Dingari, I. Barman, P. S. Prasad, S. Prabhakar and D. Narayana Rao, et al., Raman spectroscopy provides a powerful, rapid diagnostic tool for the detection of tuberculous meningitis in ex vivo cerebrospinal fluid samples, J. Biophotonics, 2013, 6, 567–572 CrossRef PubMed
.
- U. Neugebauer, S. Trenkmann, T. Bocklitz, D. Schmerler, M. Kiehntopf and J. Popp, Fast differentiation of SIRS and sepsis from blood plasma of ICU patients using Raman spectroscopy, J. Biophotonics, 2014, 7, 232–240 CrossRef PubMed
.
- H. Ding, A. W. Dupont, S. Singhal, L. D. Scott, S. Guha and M. Younes, et al., In vivo analysis of mucosal lipids reveals histological disease activity in ulcerative colitis using endoscope-coupled Raman spectroscopy, Biomed. Opt. Express, 2017, 8, 3426–3439 CrossRef PubMed
.
- J. Addis, N. Mohammed, O. Rotimi, D. Magee, A. Jha and V. Subramanian, Raman spectroscopy of endoscopic colonic biopsies from patients with ulcerative colitis to identify mucosal inflammation and healing, Biomed. Opt. Express, 2016, 7, 2022–2035 CrossRef PubMed
.
- I. J. Pence, D. B. Beaulieu, S. N. Horst, X. Bi, A. J. Herline and D. A. Schwartz, et al., Clinical characterization of in vivo inflammatory bowel disease with Raman spectroscopy, Biomed. Opt. Express, 2017, 8, 524–535 CrossRef PubMed
.
- C. Camerlingo, F. d'Apuzzo, V. Grassia, L. Perillo and M. Lepore, Micro-Raman spectroscopy for monitoring changes in periodontal ligaments and gingival crevicular fluid, Sensors, 2014, 14, 22552–22563 CrossRef PubMed
.
- M. Paraskevaidi, C. L. M. Morais, D. E. Halliwell, D. M. A. Mann, D. Allsop and P. L. Martin-Hirsch, et al., Raman Spectroscopy to Diagnose Alzheimer's Disease and Dementia with Lewy Bodies in Blood, ACS Chem. Neurosci., 2018 DOI:10.1021/acschemneuro.8b00198
.
- E. Ryzhikova, O. Kazakov, L. Halamkova, D. Celmins, P. Malone and E. Molho, et al., Raman spectroscopy of blood serum for Alzheimer's disease diagnostics: specificity relative to other types of dementia, J. Biophotonics, 2015, 8, 584–596 CrossRef PubMed
.
- L. Bokobza, J.-L. Bruneel and M. Couzi, Raman spectroscopy as a tool for the analysis of carbon-based materials (highly oriented pyrolitic graphite, multilayer graphene and multiwall carbon nanotubes) and of some of their elastomeric composites, Vib. Spectrosc., 2014, 74, 57–63 CrossRef
.
- Y. Wang, D. C. Alsmeyer and R. L. McCreery, Raman spectroscopy of carbon materials: structural basis of observed spectra, Chem. Mater., 1990, 2, 557–563 CrossRef
.
- M. S. Dresselhaus, G. Dresselhaus, R. Saito and A. Jorio, Raman spectroscopy of carbon nanotubes, Phys. Rep., 2005, 409, 47–99 CrossRef
.
- M. S. Dresselhaus, A. Jorio, M. Hofmann, G. Dresselhaus and R. Saito, Perspectives on Carbon Nanotubes and Graphene Raman Spectroscopy, Nano Lett., 2010, 10, 751–758 CrossRef PubMed
.
- A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri and F. Mauri, et al., Raman Spectrum of Graphene and Graphene Layers, Phys. Rev. Lett., 2006, 97, 187401 CrossRef PubMed
.
- A. C. Ferrari and D. M. Basko, Raman spectroscopy as a versatile tool for studying the properties of graphene, Nat. Nanotechnol., 2013, 8, 235 CrossRef PubMed
.
- M. R. Kagan and R. L. Mccreery, Reduction of Fluorescence Interference in Raman-Spectroscopy Via Analyte Adsorption on Graphitic Carbon, Anal. Chem., 1994, 66, 4159–4165 CrossRef
.
- X. Ling, L. M. Xie, Y. Fang, H. Xu, H. L. Zhang and J. Kong, et al., Can Graphene be used as a Substrate for Raman Enhancement?, Nano Lett., 2010, 10, 553–561 CrossRef PubMed
.
- L. Xie, X. Ling, Y. Fang, J. Zhang and Z. Liu, Graphene as a Substrate To Suppress Fluorescence in Resonance Raman Spectroscopy, J. Am. Chem. Soc., 2009, 131, 9890–9891 CrossRef PubMed
.
- S. Sil, N. Kuhar, S. Acharya and S. Umapathy, Is Chemically Synthesized Graphene 'Really' a Unique Substrate for SERS and Fluorescence Quenching?, Sci. Rep., 2013, 3, 3336 CrossRef PubMed
.
