Open Access Article
Open Access ArticleCudrania tricuspidata: an updated review on ethnomedicine, phytochemistry and pharmacology
Lan-Ting Xin†
 ab,
Shi-Jun Yue†ab,
Ya-Chu Fanab,
Jing-Shuai Wuab,
Dan Yanc,
Hua-Shi Guan*ab and
Chang-Yun Wang
ab,
Shi-Jun Yue†ab,
Ya-Chu Fanab,
Jing-Shuai Wuab,
Dan Yanc,
Hua-Shi Guan*ab and
Chang-Yun Wang *ab
*ab
aKey Laboratory of Marine Drugs, The Ministry of Education of China, School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, P. R. China. E-mail: changyun@ouc.edu.cn; hsguan@ouc.edu.cn; Fax: +86 532 82031536; Tel: +86 532 82031536 Tel: +86 532 82031667
bLaboratory for Marine Drugs and Bioproducts, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, P. R. China
cBeijing Shijitan Hospital, Capital Medical University, Beijing 100038, P. R. China
First published on 22nd June 2017
Abstract
Cudrania tricuspidata is a perennial plant of the family Moraceae with numerous medicinal and nutritional applications. It has been widely used in East Asia as an important traditional folk medicine for the treatment of many ailments such as eczema, mumps, tuberculosis, contusions, insomnia and acute arthritis. The whole plant of C. tricuspidata, including the roots, leaves, bark, stems and fruits, has been found to contain diverse phytochemicals, including xanthones, flavonoids, organic acids, and polysaccharides, with various bioactivities. In particular, xanthones and flavonoids, as the main active constituents, isolated from C. tricuspidata have been proven to possess notable anti-inflammatory, antioxidative, antitumor, hepatoprotective, neuroprotective and anti-obesity effects. This review summarizes the botany, traditional uses, phytochemistry and pharmacology of C. tricuspidata, and the limitations of studies on this species have also been discussed such that to serve as the basis for further research and development on this medicinal plant.
1 Introduction
Cudrania tricuspidata (Carr.) Bur. ex Lavallee, which is a deciduous thorny tree belonging to the family Moraceae, is widespread throughout East Asia1 and is known as cudrang, mandarin melon berry, silkworm thorn, storehouse bush, hariguwa (in Japanese) and che (in Chinese).2–4 In China, C. tricuspidata roots have been used as ‘Chuan-po-shi’ in traditional Chinese medicine (TCM) in the treatment of gonorrhea, rheumatism, jaundice, boils, scabies, bruising, and dysmenorrhea;5,6 its root bark has been widely used in the treatment of lumbago, hemoptysis, and contusions;7 and its roots and stems have also been applied in the forms of syrups, granules and injections to cure tumors of the digestive tract.8 In Korea, C. tricuspidata has become one of the most ubiquitous folk remedies against cancer during the last few decades.9 In addition to the above medical uses, in China the stems and roots of C. tricuspidata have been used to prepare herbal teas or functional beverages for a long period;10 the stems, which contain a reddish-yellow dye, have been noted for their use in coloring imperial garments;3 and the tender leaves of C. tricuspidata have been used as a perfect food for breeding silkworms since the Han dynasty, and the natural silk has been praised under the name of Zhe Si.11 Its edible fruits have been made into juices, jams, alcoholic beverages, dietary supplements and other health products in Korea.12 Moreover, its bark fibers have been utilized to make paper and its trunks have been used as valuable timber for furniture manufacture.13The immense medicinal and economic value of C. tricuspidata has encouraged numerous studies of its phytochemicals and pharmacological activities. C. tricuspidata extracts have been demonstrated to possess good therapeutic effects against various ailments including inflammation,14,15 tumors,16,17 obesity,18,19 and diabetes.20,21 Xanthones and flavonoids have been considered to be the two major classes of phytochemicals in C. tricuspidata. For example, prenylated xanthones and flavonoids were found to be the most important and abundant constituents in its leaves and root bark with regard to their notable anti-inflammatory,22,23 antitumor,16,24 hepatoprotective,25,26 neuroprotective,27,28 and anticoagulant29 activities; hydroxybenzyl flavonoid glycosides from the stem bark were reported to be promising natural antioxidant and antitumor agents;30 and prenylated isoflavonoids and benzylated flavonoids from the fruits displayed potential anti-inflammatory31 and antioxidant32 activities. Besides, a glycoprotein (75 kDa) from C. tricuspidata, which consisted of carbohydrate (72.5%) and protein moieties (27.5%), exhibited distinctive characteristics with anti-inflammatory,33 antioxidant,34 hepatoprotective,35 and immunomodulatory36 effects.
To date, to the best of our knowledge, no comprehensive review concerning C. tricuspidata has been available. A literature survey was conducted via an electronic search using PubMed, Scopus, ACS, Web of Science, ScienceDirect, China Knowledge Resource Integrated Database (CNKI), Google Scholar, SciFinder and a library search for ethnobotanical textbooks. The Plant List (www.theplantlist.org), the Missouri Botanical Garden's Tropicos nomenclature database (www.tropicos.org) and the Chinese Field Herbarium (www.cfh.ac.cn) were used to validate the taxonomy and also obtain information regarding subspecies and cultivars. On the basis of the literature search, we reviewed the achievements of research on the botanical characteristics, traditional uses, phytochemicals and pharmacological activities of C. tricuspidata so as to provide a systematic summary of the literature for further research on, and development of, this medicinal plant.
2 Botany and traditional uses of C. tricuspidata
2.1. Botany of C. tricuspidata
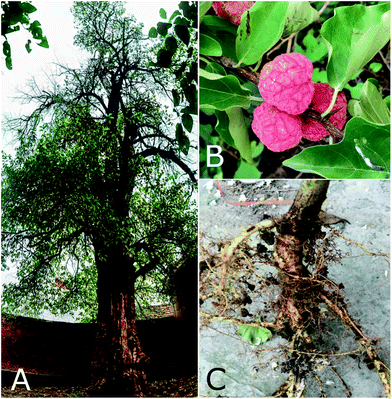
C. tricuspidata is one of six species in the genus Cudrania, which is endemic to Asia and Oceania, of the family Moraceae in the order Urticales.37 Five species, namely, C. tricuspidata, C. cochinchinensis (Lour.) Kudo et Masam, C. fruticosa (Roxb.) Wight ex Kurz, C. amboinensis (Bl.) Miq., and C. pubescens Trec., as well as C. cochinchinensis var. gerontogea (Nakai) Kudo et Masam, have been widely cultivated in Southern China, including Yunnan, Fujian and Jiangsu provinces, as well as in Hebei province, as profitable plants for producing valuable fruits and timber.38,39 In Europe C. tricuspidata was introduced into cultivation in 1870, and in the USA in 1909.40 In contrast, wild populations of this species are now under threat of extinction.As a hardy deciduous plant, C. tricuspidata is widely distributed in lowlands, foothills, forests, or dense scrub at altitudes of between 500 and 2000 m. It can eventually grow to a height of approximately 1.0–7.0 m (Fig. 1), but often exists as a broad spreading bush or small tree. Its leaves have single alternate ovate to rhombic-ovate blades with a size of 5.0–14.0 cm long and 3.0–6.0 cm wide; its flowers have a dioecious capitulum with an inflorescence length of 0.5 (male) or 1.0–1.5 cm (female); the color of its syncarpous fruits is orange-red when mature; and its roots, which are up to 50 cm long, are yellow and irregularly cylindrical.37
2.2. Traditional uses
Since ancient times, C. tricuspidata has been used as a folk medicine in oriental countries.41 The medicinal material, which is known as Zhemu in TCM, is sweet in taste, slightly warm in nature and acts on the liver and spleen meridians in TCM theory.42 Its medicinal usage was first documented in Ben Cao Shi Yi (700–800 A.D., Tang Dynasty), which is a famous masterpiece of TCM,42 followed by other Chinese medical classics such as Ben Cao Yan Yi (1116 A.D., Song Dynasty),43 Ben Cao Hui Yan (1624 A.D., Ming Dynasty),44 and the Dictionary of Chinese Herbal Medicine (2006 Edition).6 Notably, C. tricuspidata roots, together with the roots of C. cochinchinensis (Lour.) Kudo et Masam, have been recorded as ‘Chuan-po-shi’ in the Chinese Pharmacopoeia (1977 Edition).5 According to traditional applications and empirical practice, the bark of C. tricuspidata has mainly been used to strengthen the body and improve health conditions, and the trunk to invigorate the circulation of the blood and cure impaludism.42 An aqueous decoction of its fruits and leaves (15–30 g) could be taken orally to relieve rheumatoid arthritis. Scabies and eczema could be alleviated using a decoction of its roots mixed with the roots of C. cochinchinensis and glutinous rice.45 An aqueous decoction of its roots combined with Acanthus ilicifolius L. and Desmodium pulchellum (L.) Benth. was documented to treat hepatitis, in particular viral hepatitis.46,47 Currently, the bark of C. tricuspidata, together with Zhemu syrup (a traditional Chinese patent medicine), is widely employed in TCM clinics for the treatment of cancer of the alimentary system, in particular gastric carcinoma.8,24 Specifically, C. tricuspidata has been widely used with a long folkloric medicinal history by Chinese nationalities including Yi, Wa, Tong, Bai and Yao.In the Korean classic Donguibogam (1613 A.D., Joseon Dynasty), C. tricuspidata was recorded as treating eczema, mumps, tuberculosis, contusions, and acute arthritis.48 Its fruits are commonly consumed in the Korean daily diet owing to its diverse biological effects, e.g., antioxidant, anti-inflammatory and immunomodulatory activities.12 In addition, during the last few decades, the whole plant of C. tricuspidata has been exploited as an important folk remedy for cancer in Korea.49