- M. Lopez-Lopez, V. Merk, C. Garcia-Ruiz and J. Kneipp, Surface-enhanced Raman spectroscopy for the analysis of smokeless gunpowders and macroscopic gunshot residues, Anal. Bioanal. Chem., 2016, 408, 4965–4973 CrossRef PubMed
.
- S. Sil, D. Chaturvedi, K. B. Krishnappa, S. Kumar, S. N. Asthana and S. Umapathy, Density functional theoretical modeling, electrostatic surface potential and surface enhanced Raman spectroscopic studies on biosynthesized silver nanoparticles: observation of 400 PM sensitivity to explosives, J. Phys. Chem. A, 2014, 118, 2904–2914 CrossRef PubMed
.
- National Research Council, Committee on the Review of Existing and Potential Standoff Explosives Detection Techniques, The National Academies Press, Washington, DC, 2004 Search PubMed
.
- T. Hirschfeld, E. R. Schildkraut, H. Tannenbaum and D. Tannenbaum, Remote Spectroscopic Analysis of Ppm-Level Air Pollutants by Raman Spectroscopy, Appl. Phys. Lett., 1973, 22, 38–40 CrossRef
.
- J. P. Agrawal, High Energy Materials: Propellants, Explosives and Pyrotechnics, 2010 Search PubMed
.
- J. Akhavan, The Chemistry of Explosives, 3rd edn, 2011 Search PubMed.
- J. Mills, C. Farley, A. Kassu, M. Curley, A. Sharma and P. Ruffin, et al., Raman monitoring and evaluation of the aging effects of rocket propellant stabilizers, Proc. SPIE, 2017, 10382 Search PubMed
.
- M. Ghosh, V. Venkatesan, N. Sikder and A. K. Sikder, Quantitative Analysis of alpha-CL-20 Polymorphic Impurity in epsilon-CL-20 using Dispersive Raman Spectroscopy, Cent. Eur. J. Energ. Mater., 2013, 10, 419–438 Search PubMed
.
- Z. A. Dreger and Y. M. Gupta, Raman Spectroscopy of High-Pressure-High-Temperature Polymorph of Hexahydro-1,3,5-trinitro-1,3,5-triazine (epsilon-RDX), J. Phys. Chem. A, 2010, 114, 7038–7047 CrossRef PubMed
.
- V. Thottempudi and J. M. Shreeve, Synthesis and promising properties of a new family of high-density energetic salts of 5-nitro-3-trinitromethyl-1H-1,2,4-triazole and 5,5′-bis(trinitromethyl)-3,3′-azo-1H-1,2,4-triazole, J. Am. Chem. Soc., 2011, 133, 19982–19992 CrossRef PubMed
.
- K. B. Landenberger and A. J. Matzger, Cocrystal Engineering of a Prototype Energetic Material Supramolecular Chemistry of 2,4,6-Trinitrotoluene, Cryst. Growth Des., 2010, 10, 5341–5347 CrossRef
.
- R. V. Kent, R. A. Wiscons, P. Sharon, D. Grinstein, A. A. Frimer and A. J. Matzger, Cocrystal Engineering of a High Nitrogen Energetic Material, Cryst. Growth Des., 2018, 18, 219–224 CrossRef
.
- P. Ravi, D. M. Badgujar, G. M. Gore, S. P. Tewari and A. K. Sikder, Review on Melt Cast Explosives, Propellants, Explos., Pyrotech., 2011, 36, 393–403 CrossRef
.
- Y. Kholod, S. Okovytyy, G. Kuramshina, M. Qasim, L. Gorb and J. Leszczynski, An analysis of stable forms of CL-20: A DFT study of conformational transitions, infrared and Raman spectra, J. Mol. Struct., 2007, 843, 14–25 CrossRef
.
- P. Goede, N. V. Latypov and H. Ostmark, Fourier transform Raman Spectroscopy of the four crystallographic phases of alpha, beta, gamma and epsilon 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazatetracyclo[5.5.0.0(5,9).0(3,11)]dodecane (HNIW, CL-20), Propellants, Explos., Pyrotech., 2004, 29, 205–208 CrossRef
.
- A. H. Farhadian, M. K. Tehrani, M. H. Keshavarz and S. M. R. Darbani, Raman spectroscopy combined with principle component analysis to investigate the aging of high energy materials, Laser Phys., 2017, 27, 075701 CrossRef
.
- P. Matousek, I. P. Clark, E. R. C. Draper, M. D. Morris, A. E. Goodship and N. Everall, et al., Subsurface probing in diffusely scattering media using spatially offset Raman spectroscopy, Appl. Spectrosc., 2005, 59, 393–400 CrossRef PubMed
.
- O. Stevens, I. E. I. Petterson, J. C. C. Day and N. Stone, Developing fibre optic Raman probes for applications in clinical spectroscopy, Chem. Soc. Rev., 2016, 45, 1919–1934 RSC
.