3 Phytochemistry
Over recent decades, a large number of chemical constituents have been isolated from C. tricuspidata. Xanthones and flavonoids have been recognized to be the main active and structurally diverse constituents responsible for the various activities of this species, followed by organic acids, polysaccharides, phenylpropanoids, and other constituents (Table 1).| No. | Compound name(s) | Tissue(s) | Ref. |
|---|---|---|---|
| Xanthones | |||
| 1 | Cudratricusxanthone A | Whole plant | 49 |
| 2 | Cudratricusxanthone B | Roots | 17 |
| 3 | Cudratricusxanthone C | Roots | 17 |
| 4 | Cudratricusxanthone D | Roots | 17 |
| 5 | Cudratricusxanthone E | Roots | 17 |
| 6 | Cudratricusxanthone F | Roots | 17 |
| 7 | Cudratricusxanthone G | Roots | 17 |
| 8 | Cudratricusxanthone H | Roots | 17 |
| 9 | Cudratricusxanthone I | Roots | 55 |
| 10 | Cudratricusxanthone J | Roots | 50 |
| 11 | Cudratricusxanthone K | Roots | 50 |
| 12 | Cudratricusxanthone L | Roots | 50 |
| 13 | Cudratricusxanthone M | Roots | 50 |
| 14 | Cudratricusxanthone N | Roots | 143 |
| 15 | Cudratricusxanthone O | Roots | 143 |
| 16 | Cudratricusxanthone P/cudracuspixanthone A | Roots | 116 and 143 |
| 17 | Cudraxanthone A | Root bark | 144 |
| 18 | Cudraxanthone B | Root bark | 144 |
| 19 | Cudraxanthone C | Root bark | 144 |
| 20 | Cudraxanthone D | Root bark | 4 |
| 21 | Cudraxanthone E | Root bark | 146 |
| 22 | Cudraxanthone F | Root bark | 146 |
| 23 | Cudraxanthone G | Root bark | 146 |
| 24 | Cudraxanthone H | Root bark | 147 |
| 25 | Cudraxanthone I | Root bark | 147 |
| 26 | Cudraxanthone J | Root bark | 147 |
| 27 | Cudraxanthone K | Root bark | 147 |
| 28 | Cudraxanthone L | Root bark | 148 |
| 29 | Cudraxanthone M | Root bark | 148 |
| 30 | Cudraxanthone N | Root bark | 148 |
| 31 | Cudraxanthone O | Root bark | 148 |
| 32 | Cudracuspixanthone B/cudratrixanthone B | Roots | 27 and 116 |
| 33 | Cudracuspixanthone C | Roots | 116 |
| 34 | Cudracuspixanthone D | Roots | 116 |
| 35 | Cudracuspixanthone E | Roots | 88 |
| 36 | Cudracuspixanthone F | Roots | 88 |
| 37 | Cudracuspixanthone G | Roots | 88 |
| 38 | Cudratrixanthone A | Root bark | 27 |
| 39 | Cudratrixanthone C | Root bark | 27 |
| 40 | Cudratrixanthone D | Root bark | 27 |
| 41 | Cudratrixanthone E | Root bark | 27 |
| 42 | Cudratrixanthone F | Root bark | 27 |
| 43 | Cudratrixanthone G | Root bark | 27 |
| 44 | Cudratrixanthone H | Root bark | 27 |
| 45 | Cudratrixanthone I | Root bark | 27 |
| 46 | Cudratrixanthone J | Root bark | 27 |
| 47 | Cudratrixanthone K | Root bark | 27 |
| 48 | Cudratrixanthone L | Root bark | 27 |
| 49 | Cudratrixanthone M | Root bark | 27 |
| 50 | Cudratrixanthone N | Root bark | 27 |
| 51 | Cudratrixanthone O | Root bark | 27 |
| 52 | Cudratrixanthone P | Root bark | 145 |
| 53 | Cudratrixanthone Q | Root bark | 145 |
| 54 | Cudratrixanthone R | Root bark | 145 |
| 55 | Cudratrixanthone S | Root bark | 145 |
| 56 | Cudratrixanthone T | Root bark | 145 |
| 57 | Cudratrixanthone U | Root bark | 145 |
| 58 | Cudratrixanthone V | Root bark | 145 |
| 59 | Cudratrixanthone W | Root bark | 145 |
| 60 | Alvaxanthone | Roots | 88 |
| 61 | Alloathyriol | Roots | 88 |
| 62 | Dulxanthone B | Twigs | 75 |
| 63 | Gerontoxanthone A | Root bark | 25 |
| 64 | Gerontoxanthone C | Root bark | 27 |
| 65 | Gerontoxanthone I | Roots | 88 |
| 66 | Isocudraniaxanthone A | Roots | 116 |
| 67 | Isocudraniaxanthone B | Root bark | 56 |
| 68 | Isocudraxanthone K | Root bark | 25 |
| 69 | Isogentisin | Roots | 88 |
| 70 | Isoalvaxanthone | Roots | 88 |
| 71 | Laxanthone I | Root bark | 116 |
| 72 | Macluraxanthone B | Whole plant | 49 |
| 73 | Macluraxanthone C | Roots | 55 |
| 74 | Nigrolineaxanthone F | Root bark | 27 |
| 75 | Neriifolone A | Root bark | 27 |
| 76 | Toxyloxanthone B | Root bark | 145 |
| 77 | Toxyloxanthone C | Roots | 17 |
| 78 | Xanthone V1a | Roots | 17 |
| 79 | 1-Trihydroxy-3,6,7-trimethoxyxanthone | Roots | 55 |
| 80 | 1,3,5-Trihydroxy-4-prenylxanthone | Roots | 116 |
| 81 | 1,3,5-Trihydroxy-2-(3-methylbut-2-enyl)xanthone | Root bark | 27 |
| 82 | 1,3,5,6-Tetrahydroxyxanthone | Bark | 149 |
| 83 | 1,3,6,7-Tetrahydroxy-2-(3-methylbut-2-enyl)-8-(2-methylbut-3-en-2-yl)-9H-xanthen-9-one | Roots | 52 |
| 84 | 1,3,7-Trihydroxy-4-(1,1-dimethyl-2-propenyl)-5,6-(2,2-dimethylchromeno)xanthone | Roots | 52 |
| 85 | 1,5-Dihydroxy-3,6-dimethoxyxanthen-9-one | Twigs | 75 |
| 86 | 1,7-Dihydroxy-3,6-dimethoxyxanthone | Roots | 55 |
| 87 | 1,6,7-Trihydroxy-2-(1,1-dimethyl-2-propenyl)-3-methoxyxanthone | Roots | 119 |
| 88 | 1,6,7-Trihydroxy-3-methyl-4-(1,1,3-trimethyl-2-buten-1-yl)-9H-xanthen-9-one | Root bark | 23 |
| 89 | 2-Deprenylrheediaxanthone B | Root bark | 116 |
| 90 | 2,6-Dihydroxyxanthone | Roots | 116 |
| 91 | 3-O-Methylcudratrixanthone G | Root bark | 27 |
| 92 | 5-O-Methylformoxanthone C | Root bark | 27 |
| 93 | 6-Deoxyisojacareubin | Root bark | 27 |
| 94 | 6-Deoxy-γ-mangostin | Root bark | 27 |
| 95 | 7-O-Demethylcudratrixanthone C | Root bark | 145 |
| 96 | 8-Prenylxanthone | Roots | 88 |
| 97 | 16-Hydroxycudratrixanthone Q | Root bark | 145 |
| 98 | 16-Hydroxycudratrixanthone M | Root bark | 145 |
| 99 | 16-Methoxycudratrixanthone M | Root bark | 145 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Flavonoids | |||
| Flavones | |||
| 100 | Artocarpesin | Roots and stems | 17 |
| 101 | Apigenin | Fruits | 168 |
| 102 | Apigenin-7-O-β-D-glucopyranoside | Fruits | 168 |
| 103 | Cudraflavone A | Root bark | 150 |
| 104 | Cudraflavone B | Root bark | 150 |
| 105 | Cudraflavone C | Root bark | 151 |
| 106 | Cudraflavone D | Root bark | 151 |
| 107 | Cudraflavone F | Roots | 58 |
| 108 | Cudraflavone G | Roots | 58 |
| 109 | Cudraflavone H | Root bark | 145 |
| 110 | Cycloartocarpesin B | Roots | 17 |
| 111 | Cyclomorusin | Twigs | 75 |
| 112 | Cycloartocarpin | Whole plant | 152 |
| 113 | Hirsutrin/quercetin-3-O-β-D-glucopyranoside | Fruits | 66 |
| 114 | Kuwanon C | Roots | 125 |
| 115 | Licoflavone C | Leaves | 153 |
| 116 | 5,7,2′,4′-Tetrahydroxyflavone/norarthocarpetin | Stems | 154 |
| 117 | 6-Prenylapigenin | Roots | 58 |
| 118 | Kaempferol | Root bark | 134 |
| 119 | Kaempferol-3-O-β-D-glucopyranoside/astragalin | Fruits | 168 |
| 120 | Kaempferol-7-O-β-D-glucopyranoside/populnin | Whole plant | 155 |
| 121 | 6-p-Hydroxybenzyl kaempferol-7-O-β-D-glucopyranoside | Root bark | 156 |
| 122 | Morin | Root bark | 124 |
| 123 | Myricetin | Roots | 117 |
| 124 | Nicotiflorine | Fruits | 168 |
| 125 | Quercetin | Twigs, root bark and stems | 75, 106 and 157 |
| 126 | Quercetin-7-O-β-D-glucopyranoside/quercimeritrin | Root bark | 158 |
| 127 | 6-p-Hydroxybenzyl quercetin-7-O-β-D-glucopyranoside | Root bark | 156 |
| 128 | Rutin | Fruits | 66 |
| Flavanones | |||
| 129 | Cudraflavanone A | Root bark | 158 |
| 130 | Cudraflavanone B | Roots | 125 |
| 131 | Cudraflavanone C | Roots | 55 |
| 132 | Cudraflavanone D | Roots | 55 |
| 133 | Cudraflavanone E | Roots | 58 |
| 134 | Cudraflavanone F | Roots | 58 |
| 135 | Cudraflavanone G | Root bark | 145 |
| 136 | (2R)-Cudraflavanone H | Root bark | 145 |
| 137 | (2S)-Cudraflavanone H | Root bark | 145 |
| 138 | Cycloaltilisin 7 | Twigs | 75 |
| 139 | Cudracuspiflavanone A | Root bark | 124 |
| 140 | Carthamidin | Leaves | 153 |
| 141 | Dalenin | Root bark | 145 |
| 142 | Dicycloeuchrestaflavanone B | Root bark | 145 |
| 143 | Euchrestaflavanone B | Root bark | 157 |
| 144 | Euchrestaflavanone C | Root bark | 157 |
| 145 | Eriodictyol | Stem bark | 30 |
| 146 | Naringenin | Twigs and root bark | 75 and 157 |
| 147 | Pinocembrin | Root bark | 145 |
| 148 | Prunin | Fruits | 168 |
| 149 | Steppogenin | Twigs, roots and stems | 75, 159 and 160 |
| 150 | Tomentosanol D | Root barks | 145 |
| 151 | (2S)-2′,5,7-Trihydroxy-6-(3-hydroxy-3-methylbutyl)-6′′,6′′-dimethylpyrano[2′′,3′′:4′,5′]flavanone | Roots | 58 |
| 152 | 2′,5,7-Trihydroxy-4′,5′-(2,2-dimethylchromeno)-8-(3-hydroxy-3-methylbutyl)flavanone | Root bark | 157 |
| 153 | 4′-Hydroxyisolonchocarpin | Root bark | 145 |
| 154 | 5-Dehydroxybavachinone A | Root bark | 145 |
| 155 | 5,7,3′,5′-Tetrahydroxyflavanone | Root bark | 124 |
| 156 | 6-Prenylnaringenin | Roots | 161 |
| 157 | 8-Prenylnaringenin | Root bark | 124 |
| 158 | Aromadendrin/dihydrokaempferol | Root bark and twigs | 75 and 156 |
| 159 | Dihydrokaempferol-7-O-β-D-glucoside | Twigs | 75 |
| 160 | trans-Dihydromorin | Twigs and whole plant | 133 and 152 |
| 161 | Gericudranin A | Stem bark | 16 |
| 162 | Gericudranin B | Stem bark | 16 |
| 163 | Gericudranin C | Stem bark | 16 |
| 164 | Gericudranin D | Stem bark | 58 |
| 165 | Gericudranin E | Stem bark | 58 |
| 166 | Taxifolin/dihydroquercetin | Twigs and stems | 75 and 160 |
| 167 | Taxifolin-7-methyl ether | Twigs | 75 |
| 168 | Taxifolin-7-O-β-D-glucopyranoside | Twigs | 75 |
| 169 | Tricusposide | Bark | 149 |
| 170 | (2S,3S)-2,3-trans-Dihydromorin-7-O-β-D-glucoside | Twigs | 75 |
| 171 | (2R,3R)-2,3-Dihydro-3,5,6,7-tetrahydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one | Root bark | 124 |
| 172 | 3,5,7,2′,4′-Pentahydroxydihydroflavonol | Whole plant | 152 |
| 173 | 5,7,4′-Trihydroxy-8-p-hydroxybenzyldihydroflavonol | Root bark | 124 |
| Isoflavones | |||
| 174 | Cudraisoflavone B | Fruits | 28 |
| 175 | Cudraisoflavone C | Fruits | 28 |
| 176 | Cudraisoflavone D | Fruits | 28 |
| 177 | Cudraisoflavone E | Fruits | 28 |
| 178 | Cudraisoflavone F | Fruits | 28 |
| 179 | Cudraisoflavone G | Fruits | 28 |
| 180 | Cudraisoflavone H | Fruits | 28 |
| 181 | Cudraisoflavone I | Fruits | 28 |
| 182 | Cudraisoflavone J | Fruits | 28 |
| 183 | Cudraisoflavone K | Fruits | 28 |
| 184 | Cudraisoflavone L (1) | Leaves | 87 |
| 185 | Cudraisoflavone L (2) | Fruits | 168 |
| 186 | Cudraisoflavone M | Fruits | 168 |
| 187 | Cudraisoflavone N | Fruits | 168 |
| 188 | Cudraisoflavone O | Fruits | 168 |
| 189 | Cudraisoflavone P | Fruits | 168 |
| 190 | Cudraisoflavone Q | Fruits | 168 |
| 191 | Cudraisoflavone R | Fruits | 168 |
| 192 | Cudraisoflavone S | Fruits | 168 |
| 193 | Cudraisoflavone T | Fruits | 168 |
| 194 | Cudracusisoflavone A | Fruits | 66 |
| 195 | Cudracusisoflavone B | Fruits | 66 |
| 196 | Auriculasin | Fruits | 28 |
| 197 | Anagyroidisoflavone A | Fruits | 28 |
| 198 | Alpinumisoflavone | Fruits | 32 |
| 199 | Biochanin A | Root bark | 124 |
| 200 | Erysenegalensein E | Fruits | 31 |
| 201 | Isoerysenegalensein E | Fruits | 31 |
| 202 | Erythrinin B/wighteone/6-isopentenylgenistein | Twigs and fruits | 28 and 102 |
| 203 | Erythrinin C | Fruits | 28 |
| 204 | Eryvarin B | Fruits | 28 |
| 205 | Erysubin A | Leaves | 87 |
| 206 | Euchrenone b8 | Fruits | 28 |
| 207 | Euchrenone b9 | Fruits | 28 |
| 208 | Euchrenone b10 | Fruits | 28 |
| 209 | Erythrivarone A | Leaves | 153 |
| 210 | Derrone | Fruits | 28 |
| 211 | Derrone-4′-O-methyl ether | Fruits | 66 |
| 212 | Flaniostatin | Leaves | 162 |
| 213 | Flemiphilippinin B | Whole plants | 163 |
| 214 | Flemiphilippinin G | Fruits | 28 |
| 215 | Furowanin B | Leaves | 86 |
| 216 | Gancaonin A | Fruits | 116 |
| 217 | Gancaonin B | Fruits | 28 |
| 218 | Genistein | Twigs and bark | 75 and 149 |
| 219 | Genistein-4′-O-β-glucopyranoside/sophorobioside | Fruits | 168 |
| 220 | Genistin | Bark | 149 |
| 221 | Glycyrrhisoflavone | Twigs | 75 |
| 222 | Isolupalbigenin | Leaves | 86 |
| 223 | Isochandalone | Fruits | 66 |
| 224 | Lupiwighteone | Fruits | 28 |
| 225 | Lupalbigenin | Leaves | 87 |
| 226 | Laburnetin | Root bark | 145 |
| 227 | Millewanin H | Leaves | 87 |
| 228 | Millewanin G | Leaves | 153 |
| 229 | Osajin | Fruits | 32 |
| 230 | Orobol | Fruits | 31 |
| 231 | Oroboside | Fruits | 66 |
| 232 | Orobol-8-C-glucoside | Twigs | 75 |
| 233 | Pomiferin | Fruits | 32 |
| 234 | Parvisoflavone A | Root bark | 145 |
| 235 | Senegalensin | Fruits | 31 |
| 236 | Santal | Twigs | 75 |
| 237 | Sphaerobioside | Twigs | 75 |
| 238 | Ulexin B | Fruits | 66 |
| 239 | Ulexone B | Fruits | 66 |
| 240 | Warangalon | Fruits | 28 |
| 241 | 3′-O-Methylorobol | Root bark | 124 |
| 242 | 4′-O-Methylalpinumisoflavone | Fruits and stem bark | 105 and 116 |
| 243 | 4′-O-Methylcudraisoflavone O | Fruits | 168 |
| 244 | 4′-O-Methylcudraisoflavone P | Fruits | 168 |
| 245 | 4′-O-Methylerythrinin C | Fruits | 168 |
| 246 | 4′,7-Dihydroxy-5-methoxyisoflavone/5-O-methylgenistein | Stems | 154 |
| 247 | 5,3′-Dihydroxy-4′-methoxy-2′′,2′′-dimethylpyrano[5′′,6′′;6,7]isoflavone | Fruits | 31 |
| 248 | 5,3′,4′-Trihydroxy-6′′,6′′-dimethylpyrano[2′′,3′′;7,6]isoflavone | Fruits | 66 |
| 249 | 5,4′-Dihydroxy-8-(3′′-methylbut-2′′-enyl)-2′′′-(4′′′-hydroxy-4′′′-methylethyl)furano[4′′′,5′′′;6,7]isoflavone | Fruits | 31 |
| 250 | 5,4′-Dihydroxy-6-(3′′-methylbut-2′′-enyl)-2′′′-(4′′′-hydroxy-4′′′-methylethyl)-3′′′-methoxydihydrofurano[4′′′,5′′′;7,8]isoflavone | Fruits | 31 |
| 251 | 5,7-Dihydroxy-6-(2′′-hydroxy-3′′-methylbut-3′′-enyl)-4′-methoxyisoflavone | Fruits | 31 |
| 252 | 5,7,4′-Trihydroxy-6,8-diprenylisoflavone/6,8-diprenylgenistein/8-(γ,γ-dimethylallyl)wighteone | Fruits | 32 and 75 |
| 253 | 5,7,4′-Trihydroxydihydroisoflavone | Whole plant | 152 |
| 254 | 6,8-Diprenylorobol/5,7,3′,4′-tetrahydroxy-6,8-diprenylisoflavone | Twigs and fruits | 28 and 75 |
| 255 | 6-Prenylorobol | Leaves | 153 |
| 256 | 7,4′-Dimethoxy-5-hydroxyisoflavone | Fruits | 66 |
| 257 | 8-Hydroxygenistein | Root bark | 145 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Organic acids | |||
| 258 | Butyl citrate | Trunk | 15 |
| 259 | Benzoic acid | Fruits | 76 |
| 260 | Boric acid | Fruits | 76 |
| 261 | Citric acid | Fruits | 70 |
| 262 | Mandelic acid | Fruits | 76 |
| 263 | Methyl linoleate | Trunk | 15 |
| 264 | Malic acid | Fruits | 70 |
| 265 | Oxalic acid | Fruits | 70 |
| 266 | Palmitic acid | Trunk | 15 |
| 267 | Palmitic acid methyl ester | Trunk | 15 |
| 268 | Palmitic acid β-monoglyceride | Trunk | 15 |
| 269 | Protocatechuic acid | Twigs | 75 |
| 270 | Succinic acid | Fruits | 70 |
| 271 | Stearic acid | Trunk | 15 |
| 272 | Syringic acid | Trunk | 15 |
| 273 | Tartaric acid | Fruits | 70 |
| 274 | threo-9,10-O-Isopropylidene-13-hydroxy-(11E)-octadecenoic acid | Roots | 164 |
| 275 | n-Decanoic acid | Roots and stems | 10 |
| 276 | n-Nonanoic acid | Roots | 10 |
| 277 | n-Pentanoic acid | Roots and stems | 10 |
| 278 | n-Hexanoic acid | Roots and stems | 10 |
| 279 | n-Heptanoic acid | Roots | 10 |
| 280 | n-Octanoic acid | Roots | 10 |
| 281 | (E)-2-Decenoic acid | Roots | 10 |
| 282 | (E)-2-Octenoic acid | Roots and stems | 10 |
| 283 | 2′,3′-Dihydroxypropyl pentadecanoate | Roots | 165 |
| 284 | 4-Hydroxybenzoic acid | Fruits | 168 |
| 285 | 9,12-Octadecadienoic acid | Trunk | 15 |
| 286 | 9,12,15-Octadecatrienoic acid methyl ester | Trunk | 15 |
| 287 | 9,12,15-Octadecatrien-1-ol | Trunk | 15 |
| 288 | 9,17-Octadecadienal | Trunk | 15 |
| 289 | γ-Hexadecalactone | Root bark | 145 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Polysaccharides | |||
| 290 | CTP-B1 | Roots | 73 |
| 291 | CTPS-01 | Roots | 72 |
| 292 | CPS-0 | Roots | 71 |
| 293 | CTPS-1A | Roots | 71 |
| 294 | CTPS-2B | Roots | 71 |
| 295 | CTPS-3A | Roots | 71 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Phenylpropanoids | |||
| 296 | Bergapten | Root bark | 145 |
| 297 | Cudrastilbene | Roots | 159 |
| 298 | cis-3′,4′-Diisovalerylkhellactone | Root bark | 145 |
| 299 | Demethylsuberosin | Whole plant | 163 |
| 300 | Decursinol angelate | Root bark | 145 |
| 301 | Gomisin A | Roots | 166 |
| 302 | Gomisin H | Roots | 166 |
| 303 | Hyuganin C | Root bark | 145 |
| 304 | Imperatorin | Whole plant | 163 |
| 305 | Isoimperatorin | Whole plant | 163 |
| 306 | Oxyresveratrol | Twigs | 75 |
| 307 | Scopoletin | Trunk | 15 |
| 308 | Schizandrin | Whole plant | 166 |
| 309 | Syringaresinol | Whole plant | 166 |
| 310 | Umbelliferone | Root bark | 151 |
| 311 | Xanthyletin | Root bark | 145 |
| 312 | 7-Hydroxy-2H-1-benzopyran-2-one | Trunk | 15 |
| 313 | 5-Methoxy-4,5-diphenyl-2(5H)-furanone | Twigs | 75 |
| 314 | 3-Methyl-2(5H)-furanone | Roots | 10 |
| 315 | 5-Ethyl-2(5H)-furanone | Roots | 10 |
| 316 | 5,5-Dimethyl-2(5H)-furanone | Roots | 10 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Other ingredients | |||
| 317 | Betulin | Roots | 165 |
| 318 | Butyrospermol | Fruits | 168 |
| 319 | Camphene | Roots | 10 |
| 320 | Drimenol | Roots | 10 |
| 321 | Dihydroctinidiolide | Stems | 10 |
| 322 | Glutinol | Root bark | 145 |
| 323 | Lupeol | Roots | 165 |
| 324 | Lanosta-8-24-dien-3β-ol-acetate | Trunk | 15 |
| 325 | Lanosta-8-en-3-one | Trunk | 15 |
| 326 | Lanosta-7,24-diene-3β-ol | Whole plant | 152 |
| 327 | Lanosta-7,24-diene-3β-O-acetate | Whole plant | 152 |
| 328 | Olean-12-ene | Trunk | 15 |
| 329 | Taraxerone | Stems | 160 |
| 330 | Terpin hydrate | Roots | 10 |
| 331 | Ursolic acid | Roots | 165 |
| 332 | (E)-Geranylacetone | Roots | 10 |
| 333 | (E)-β-Ionone | Roots and stems | 10 |
| 334 | (E)-Linalool oxide | Roots | 10 |
| 335 | (Z)-Linalool oxide | Roots | 10 |
| 336 | (E)-α-Terpineol | Roots and stems | 10 |
| 337 | α-Amyrin | Root bark | 145 |
| 338 | Campesterol | Trunk | 15 |
| 339 | Daucosterol | Roots | 165 |
| 340 | Itesmol | Roots | 165 |
| 341 | β-Sitosterol | Roots | 165 |
| 342 | γ-Sitosterol | Trunk | 15 |
| 343 | Achilleol A | Whole plant | 163 |
| 344 | Antiarol | Fruits | 76 |
| 345 | Anisaldehyde | Roots | 10 |
| 346 | Aristolone | Trunk | 15 |
| 347 | Adacene 12 | Fruits | 76 |
| 348 | Brosimine B | Root bark | 145 |
| 349 | Benzophenone | Stems | 10 |
| 350 | Benzylhydrazine | Fruits | 76 |
| 351 | Bis(2-azabicyclo[2.2.1]hept-5-en-2-yl)diazene | Fruits | 76 |
| 352 | Butylated hydroxytoluene | Fruits | 76 |
| 353 | Cudracuspiphenone A | Roots | 116 |
| 354 | Cudracuspiphenone B | Roots | 116 |
| 355 | Cudrachromone A | Root bark | 145 |
| 356 | Cudraphenol A | Root bark | 145 |