- R. J. Stokes, M. Bailey, S. Bonthron, T. Stone, G. Maskall and O. Presly, et al., New capability for hazardous materials ID within sealed containers using a portable spatially offset Raman spectroscopy (SORS) device, Optics and Photonics for Counterterrorism, Crime Fighting, and Defence Xii, 2016, vol. 9995 Search PubMed
.
- P. Matousek, C. Conti, M. Realini and C. Colombo, Micro-scale spatially offset Raman spectroscopy for non-invasive subsurface analysis of turbid materials, Analyst, 2016, 141, 731–739 RSC
.
- B. Schrader and G. Bergmann, Die Intensität des Ramanspektrums polykristalliner Substanzen, Fresenius' J. Anal. Chem., 1967, 225, 230–247 CrossRef
.
- P. Matousek and A. W. Parker, Bulk Raman analysis of pharmaceutical tablets, Appl. Spectrosc., 2006, 60, 1353–1357 CrossRef PubMed
.
- C. Eliasson, N. A. Macleod, L. C. Jayes, F. C. Clarke, S. V. Hammond and M. R. Smith, et al., Non-invasive quantitative assessment of the content of pharmaceutical capsules using transmission Raman spectroscopy, J. Pharm. Biomed. Anal., 2008, 47, 221–229 CrossRef PubMed
.
- J. Johansson, A. Sparen, O. Svensson, S. Folestad and M. Claybourn, Quantitative transmission Raman spectroscopy of pharmaceutical tablets and capsules, Appl. Spectrosc., 2007, 61, 1211–1218 CrossRef PubMed
.
- M. Edinger, M. M. Knopp, H. Kerdoncuff, J. Rantanen, T. Rades and K. Lobmann, Quantification of microwave-induced amorphization of celecoxib in PVP tablets using transmission Raman spectroscopy, Eur. J. Pharm. Sci., 2018, 117, 62–67 CrossRef PubMed
.
- D. Andrews, K. Geentjens, B. Igne, G. McGeorge, A. Owen and N. Pedge, et al., Analytical Method Development Using Transmission Raman Spectroscopy for Pharmaceutical Assays and Compliance with Regulatory Guidelines—Part I: Transmission Raman Spectroscopy and Method Development, J. Pharm. Innov., 2018, 13, 121–132 CrossRef
.
- M. Hartmann, Light scattering by small particles. By H. C. van de Hulst. New York (John Wiley and Sons), London (Chapman and Hall), 1957. Pp. xiii, 470; 103 Figs.; 46 Tables. 96s, Q. J. R. Metereol. Soc., 1958, 84, 198–199 Search PubMed
.
- C. F. Bohren and D. R. Huffman, Absorption and Scattering of Light by Small Particles, Wiley, 1998 Search PubMed
.
- A. Ishimaru, Wave Propagation and Scattering in Random Media-Multiple Scattering, Turbulence, Rough Surfaces, and Remote Sensing, Academic Press, 1978 Search PubMed
.
- S. Sasic and Y. Ozaki, Raman, Infrared, and Near-Infrared Chemical Imaging, 2010 Search PubMed
.
- D. J. Pine, D. A. Weitz, P. M. Chaikin and E. Herbolzheimer, Diffusing-Wave Spectroscopy, Phys. Rev. Lett., 1988, 60, 1134–1137 CrossRef PubMed
.
- I. E. Petterson, M. Lopez-Lopez, C. Garcia-Ruiz, C. Gooijer, J. B. Buijs and F. Ariese, Noninvasive detection of concealed explosives: depth profiling through opaque plastics by time-resolved Raman spectroscopy, Anal. Chem., 2011, 83, 8517–8523 CrossRef PubMed
.
- S. Umapathy, S. Sil, J. Kiran, Method and a system for detection of hazardous chemicals in a non-metallic container, Ind. Pat., IN2311CH2013, 2013, US Pat. 9,606,062B2, 2017
.
- S. Umapathy, S. Sil, G. Dhal, F. Ariese, Chemical signature resolved detection of concealed objects, Ind. Pat., IN6626/CHE/2014, 2014, US Pat. App. 15/122,170, 2016
.
- S. Umapathy, S. Sil, J. Kiran, Method and an apparatus for obtaining sample specific signatures, Ind. Pat., IN2312CH2013, 2013, US Pat., US9983136B2, 2018
.
- V. Periyasamy, S. Sil, G. Dhal, F. Ariese, S. Umapathy and M. Pramanik, Experimentally validated Raman Monte Carlo simulation for a cuboid object to obtain Raman spectroscopic signatures for hidden material, J. Raman Spectrosc., 2015, 46, 669–676 CrossRef
.
- S. Sil and S. Umapathy, Raman spectroscopy explores molecular structural signatures of hidden materials in depth: Universal Multiple Angle Raman Spectroscopy, Sci. Rep., 2014, 4, 5308 CrossRef PubMed
.
- W. Keifer, Recent advances in linear and nonlinear Raman spectroscopy II, J. Raman Spectrosc., 2008, 39, 1710–1725 CrossRef
.
Footnote |
| † All the authors have contributed equally and the names have been provided in alphabetical order. |
| This journal is © The Royal Society of Chemistry 2018 |