| 357 | Cudraphenol B | Root bark | 145 |
| 358 | Cudraphenol C | Root bark | 145 |
| 359 | Cudraphenone E | Root bark | 145 |
| 360 | Cudradihydrochalcone A | Fruits | 168 |
| 361 | Cudrabibenzyl A | Fruits | 168 |
| 362 | (E)-Cinnamic aldehyde | Roots and stems | 10 |
| 363 | Dopamine | Fruits | 76 |
| 364 | Demeton-O-methyl | Fruits | 76 |
| 365 | Diethyl phthalate | Fruits | 76 |
| 366 | Ethyl-N-methylcarbamate | Fruits | 76 |
| 367 | Eriosematin A | Root bark | 145 |
| 368 | Ethyl p-tert-butylbenzoic acid | Fruits | 76 |
| 369 | Eugenol | Roots and stems | 10 |
| 370 | Isoeugenol | Roots | 10 |
| 371 | Isoencecalin | Root bark | 145 |
| 372 | Indene | Fruits | 76 |
| 373 | Lavender lactone | Roots | 10 |
| 374 | Lycopene | Fruits | 77 |
| 375 | Lutein | Fruits | 77 |
| 376 | Palustrol | Fruits | 76 |
| 377 | Peonoside | Bark | 149 |
| 378 | Phenol | Fruits, roots and stems | 10 and 76 |
| 379 | Phytofluene | Fruits | 77 |
| 380 | Phenylethyl alcohol | Roots and stems | 10 |
| 381 | Pyridine | Roots | 10 |
| 382 | Pyrrole-2-carboxaldehyde | Stems | 10 |
| 383 | Ruboxanthin | Fruits | 77 |
| 384 | Salicylamide | Fruits | 76 |
| 385 | Scyllitol | Fruits | 76 |
| 386 | Sucrose | Bark | 149 |
| 387 | Stachydrine | Roots | 167 |
| 388 | Tridecanol | Fruits | 76 |
| 389 | Undecane | Fruits | 76 |
| 390 | Vanillin | Root bark | 145 |
| 391 | Zeaxanthin | Fruits | 77 |
| 392 | p-Vinylguaiacol | Roots and stems | 10 |
| 393 | n-Hexanal | Roots | 10 |
| 394 | n-Hexanol | Roots | 10 |
| 395 | n-Butanol | Roots | 10 |
| 396 | n-Nonanal | Roots and stems | 10 |
| 397 | N-Acetylnorephedrine | Fruits | 76 |
| 398 | 1-Phenyl-1-cyclohexylethane | Fruits | 76 |
| 399 | 1-Methyl-2-pyrrolidone | Roots and stems | 10 |
| 400 | 1-[(2,4,6-Trimethylphenyl)methyl]imidazole | Fruits | 76 |
| 401 | (4S)-1,1-Difluoro-4 vinylspiropentane | Fruits | 76 |
| 402 | 2-Deuteriophenylalanine | Fruits | 76 |
| 403 | 2-Furanmethanol | Trunk | 15 |
| 404 | 2-Ethyl-1-hexanol | Roots and stems | 10 |
| 405 | 2-Acetylpyrrole | Stems | 10 |
| 406 | 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | Trunk | 15 |
| 407 | 2,4-Bis(4-hydroxybenzyl)phenol | Root bark and stems | 10 and 145 |
| 408 | 2,5-Furandione | Trunk | 15 |
| 409 | 3-Methoxycarbonylindole | Root bark | 145 |
| 410 | 3-Methyl-2,5-furandione | Trunk | 15 |
| 411 | 4-Acetylpyrazole | Roots | 10 |
| 412 | 4-Ethylguaiacol | Roots and stems | 10 |
| 413 | 4-Hydroxybenzalacetone | Root bark | 145 |
| 414 | 4-Hydroxymethylbenzoate | Root bark | 145 |
| 415 | 4-(Methoxymethyl)phenol | Whole plant | 163 |
| 416 | 4-Methyltridecane | Fruits | 76 |
| 417 | 4-Methylguaiacol | Roots | 10 |
| 418 | 4-Hydroxybenzaldehyde/p-hydroxybenzaldehyde | Trunk | 15 |
| 429 | 4-Valerolactone | Roots and stems | 10 |
| 420 | 4,4-Diphenyl-5-methyl-2-cyclohexenone | Fruits | 76 |
| 421 | p-Hydroxybenzyl alcohol | Trunk | 15 |
| 422 | 5-(Hydroxymethyl)-2-furancarboxaldehyde | Trunk | 15 |
| 423 | 5-Hydroxy-2,2-dimethyl-2H,6H-benzodipyran-6-one | Root bark | 124 |
| 424 | 5-Methyl-1H-pyrrole-2-carboxaldehyde | Stems | 10 |
| 425 | 5,7-Dihydroxychromone | Root bark | 124 |
| 426 | 6-Pentyl-5,6-dihydro-2H-pyran-2-one | Roots and stems | 10 |
| 427 | 8-Chloro-6-(2-fluorophenyl)imidazole[1,2-a][1,4]benzodiazepine | Fruits | 76 |
| 428 | α-Carotene | Fruits | 77 |
| 429 | β-Carotene | Fruits | 77 |
| 430 | Neo-β-carotene | Fruits | 77 |
| 431 | γ-Amylbutyrolactone | Roots and stems | 10 |
| 432 | γ-Butylbutyrolactone | Roots and stems | 10 |
| 433 | γ-Butyrolactone | Roots | 10 |
| 434 | γ-Caprolactone | Roots | 10 |
| 435 | γ-Crotonolactone | Roots | 10 |
| 436 | γ-Dodecalactone | Roots and stems | 10 |
| 437 | γ-Palmitolactone | Roots | 10 |
| 438 | Arginine | Roots | 167 |
| 439 | Alanine | Roots | 167 |
| 440 | Aspartate | Roots | 167 |
| 441 | Glutamic acid | Roots | 167 |
| 442 | Proline | Roots | 167 |
| 443 | Polycopene | Fruits | 77 |
3.1. Xanthones (1–99)
The presence of abundant xanthones substituted by a variety of isoprenoid, phenolic and methoxy groups has been considered to be a taxonomic feature of C. tricuspidata (Fig. 2).17,49,50 Among these, from the perspective of the structure–activity relationship (SAR), xanthones substituted by isoprenoid groups display better biological activities. For instance, cudratricusxanthone A (CTXA, 1), cudraxanthones L (28) and M (29), and macluraxanthone B (72) are notable isoprenylated xanthones with anti-inflammatory,22 antitumor,17 neuroprotective,51 hepatoprotective,26 monoamine oxidase (MAO)-inhibiting,50 anticoagulant,29 antidiabetic,52 and neuraminidase-inhibiting effects.53 Cudratricusxanthones B–E (2–5) and G (7), cudraxanthones D (20), L and M, and macluraxanthones B and C (73), which are classed as catecholic xanthones, could be converted into quinone methide intermediates in an enzymatic or a non-enzymatic manner54 and were reported to have significant antitumor activity.17,49,55,56 In addition, catecholic xanthones, specifically cudraxanthone C (19) and 1,3,7-trihydroxy-4-(1,1-dimethyl-2-propenyl)-5,6-(2,2-dimethylchromeno)xanthone (84), exhibited both potent superoxide- and hydroxyl radical-scavenging activities, which could be rationalized by their chelating effect with Fe2+ ions.56It should be noted that investigations into quantitative analysis of the characteristic xanthones in C. tricuspidata are scarce. On the basis of HPLC analysis, cudratricusxanthones B, D and F (6) and macluraxanthone B in C. tricuspidata root bark were found to account for 0.017%, 0.026%, 0.025% and 0.071%, respectively.57 The quantitative analysis of other xanthones in C. tricuspidata is worth investigating in the future.
3.2. Flavonoids (100–257)
Flavonoids account for the largest proportion of C. tricuspidata and have attracted particular interest because of their well-defined pharmacological activities. To date, more than 120 flavonoids (Fig. 2) have been isolated from C. tricuspidata and can be classified into flavones (100–128), flavanones (129–173), and isoflavones (174–257). Structurally, the majority of them possess prenylated, benzylated, and methoxy groups substituted on their aromatic rings. Cudraflavanone E (133), which is isolated from C. tricuspidata roots, features a rare flavanone skeleton with the B-ring fused to a furan ring.58 Lee et al.16,59 isolated a series of rare benzyl-substituted flavonoids, i.e., gericudranins A–E (161–165), from C. tricuspidata. Notably, prenylated flavonoids have been regarded as attractive specialized metabolites with diverse biological activities. Specifically, cudraflavone B (104), which is a prominent prenylated flavonoid from the roots of C. tricuspidata, exhibited MAO-inhibiting,50 antiatherosclerotic,9 anti-inflammatory,60 hepatoprotective,25 antitumor,61 and neuroprotective62 effects. Euchrestaflavanones B (143) and C (144) displayed antibacterial activity against Gram-positive bacteria, Staphylococcus aureus, Bacillus subtilis and Bacillus cereus.63The investigation of the biosynthesis of the prenylflavonoids in C. tricuspidata has been attempted. Dai et al.64 established a cell suspension culture of C. tricuspidata for the enzymatic preparation of prenylflavonoids. A flavonoid prenyltransferase was identified as C. tricuspidata isoliquiritigenin 3′-dimethylallyltransferase.64 This enzyme was found to be able to regioselectively introduce dimethylallyl diphosphate at the ortho-position of the phenolic moiety in the common 2,4-dihydroxyacetophenone substructure shared by the three types of flavonoids, i.e., chalcones, isoflavones, and flavones.64,65 These studies could improve our knowledge of the mechanism of the biosynthesis and accumulation of prenylated flavonoids in C. tricuspidata.
The constituents and quantities of the flavonoids in C. tricuspidata fruits have been demonstrated to change in accordance with their maturation stage. Unripe fruits of C. tricuspidata were found to have a higher content of total flavonoids in comparison with ripe fruits. An analysis of the chemical constituents revealed that flavonoids with a side chain of cyclized prenyl 2,2-dimethylpyran rings were predominant in the unripe fruits, whereas flavonoids with a linear prenyl side chain were the main constituents in ripe fruits.66
Only a few studies have been reported concerning the quantitative analysis of representative flavonoids in C. tricuspidata. By UV-vis spectrophotometry, the concentration of total flavonoid glycosides in C. tricuspidata roots was measured to be up to 3.96 mg g−1 (as rutin equivalents).67 By HPLC analysis, the flavonoids kaempferol (118), quercetin (125), naringenin (146) and taxifolin (166) in C. tricuspidata were found to occur at 0.30, 0.09, 1.94 and 0.63 mg g−1 in the roots and 0.08, 0.04, 0.90 and 0.62 mg g−1 in the stems, respectively.68 Jeon et al.69 reported the isolation of prenylated isoflavonoids from an n-hexane extract of C. tricuspidata fruits using centrifugal partition chromatography and found that the main flavonoids 4′-O-methylalpinumisoflavone (242), 6,8-diprenylgenistein (252) and 6,8-diprenylorobol (254) amounted to 2.7%, 7.6% and 6.4%, respectively.
3.3. Organic acids (258–289)
To date, thirty-one organic acids and their esters have been isolated from C. tricuspidata. The stem extract was reported to contain n-hexanoic acid (278) in the greatest concentration (9.89 μg g−1) followed by 2-acetylpyrrole (405, 1.86 μg g−1), whereas the root extract was found to have n-hexanoic acid (13.13 μg g−1) in the greatest concentration followed by n-heptanoic acid (279, 2.05 μg g−1).10 Jung et al.70 reported that the levels of organic acids such as citric acid (261), malic acid (264), oxalic acid (265), succinic acid (270) and tartaric acid (273) in C. tricuspidata fruits varied with the maturation stage, and malic acid was the most abundant.3.4. Polysaccharides (290–295)
The yield of total polysaccharides (CTPS) amounted to 1.0% in the roots of C. tricuspidata.71 Six polysaccharides with strong immunomodulatory activities, namely, CTP-B1 (290), CTPS-01 (291), CPS-0 (292), CTPS-1A (293), CTPS-2B (294) and CTPS-3A (295), were obtained from the roots of C. tricuspidata.72–74 Their backbones were revealed to be commonly substituted with α-D-glucuronic acid, 4-O-methyl-α-D-glucuronic acid, and neutral sugar units such as α-L-arabinose, α-D-xylose and α-D-galactose.743.5. Phenylpropanoids (296–316)
Twenty-one phenylpropanoids have been reported in C. tricuspidata, such as oxyresveratrol (306),75 scopoletin (307),15 3-methyl-2(5H)-furanone (314),10 and 5-ethyl-2(5H)-furanone (315).10 Oxyresveratrol, as a representative phenylpropanoid, was isolated from the twigs of C. tricuspidata, exhibited potent inhibitory activity against mushroom tyrosinase and might serve as an anti-browning agent for food.753.6. Others (317–443)
In addition to the aforementioned components, a large number of other components have also been identified in C. tricuspidata. Twenty-nine compounds were identified in the essential oil of C. tricuspidata fruits, which accounted for 94.46% of the essential oil, such as demeton-O-methyl (364), diethyl phthalate (365), ethyl-N-methylcarbamate (366), indene (372), scyllitol (385), tridecanol (388) and 1-phenyl-1-cyclohexylethane (398).76 It should be mentioned that a series of carotenoids were identified in C. tricuspidata fruits, including lycopene (374), lutein (375), phytofluene (379), ruboxanthin (383), zeaxanthin (391), α-carotene (428), β-carotene (429), neo-β-carotene (430) and polycopene (443).774 Pharmacological properties
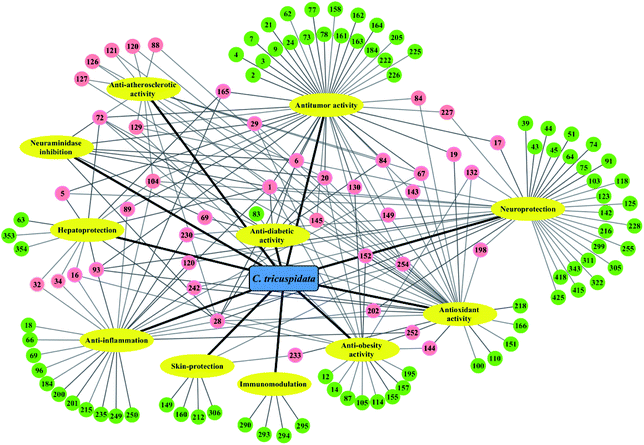
Accumulated studies have revealed that the extracts and components of C. tricuspidata exhibited a broad spectrum of pharmacological activities, including anti-inflammatory,22,23 antioxidant,78,79 antitumor,16,24 hepatoprotective,25,26 neuroprotective,27,28 antiobesity,18,19 immunomodulatory,70,71 antiatherosclerotic,80,81 antimicrobial,11,76 skin-protecting,79,82 and antidiabetic21,52 effects. The presence of a variety of bioactive compounds may be synergistically or individually responsible for the various activities of this species (Fig. 3). Among these, xanthones and flavonoids are representative ingredients that mainly possess anti-inflammatory, antioxidant and antitumor activities.4.1. Anti-inflammatory activity
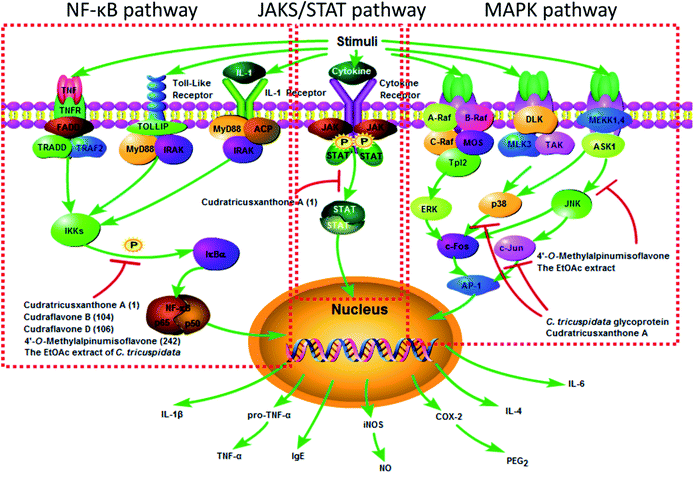
There has been strong evidence that diseases associated with inflammation may be ameliorated by C. tricuspidata. The anti-inflammatory molecular mechanisms could be elucidated on the basis of the effects of the extracts and compounds from C. tricuspidata (Fig. 4). It has been reported that a methanolic extract of C. tricuspidata could decrease the production of the pro-inflammatory cytokines interleukin-2 (IL-2) and interferon-γ (IFN-γ) by selectively inhibiting the proliferation of anti-CD3/CD28-mediated CD4+CD25− T-cells.15 The chloroform (CHCl3) fraction of C. tricuspidata was observed to inhibit the overproduction of nitric oxide (NO) and prostaglandin E2 (PGE2) by decreasing the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) and reducing the levels of tumor necrosis factor-α (TNF-α), IL-1β and IL-6 in RAW 264.7 mouse macrophage cells stimulated with lipopolysaccharide (LPS).83 The ethyl acetate (EtOAc) fraction of the stem bark could suppress the production of NO and expression of iNOS in RAW 264.7 cells stimulated with IFN-γ/LPS via the inactivation of nuclear factor-κB (NF-κB).84 The EtOAc fraction of C. tricuspidata stem bark could inhibit the differentiation of osteoclasts stimulated by IL-1β and mediated by receptor activator of NF-κB ligand, the phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 and p38 mitogen-activated protein kinase (MAPK), and the expression of c-Fos and nuclear factor of activated T-cells c1 (NFATc1).85 The EtOAc fraction of the whole plant was also found to reduce the expression of IL-1β, matrix metalloproteinases (MMPs), COX-2 and PGE2 by inhibiting the phosphorylation of MAPK and the activation of NF-κB signalling pathways in rheumatoid synovial fibroblasts.86 The above research suggested that C. tricuspidata may be useful for managing bone destruction in inflammatory diseases, such as rheumatoid arthritis (RA).Numerous compounds from C. tricuspidata possess noticeable anti-inflammatory properties. Prenylated isoflavones from the leaves of C. tricuspidata, including cudraisoflavone L (184), wighteone (202) and furowanin B (215) exhibited potential anti-inflammatory activity by inhibiting the production of NO in LPS-stimulated RAW 264.7 cells, with inhibition values of 72.5 ± 2.4%, 66.9 ± 1.8%, and 55.4 ± 2.7% at a concentration of 10 μM, respectively.87 It was found that the position of hydroxyl groups in the xanthone moiety was important for the NO-inhibiting activity, and the catechol moiety was partially responsible for the inhibitory activity (Table 2).88 A C. tricuspidata glycoprotein suppressed the expression of iNOS and COX-2 via the regulation of NF-κB in LPS-stimulated RAW 264.7 cells.34 The xanthone CTXA, as an effective inducer of heme oxygenase-1 (HO-1), significantly inhibited the production of PGE2, NO, TNF-α, and IL-1β and increased the activity of HO in LPS-stimulated RAW 264.7 macrophages.22 Moreover, CTXA could exert anti-soluble endothelial cell protein C receptor (anti-sEPCR) shedding activity against vascular inflammation via inhibiting the expression of TNF-α-converting enzyme induced by phorbol-12-myristate-13-acetate in endothelial cells.89 Cudraflavone B was not only a potent inhibitor of TNF-α by blocking the translocation of NF-κB from the cytoplasm to the nucleus in macrophages derived from a THP-1 human monocytic leukemia cell line, but was also an inhibitor of COX-1 and COX-2 with higher selectivity toward COX-2, which suggested that it could be used as a lead for the development of non-steroidal anti-inflammatory drugs.60
Allergic inflammation affects roughly one-quarter of people in the world.90 5,7,3′,4′-Tetrahydroxy-6,8-diprenylisoflavone (254) not only interfered with the interaction between IgE and high-affinity IgE receptor (FcεRI) and the expression of FcεRIβ mRNA but also inhibited the redistribution of F-actin and downstream signalling by suppressing the activation of FcεRI-mediated spleen tyrosine kinase in mast cells, which was suggestive of therapeutic potential for controlling mast cell activation in allergic processes.91 Treatment with the C. tricuspidata glycoprotein resulted in degranulation for allergic response (β-hexosaminidase) and the activation of MAPK/activator protein-1 (AP-1) and NF-κB, as well as the expression of cytokines related to allergic inflammation (IL-4, IL-6, TNF-α, IFN-γ, and IL-1β), which are indirectly activated by bisphenol A or di(2-ethylhexyl) phthalate in HMC-1 and RBL-2H3 cells.33,92–96
4.2. Antioxidant activity
Evidence has mounted that C. tricuspidata could act as an efficient free-radical scavenger and thus help the antioxidant defense system (Table 3). C. tricuspidata leaves, in comparison with other parts, exhibited the highest scavenging activities against the 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radicals and the highest ferric reducing/antioxidant power (FRAP), which was correlated with their high level of polyphenols (73.60 ± 0.28 mg g−1), in particular quercetin.97,98 It has been reported that C. tricuspidata leaves could produce more quercetin and kaempferol aglycones via Lactobacillus-mediated fermentation, which would increase antioxidant activities (DPPH and ABTS assays), and thereby could be developed as high-value-added food materials and functional foods.99–102 An aqueous extract of C. tricuspidata (2 mg mL−1) exhibited significant scavenging activities of 50.2% (ABTS) and 40.5% (FRAP), respectively.103 The antioxidant activity of C. tricuspidata fruits was revealed to change depending on the maturation stage and was positively associated with the contents of prenylflavonoids such as artocarpesin (100), alpinumisoflavone (198), 6-isopentenylgenistein (202), 4′-O-methylalpinumisoflavone and 6,8-diprenylgenistein.104 The prenyl group on the A-ring of isoflavone was a potent contributor against the ABTS radical system.105 The C. tricuspidata glycoprotein (100 μg mL−1) exhibited strong scavenging activities against DPPH, superoxide anions and hydroxyl radicals, with no pro-oxidant activity in vitro.34 It should be interesting to investigate the in vivo antioxidant potentials of these compounds for preventing various radical-mediated injuries in pathological situations.| Sample | IC50 | Ref. | ||
|---|---|---|---|---|
| DPPH | ABTS | TBARS | ||
| a N.A = not available. | ||||
| The MeOH extract of leaves | 13.29 μg mL−1 | N.A | N.A | 78 |
| The MeOH extract of root bark | 54.48 mg mL−1 | N.A | 15.13 mg mL−1 | 156 |
| The ethyl ether fraction of MeOH extract of root bark | 30.78 mg mL−1 | N.A | 7.72 mg mL−1 | 156 |
| The EtOAc fraction of MeOH extract of root bark | 20.32 mg mL−1 | N.A | 7.46 mg mL−1 | 156 |
| The n-BuOH fraction of MeOH extract of root bark | 93.37 mg mL−1 | N.A | >20 mg mL−1 | 156 |
| Compd 20 | N.A | N.A | 6.2 μM | 23 |
| Compd 28 | N.A | N.A | 3.8 μM | 23 |
| Compd 29 | N.A | N.A | 2.2 μM | 23 |
| Compd 67 | N.A | N.A | 0.8 μM | 23 |
| Compd 72 | N.A | N.A | 4.5 μM | 23 |
| Compd 84 | N.A | N.A | 12.6 μM | 23 |
| Compd 88 | N.A | N.A | 2.6 μM | 23 |
| Compd 104 | >300 μM | 4.2 μM | N.A | 157 |
| Compd 110 | >300 μM | 8.2 μM | N.A | 157 |
| Compd 120 | 4.14 μg mL−1 | N.A | 3.65 μg mL−1 | 156 |
| Compd 121 | 5.94 μg mL−1 | N.A | 3.24 μg mL−1 | 156 |
| Compd 126 | 4.04 μg mL−1 | N.A | 3.72 μg mL−1 | 156 |
| Compd 127 | 5.50 μg mL−1 | N.A | 3.71 μg mL−1 | 156 |
| Compd 143 | >300 μM | 5.4 μM | N.A | 157 |
| Compd 144 | >300 μM | 6.0 μM | N.A | 157 |
| Compd 145 | N.A | N.A | 3 μg mL−1 | 30 |
| Compd 149 | N.A | N.A | 10 μg mL−1 | 30 |
| Compd 152 | >300 μM | 8.3 μM | N.A | 157 |
| Compd 166 | N.A | N.A | 6 μg mL−1 | 30 |
| Compd 230 | N.A | N.A | 3 μg mL−1 | 30 |
| Compd 254 | >200 μM | 16.3 μM | N.A | 105 |
4.3. Antitumor activity
During recent decades, C. tricuspidata has been demonstrated to possess promising antitumor and cytotoxic activities, and its cortices and root bark have been widely employed in TCM clinics for the treatment of cancer of the alimentary system, in particular gastric carcinoma.7,55 An EtOAc extract of C. tricuspidata stem bark displayed significant cytotoxicity against HL-60 cells, and the mechanism underlying its cytotoxicity may be due to apoptosis.106 A CHCl3 extract of C. tricuspidata roots exhibited significant cytotoxicity against human gastric carcinoma cell lines (SGC-7901 and BGC-823).17 An MeOH extract of C. tricuspidata stems could induce the apoptosis of cervical cancer cells via the extrinsic pathway, as well as via the repression of human papillomavirus type-16 oncoproteins E6 and E7 and the alteration of p53 and p-pRb protein levels, instead of cytotoxicity.107 In nude mouse models of B16 melanoma and human SK-OV3 xenografted tumors, the tumor-inhibiting rates of the total flavonoids (250 mg kg−1) from C. tricuspidata were 50.54% and 46.38%, respectively.108Several compounds isolated from C. tricuspidata displayed considerable inhibitory activity against various tumor cells in an MTT assay (Table 4). The isoprenylated xanthones 20, 28, and 29 exhibited potent cytotoxic activity against HL-60 cells owing to apoptosis in a DNA fragmentation assay.56 Gericudranins A–E isolated from the stem bark of C. tricuspidata exhibited cytotoxicity against human tumor cell lines such as CRL1579 (skin), LOX-IMVI (skin), MOLT-4F (leukemia), KM12 (colon) and UO-31 (renal).16,58 The p-hydroxybenzyl moiety at C-6 was revealed to be essential for the cytotoxic activity.16,58 2′,5,7-Trihydroxy-4′,5′-(2,2-dimethylchromeno)-8-(3-hydroxy-3-methylbutyl)flavanone (152) could inhibit the activity of topoisomerase I (IC50 = 1.0 mM) and induce apoptotic cell death of U937 human leukemia cells, at least in part, via the inhibition of DNA topoisomerase I activity.109 Cudraflavanone A (129) inhibited mammalian topoisomerase I with an IC50 of 0.4 mM and inhibited the activity of protein kinase C with an IC50 of 150 μM.110 Euchrestaflavanone B could inhibit the activity of protein kinase CKII with an IC50 of 78 μM.111 Cudraflavone B was demonstrated to be a lead for the development of a potential candidate for treating human oral squamous cell carcinoma cells via the activation of MAPK and NF-κB, as well as the silent information regulator 1 (SIRT1) pathway.61 Cudraxanthone H (24) and isocudraxanthone K (62) exerted significant antiproliferative and apoptosis-inducing effects in oral squamous cell carcinoma cells (IC50 values of 14.31 and 17.91 μM for HNSCC4 and 14.91 and 20.01 μM for HNSCC12 after treatment for 72 h) via the NF-κB and NIMA-interacting 1 pathways and mitochondrial death receptor, MAPK, NF-κB, and HIF-1α signalling pathways, respectively.112,113 Likewise, the cytotoxic effect of cudraflavone B was also documented against HNSCC4 cells (IC50 of 18.3, 12.6, and 10.9 μM after treatment for 24, 48, and 72 h) and HNSCC12 cells (IC50 of 19.5, 12.0, and 10.7 μM after treatment for 24, 48, and 72 h).61 CTXA could suppress the migration and invasion of MCF-7 and MDA-MB-231 breast cancer cells by downregulating MMP-9 and induce apoptosis by activating the mitochondrial-associated apoptotic signalling pathway, which suggests that it may be a novel antitumor agent for breast cancer therapy.114 Cudratricusxanthone G could inhibit the proliferation, migration and invasion of SW620 human colorectal carcinoma cells instead of displaying cytotoxicity by targeting MMP-2, thereby regulating the activation of Rac1, Cdc42 and their downstream target AP-1.24 Notably, the chemical and biogenic synthesis and molecular modification of unique compounds isolated from C. tricuspidata have attracted attention. For example, from gericudranin A a series of derivatives were synthesized by structural modification, some of which exhibited strong cytotoxicity against several cancer cell lines such as SNB19, MOLT-4F, and K562 cells in a sulforhodamine B assay.115 It is suggested that more attention should be paid to the SAR and in vivo antitumor mechanisms of the antitumor constituents of C. tricuspidata.
| Sample | Model | Active concentration | Ref. | Sample | Model | Active concentration | Ref. |
|---|---|---|---|---|---|---|---|
| a HL-60 = promyelocytic leukemia cell line.b U937 = human leukemia cell line.c HeLa = human carcinoma cell line.d MCF-7 = human breast cancer cell line.e HepG2 = human hepatoma cell line.f MDA-MB-231 = human breast cancer cell line.g BGC-823 = stomach cancer cell line.h A549 = lung carcinoma cell line.i L1210 = mouse leukemia cell line.j SK-OV3 = human ovarian cancer cell line.k HT-29 = human colon carcinoma cell line.l AGS = human lung cancer cell line.m HCT-116 = human colon carcinoma cell line.n SMMC-7721 = human hepatocellular carcinoma cell line.o SGC-7901 = human gastric cancer cell line.p P388 = mouse leukemia cell line.q CRL1579 = human skin cancer cell line.r LOX-IMVI = human melanoma cell line.s MOLT-4F = human leukemia cell line.t KM12 = human colon carcinoma cell line.u UO-31 = human renal cell line. | |||||||
| The EtOAc extract of stem bark | HL-60a | 30 μg mL−1 (IC50) | 106 | The EtOAc fraction of fruits | MCF-7 | 66.8 μg mL−1 (IC50) | 142 |
| U937b | 40 μg mL−1 (IC50) | 106 | MDA-MB-231f | 75.4 μg mL−1 (IC50) | 142 | ||
| HeLac | 58 μg mL−1 (IC50) | 106 | Total flavonoids | BGC-823g | 6.11 μg mL−1 (IC50) | 107 | |
| MCF-7d | 44 μg mL−1 (IC50) | 106 | A549h | 12.20 μg mL−1 (IC50) | 107 | ||
| HepG2e | 69 μg mL−1 (IC50) | 106 | L1210i | 12.73 μg mL−1 (IC50) | 107 | ||
| Compd 1 | BGC-823 | 15.2 μg mL−1 (IC50) | 17 | Compd 72 | A549 | 2.8 μM (IC50) | 49 |
| A549 | 5.93 μM (IC50) | 49 | 25.8 μM (LD50) | 56 | |||
| 45.8 μM (LD50) | 56 | SK-OV3 | 4.24 μM (IC50) | 49 | |||
| SK-OV3j | 7.09 μM (IC50) | 49 | 23.1 μM (LD50) | 56 | |||
| 43.2 μM (LD50) | 56 | HT-29 | 28.0 μM (LD50) | 56 | |||
| HT-29k | 41.4 μM (LD50) | 56 | HL-60 | 29.5 μM (LD50) | 56 | ||
| HL-60 | 32.8 μM (LD50) | 56 | AGS | 15.2 μM (LD50) | 56 | ||
| AGSl | 32.8 μM (LD50) | 56 | Compd 73 | HCT-116 | 6.66 μM (IC50) | 55 | |
| Compd 2 | HCT-116m | 3.9 μg mL−1 (IC50) | 17 | SMMC-7721 | 5.13 μM (IC50) | 55 | |
| SMMC-7721n | 6.9 μg mL−1 (IC50) | 17 | SGC-7901 | 3.63 μM (IC50) | 55 | ||
| SGC-7901o | 4.3 μg mL−1 (IC50) | 17 | BGC-823 | 3.11 μM (IC50) | 55 | ||
| Compd 3 | HCT-116 | 12.2 μg mL−1 (IC50) | 17 | Compd 77 | HCT-116 | 2.8 μg mL−1 (IC50) | 17 |
| SMMC-7721 | 8.9 μg mL−1 (IC50) | 17 | SMMC-7721 | 8.8 μg mL−1 (IC50) | 17 | ||
| Compd 4 | HCT-116 | 4.1 μg mL−1 (IC50) | 17 | SGC-7901 | 11.8 μg mL−1 (IC50) | 17 | |
| SMMC-7721 | 4.2 μg mL−1 (IC50) | 17 | BGC-823 | 5.2 μg mL−1 (IC50) | 17 | ||
| SGC-7901 | 9.8 μg mL−1 (IC50) | 17 | Compd 78 | HCT-116 | 1.3 μg mL−1 (IC50) | 17 | |
| Compd 5 | HCT-116 | 4.7 μg mL−1 (IC50) | 17 | SMMC-7721 | 6.2 μg mL−1 (IC50) | 17 | |
| SMMC-7721 | 4.2 μg mL−1 (IC50) | 17 | SGC-7901 | 3.4 μg mL−1 (IC50) | 17 | ||
| SGC-7901 | 5.4 μg mL−1 (IC50) | 17 | Compd 84 | A549 | 61.9 μM (LD50) | 56 | |
| BGC-823 | 1.6 μg mL−1 (IC50) | 17 | SK-OV3 | 70.4 μM (LD50) | 56 | ||
| Compd 6 | HCT-116 | 21.31 μM (IC50) | 55 | HT-29 | 46.3 μM (LD50) | 56 | |
| SMMC-7721 | 50.7 μM (IC50) | 55 | HL-60 | 35.9 μM (LD50) | 56 | ||
| SGC-7901 | 26.34 μM (IC50) | 55 | AGS | 44.7 μM (LD50) | 56 | ||
| BGC-823 | 17.62 μM (IC50) | 55 | Compd 129 | SMMC-7721 | 32.04 μM (IC50) | 55 | |
| Compd 7 | HCT-116 | 1.8 μg mL−1 (IC50) | 17 | SGC-7901 | 28.68 μM (IC50) | 55 | |
| SMMC-7721 | 2.7 μg mL−1 (IC50) | 17 | BGC-823 | 26.90 μM (IC50) | 55 | ||
| SGC-7901 | 3.4 μg mL−1 (IC50) | 17 | U937 | 6.0 μM (IC50) | 110 | ||
| BGC-823 | 1.6 μg mL−1 (IC50) | 17 | Compd 130 | HCT-116 | 24.37 μM (IC50) | 55 | |
| Compd 9 | SMMC-7721 | 11.7 μg mL−1 (IC50) | 17 | SMMC-7721 | 28.94 μM (IC50) | 55 | |
| SGC-7901 | 1.8 μg mL−1 (IC50) | 17 | SGC-7901 | 65.86 μM(IC50) | 55 | ||
| BGC-823 | 9.2 μg mL−1 (IC50) | 17 | BGC-823 | 28.68 μM (IC50) | 55 | ||
| Compd 19 | A549 | 61.7 μM (LD50) | 56 | Compd 143 | U937 | 0.8 μM (IC50) | 111 |
| SK-OV3 | 74.5 μM (LD50) | 56 | HeLa | 0.8 μM (IC50) | 111 | ||
| HT-29 | 50.7 μM (LD50) | 56 | Compd 145 | P388p | 3.3 μg mL−1 (IC50) | 30 | |
| HL-60 | 40.8 μM (LD50) | 56 | Compd 149 | P388 | 6.2 μg mL−1 (IC50) | 30 | |
| AGS | 49.5 μM (LD50) | 56 | Compd 152 | U937 | 10.0 μM (IC50) | 109 | |
| Compd 20 | A549 | 16.3 μM (LD50) | 56 | Compd 158 | P388 | 15.0 μg mL−1 (IC50) | 30 |
| SK-OV3 | 23.8 μM (LD50) | 56 | Compd 161 | CRL1579q | 3.65 μM (EC50) | 16 | |
| HT-29 | 20.7 μM (LD50) | 56 | LOX-IMVIr | 11.99 μM (EC50) | 16 | ||
| HL-60 | 6.2 μM (LD50) | 56 | MOLT-4Fs | 2.65 μM (EC50) | 16 | ||
| AGS | 4.7 μM (LD50) | 56 | KM12t | 13.70 μM (EC50) | 16 | ||
| Compd 21 | HCT-116 | 26.05 μM (IC50) | 55 | UO-31u | 6.99 μM (EC50) | 16 | |
| SMMC-7721 | 38.32 μM (IC50) | 55 | Compd 162 | CRL1579 | 13.12 μM (EC50) | 16 | |
| SGC-7901 | 32.04 μM (IC50) | 55 | LOX-IMVI | 31.26 μM (EC50) | 16 | ||
| BGC-823 | 40.24 μM (IC50) | 55 | MOLT-4F | 23.07 μM (EC50) | 16 | ||
| Compd 24 | HCT-116 | 5.50 μM (IC50) | 55 | KM12 | 28.05 μM (EC50) | 16 | |
| SMMC-7721 | 5.67 μM (IC50) | 55 | UO-31 | 9.78 μM (EC50) | 16 | ||
| SGC-7901 | 3.07 μM (IC50) | 55 | Compd 163 | CRL1579 | 3.34 μM (EC50) | 16 | |
| BGC-823 | 2.82 μM (IC50) | 55 | LOX-IMVI | 13.46 μM (EC50) | 16 | ||
| Compd 28 | A549 | 3.15 μM (IC50) | 49 | MOLT-4F | 7.62 μM (EC50) | 16 | |
| 33.5 μM (LD50) | 56 | KM12 | 13.84 μM (EC50) | 16 | |||
| SK-OV3 | 4.72 μM (IC50) | 49 | UO-31 | 16.82 μM (EC50) | 16 | ||
| 38.0 μM (LD50) | 56 | Compd 164 | CRL1579 | 9.50 μM (EC50) | 59 | ||
| HT-29 | 11.4 μM (LD50) | 56 | LOX-IMVI | 16.60 μM (EC50) | 59 | ||
| HL-60 | 8.6 μM (LD50) | 56 | MOLT-4F | 8.90 μM (EC50) | 59 | ||
| AGS | 3.9 μM (LD50) | 56 | KM12 | 5.00 μM (EC50) | 59 | ||
| Compd 29 | HCT-116 | 3.4 μg mL−1 (IC50) | 17 | UO-31 | 5.20 μM (EC50) | 59 | |
| SMMC-7721 | 5.1 μg mL−1 (IC50) | 17 | Compd 165 | CRL1579 | 2.90 μM (EC50) | 59 | |
| SGC-7901 | 9.5 μg mL−1 (IC50) | 17 | LOX-IMVI | 12.50 μM (EC50) | 59 | ||
| BGC-823 | 2.6 μg mL−1 (IC50) | 17 | MOLT-4F | 10.7 μM (EC50) | 59 | ||
| A549 | 11.8 μM (LD50) | 56 | KM12 | 11.9 μM (EC50) | 59 | ||
| SK-OV3 | 14.6 μM (LD50) | 56 | UO-31 | 7.60 μM (EC50) | 59 | ||
| HT-29 | 12.1 μM (LD50) | 56 | Compd 202 | HL-60 | 18.0 μM (IC50) | 87 | |
| HL-60 | 8.2 μM (LD50) | 56 | Compd 205 | HL-60 | 4.3 μM (IC50) | 87 | |
| AGS | 4.1 μM (LD50) | 56 | Compd 215 | HL-60 | 6.7 μM (IC50) | 87 | |
| Compd 67 | A549 | 57.8 μM (LD50) | 56 | Compd 222 | HL-60 | 5.1 μM (IC50) | 87 |
| SK-OV3 | 71.3 μM (LD50) | 56 | Compd 225 | HL-60 | 8.8 μM (IC50) | 87 | |
| HT-29 | 65.0 μM (LD50) | 56 | Compd 226 | HL-60 | 10.1 μM (IC50) | 87 | |
| HL-60 | 45.2 μM (LD50) | 56 | Compd 227 | HL-60 | 5.2 μM (IC50) | 87 | |
| AGS | 43.9 μM (LD50) | 56 | Compd 246 | P388 | 0.18 μg mL−1 (IC50) | 30 | |
| Compd 184 | HL-60 | 9.5 μM (IC50) | 87 | Compd 254 | HL-60 | 4.3 μM (IC50) | 87 |
4.4. Hepatoprotective activity
Liver disease remains one of the most serious health problems without satisfactory drugs. The CHCl3 fraction of an MeOH extract of C. tricuspidata root bark exhibited a significant hepatoprotective effect on tacrine-induced cytotoxicity in HepG2 cells.26 CTXA, cudratricusxanthone E, cudraxanthone L and macluraxanthone B, which were isolated from the CHCl3 fraction, displayed the strongest hepatoprotective effects on tacrine-induced cytotoxicity in HepG2 cells at 10 μg mL−1.26 Cudraflavone B and gericudranin E were further isolated from this MeOH extract and displayed significant protective effects against tacrine-induced cytotoxicity in HepG2 cells, with EC50 values of 37.39 and 39.87 μM, respectively.25 Cudracuspixanthone A (16) and cudracuspiphenones A (353) and B (354) exhibited moderate antiproliferative activity against HSC-T6 cells, with IC50 values of 9.7, 3.3, and 7.1 μM, respectively.116 It was demonstrated that 1,1-dimethylallyl or 2,3,3-trimethyl-2,3-dihydrofuran moieties in the xanthones played important roles for the inhibitory activity.116 The glycoprotein (75 kDa) isolated from C. tricuspidata fruits was effective in preventing CCl4-induced liver damage in A/J mice by significantly increasing the activities of superoxide dismutase, catalase, and glutathione peroxidase, as well as decreasing the production of TBARS, lactate dehydrogenase (LDH) and NO.35 These constituents might be preferred alternatives for liver disease, and in vivo assays are essential to ascertain their hepatoprotective role fully.4.5. Neuroprotective activity
An aqueous extract of C. tricuspidata roots exhibited a stronger protective effect against neurotoxicity induced by oxidative stress than those of leaves, stems, and fruits, which was correlated with its high level of phenolic compounds, in particular kaempferol, myricetin (123) and quercetin.117 CTXA and cudraflavone B displayed significant neuroprotective activity against glutamate-induced neurotoxicity via the induction of HO-1 in HT22 mouse hippocampal cells.51,62 The neuroprotective effect of cudraflavone B was probably regulated by the phosphatidylinositol 3-kinase (PI3K)/AKT pathways.62MAOs are responsible for the degradation of neurotransmitters including noradrenaline, dopamine, and 5-hydroxytryptamine in the central nervous system.118 The dichloromethane (CH2Cl2) fraction of C. tricuspidata fruits was active in inhibiting mouse brain MAO, and gancaonin A (216), 4′-O-methylalpinumisoflavone, and alpinumisoflavone inhibited MAO in a concentration-dependent manner, with IC50 values of 19.4, 23.9, and 25.8 μM, respectively. Of these, gancaonin A exhibited a selective inhibitory effect against MAO-B (IC50 = 0.8 μM) in comparison with MAO-A (IC50 > 800 μM).118 CTXA, cudraflavanone A and cudraflavone B exhibited moderate inhibitory effects against mouse brain MAO, with IC50 values of 88.3, 89.7, and 80.0 μM, respectively.50
The neuroprotective potential of the flavonoids orobol (230), 6-prenylorobol (255) and 6,8-diprenylorobol was evaluated via enhancing the ubiquitin/proteasome-dependent degradation of α-synuclein and synphilin-1 in SH-SY5Y human neuroblastoma cells induced by 6-hydroxydopamine (6-OHDA) (Table 5), which signified that they might be possible candidates for the treatment of neurodegenerative diseases.119 5,7-Dihydroxychromone (426) could prevent 6-OHDA-induced oxidative stress and apoptosis in SH-SY5Y cells via the activation of the Nrf2/ARE signalling pathway and the overexpression of antioxidant enzymes, including HO-1, NAD(P)H: quinone oxidoreductase and the glutamate-cysteine ligase catalytic subunit.120 In LPS-stimulated BV2 mouse microglia, CTXA (IC50 = 0.98 μM) decreased the production of TNF-α, IL-1β, and IL-12, inhibited the phosphorylation and degradation of IκB-α, and blocked the nuclear translocation of p50 and p65 by inhibiting the NF-κB and MAPK pathways.121 Cudraflavanone D (132) could suppress the production of NO in LPS-induced BV2 microglial cells with an IC50 value of 6.28 μM and exert anti-neuroinflammatory activity by targeting iNOS and COX-2 via the MAPK and NF-κB pathways.1 Demethylsuberosin (299), as a potent proteasome activator, attenuated the 1-methyl-4-phenylpyridinium-induced dysfunction of the chymotrypsin-like and caspase-like activities of proteasomes in SH-SY5Y cells with EC50 values of 0.76 μM and 0.82 μM, respectively, and protected SH-SY5Y cells against 1-methyl-4-phenylpyridinium-induced cell death, with an EC50 value of 0.17 μM.122 4′-O-Methylalpinumisoflavone isolated from C. tricuspidata fruits exerted anti-neuroinflammatory effects against LPS-induced microglial activation in BV2 cells by decreasing NF-κB signalling and the phosphorylation of MAPKs.123 The above results demonstrated that those compounds with neuroprotective activities could be considered as candidates for further research for therapeutic purposes into neurodegenerative diseases such as Parkinson's disease.
| Compd | EC50 (μM) | Ref. | Compd | EC50 (μM) | Ref. |
|---|---|---|---|---|---|
| 17 | 4.5 | 27 | 142 | 9.1 | 145 |
| 28 | 8.2 | 27 | 227 | 15.2 | 119 |
| 39 | 7.2 | 27 | 228 | 18.5 | 119 |
| 43 | 16.6 | 27 | 230 | 6.4 | 119 |
| 44 | 2.4 | 27 | 254 | 10.1 | 119 |
| 45 | 2.2 | 27 | 255 | 4.5 | 119 |
| 51 | 0.8 | 27 | 305 | 9.2 | 145 |
| 64 | 3.0 | 27 | 311 | 8.0 | 145 |
| 74 | 15.5 | 27 | 322 | 12.9 | 145 |
| 75 | 0.7 | 27 | 343 | 6.2 | 145 |
| 91 | 2.3 | 27 | 416 | 11.2 | 168 |
| 97 | 5.1 | 27 | 419 | 30.2 | 145 |
| 103 | 15.5 | 145 | 426 | 1.9 | 145 |
4.6. Antiobesity activity
Excess body weight and obesity are severe threats to public health worldwide. The leaves of C. tricuspidata, in comparison with other parts, exhibited the most pronounced inhibitory effect against pancreatic lipase (PL), which is a key enzyme for lipid absorption, with an IC50 value of 9.91 μg mL−1 in vitro, and were able to reduce plasma triacylglycerol levels and delay dietary fat absorption in vivo.18 The optimal conditions for the maximum PL-inhibiting activity and extraction yield of C. tricuspidata fruits were determined using response surface methodology to be an ethanol concentration of 74.5%, a temperature of 61.9 °C, and an extraction time of 13.5 h.12 Flavonoids isolated from C. tricuspidata, namely, cudraflavanones A and D and 5,7,4′-trihydroxy-6,8-diprenylisoflavone, inhibited PL, with IC50 values of 9.0, 6.5, and 65.0 μM, respectively.12,124 Further SAR studies highlighted that the prenyl moiety and number and position of hydroxyl groups of the flavonoids seemed to affect the PL-inhibiting activity, which needs to be clarified using more derivatives. The PL-inhibiting activity of C. tricuspidata fruits has been proven to vary with their maturation stage.66 Unripe fruits of C. tricuspidata, in accordance with their higher content of total phenolic compounds and flavonoids, exhibited stronger PL-inhibiting activity in comparison to ripe fruits.66 In addition, an isoflavone, namely, cudracusisoflavone B (195), from unripe fruits exhibited strong PL-inhibiting activity, with an IC50 value of 16.8 μM, in a non-competitive manner.66 Therefore, the maturation stage is an important factor for the efficacy, and unripe fruits appeared to be a good source of agents for the regulation of obesity. Protein-tyrosine phosphatases (PTP1B) are also important risk factors for obesity-related metabolic diseases. The leaves of C. tricuspidata displayed a strong inhibitory effect against PTP1B and substantially inhibited fat accumulation in 3T3-L1 cells in a dose-dependent manner.19 Xanthones and flavonoids isolated from the roots of C. tricuspidata, including CTXA, cudratricusxanthones L (12) and N (14), cudracuspixanthone A, cudraxanthones D, L, and M, macluraxanthone B, 1,6,7-trihydroxy-2-(1,1-dimethyl-2-propenyl)-3-methoxyxanthone (87), cudraflavone C (105), kuwanon C (114), cudraflavanone D and euchrestaflavanone C, displayed a significant inhibitory activity against PTP1B in a dose-dependent manner, with IC50 values ranging from 1.9 to 13.6 μM.125 In comparison with flavonoids, prenylated xanthones displayed stronger PTP1B-inhibiting effects, which suggested that they may be promising agents for the future discovery of novel PTP1B inhibitors.An aqueous extract of C. tricuspidata leaves that underwent fermentation mediated by lactic acid bacteria was proven to be beneficial for promoting osteogenic differentiation of osteoblastic cells and inhibiting fat accumulation in adipocytes.99 In diet-induced obesity (DIO) mice, this extract could decrease levels of aspartate aminotransferase, alanine aminotransferase, total fat mass, triglycerides, and blood glucose and was also found to promote the phosphorylation of IRS-1 and Akt in liver tissues and improve insulin secretion.19 Correspondingly, the leaves of C. tricuspidata could be used as materials to produce a functional food product with antiobesity effects.126 6,8-Diprenylgenistein, which is a flavonoid isolated from C. tricuspidata, was proven to decrease body weight, epididymal fat and serum triglyceride levels in DIO mice.127 The underlying mechanism of this compound has been demonstrated, namely, that it could inhibit lipogenic genes by the regulation of transcription factors such as peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer-binding protein α (C/EBPα) and hormones such as leptin and adiponectin.127 6,8-Diprenylgenistein was also found to regulate acetyl-CoA carboxylase (ACC) and hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) by the activation of AMP-activated protein kinase (AMPK).127 Further investigation is warranted to determine whether their beneficial effects are associated with gut microbiota, which is a topic of recent and growing interest.
4.7. Immunomodulatory effects
Emerging evidence has suggested that C. tricuspidata is a potent immunomodulator. An aqueous extract of C. tricuspidata displayed potent adjuvant activity to enhance antigen-specific antibody responses and cellular immune responses against keyhole limpet hemocyanin.128 In recent years, plant polysaccharides have emerged as an important class of bioactive natural products that are ideal therapeutic candidates for immunomodulatory functions with low toxicity. The in vitro immunomodulatory activities of the polysaccharides from C. tricuspidata roots were investigated in relation to the activation of mouse peritoneal macrophages.71,74 The results showed that the four water-soluble polysaccharides, namely, CTPS-1A, CTPS-2B, CTPS-3A, and CTP-B1, could directly stimulate the proliferation of mouse splenocytes alone or in combination with concanavalin A or LPS within the concentration range of 6.25 to 100 μg mL−1, in a comparable way to the immunomodulator lentinan.71,74 T-helper type 1 (Th1) and Th2 cytokines have been demonstrated to interact reciprocally to maintain a balanced immune network. The C. tricuspidata glycoprotein could prevent the development of immune diseases related to Th2 cell responses, such as autoimmune diseases, viral infections, and allergies.36,129 The precise mechanism of the differentiation of Th cells into Th1 or Th2 cells as induced by the C. tricuspidata glycoprotein remains to be elucidated.4.8. Antiatherosclerotic activity
CTXA from C. tricuspidata was found to exert inhibitory effects on the synthesis and proliferation of DNA in vascular smooth muscle cells stimulated by platelet-derived growth factor (PDGF)-BB by suppressing the PDGF receptor β-chain and downregulating the Ras-Raf-MEK-ERK1/2 signalling pathways, and may serve as an antiatherosclerotic lead compound.80 Likewise, cudraflavanone A was useful in the prevention of atherosclerosis or restenosis after angioplasty, and the molecular mechanism was found to be that it inhibited the PDGF-BB-induced growth of rat aortic smooth muscle cells via an Akt-dependent pathway.81 In addition, cudraflavone B was observed to inhibit the proliferation of rat aortic smooth muscle cells by inducing the expression of p21cip1 and p27kip1 and subsequent cell cycle arrest with a reduction in the phosphorylation of pRb at the G1-S phase, which suggests its therapeutic potential for treating cardiovascular disease.9 Low-density lipoprotein (LDL) has been known to play a crucial role in the development of atherosclerosis and hypercholesterolemia.130 Many compounds isolated from C. tricuspidata have been confirmed to be effective in preventing the oxidation of LDL in a TBARS assay (Table 3).4.9. Antimicrobial activity
The essential oil of C. tricuspidata fruits was proven to be able to disrupt the membrane functions of both Gram-positive and Gram-negative bacteria, which led to its effective use as a natural antimicrobial agent to control food-borne pathogens in the food industry. The antibacterial activity of the essential oil was investigated against Bacillus cereus ATCC 13061, Staphylococcus aureus ATCC 12600, Listeria monocytogenes ATCC 7644, Salmonella typhimurium ATCC 43174 and Escherichia coli O157:H7 ATCC 43889.76 The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the essential oil were in the range of 250–500 μg mL−1 and 500–1000 μg mL−1, respectively.76 In addition, a methanolic extract of C. tricuspidata roots exhibited high antifungal activity against Gymnosporangium haraeanum Syd., Pyricularia oryzae Cav., Rhizoctonia solani Kühn, and Colletotrichum graminicola (Ces.) Wilson, with EC50 values of 803, 997, 981 and 930 μg mL−1, respectively.114.10. Skin protection
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by elevated immunoglobulin E (IgE) levels, mast cell infiltration and skin lesions including pruritus, erythema and eczema.131 An ethanolic extract of C. tricuspidata stems could be applied topically to decrease serum IgE levels and mast cell counts in the dermis of the skin in an AD-like NC/Nga mouse model induced by Dermatophagoides farinae extract.131 Similarly, an aqueous extract of C. tricuspidata fruits was also found to inhibit the development of AD-like skin lesions induced by repeated applications of D. farinae in sensitized NC/Nga mice by reducing plasma concentrations of mouse thymus and activation-regulated chemokine (mTARC), histamine and IgE.132 Nevertheless, the definite active compounds responsible for the anti-atopic dermatitis activity remain to be identified.A methanolic extract of C. tricuspidata stems was demonstrated to prevent skin inflammation and skin aging via suppressing the solar ultraviolet-induced expression of COX-2.82 The EtOAc fraction (IC50 = 24.4 ppm) and the n-BuOH fraction (IC50 = 88.3 ppm) of the C. tricuspidata stem extract could reduce the activity of tyrosinase and the melanin content in a concentration-dependent manner.79 It was found that the flavonoids steppogenin (149, IC50 = 2.52 μM) and trans-dihydromorin (160, IC50 = 21.54 μM) and the phenylpropanoid oxyresveratrol (IC50 = 2.85 μM) from the twigs of C. tricuspidata displayed potent inhibitory activities against mushroom tyrosinase and the melanogenesis process in melanocytes, which suggested their potential to be developed as skin-whitening agents in cosmetics and anti-browning agents in food.75 The tyrosinase-inhibiting activity of the flavonoids could be affected by the hydroxyl groups substituted at the 2- and 4-positions of the aromatic ring.75 Oxyresveratrol and trans-dihydromorin, as hypopigmenting agents, could induce post-transcriptional degradation of microphthalmia-associated transcription factor (MITF), leading to significant decreases in the production of tyrosinase-related protein 1 (TRP-1) and tyrosinase-related protein 2 (TRP-2) in b16 and melan-a cells.133 Besides, 6,8-diprenylorobol and pomiferin (233) could inhibit the photooxidation of A2E, which is an important constituent of lipofuscin in the retinal pigment epithelium, in a dose-dependent manner.32 Collectively, these studies clearly showed that C. tricuspidata and the isolated bioactive compounds could be used as cosmeceutical materials and food constituents for the promotion of skin health.
4.11. Antidiabetic activity
Lee et al.20 reported that the aqueous extract of C. tricuspidata leaves could significantly improve hepatic insulin resistance and hyperglycemia by controlling obesity-induced stress in the hepatic endoplasmic reticulum and inflammation in the liver of db/db mice. Furthermore, an in vitro study demonstrated that both C. tricuspidata leaves and the isolated compound kaempferol could reduce hepatic insulin resistance by suppressing insulin receptor substrate signalling and the inflammatory response in HepG2 cells induced by endoplasmic reticulum stress.134 In addition, the α-glucosidase-inhibiting activities of aqueous extracts of C. tricuspidata stems and roots depended on the harvesting time and climate.21 A root extract exerted potent inhibitory effects on α-glucosidase activity, with 77% inhibition at a concentration of 300 μg mL−1, which signified that the root could serve as an antidiabetic biomaterial.21 Xanthones, including CTXA, cudratricusxanthone F, cudraxanthones D and L, macluraxanthone B, 1,3,6,7-tetrahydroxy-2-(3-methylbut-2-enyl)-8-(2-methylbut-3-en-2-yl)-9H-xanthen-9-one (83) and 1,3,7-trihydroxy-4-(1,1-dimethyl-2-propenyl)-5,6-(2,2-dimethylchromeno)xanthone (84), displayed inhibitory activities against α-glucosidase, with IC50 values of 16.2–52.9 μM.52 CTXA was also proven to prevent the production of NO, the expression of iNOS, and the activation of JAK/STAT and NF-κB in RINm5F cells induced by IL-1β and IFN-γ and to inhibit the glucose-stimulated secretion of insulin in pancreatic islets.135 The above results suggested that C. tricuspidata may be a promising therapeutic material in the treatment of diabetes.4.12. Others
Besides the above pharmacological properties, other biological activities of C. tricuspidata have also been reported. An aqueous extract of C. tricuspidata stems could decrease systolic blood pressure in hypertension induced by NG-nitro-L-arginine methyl ester, in part by enhancing the generation of vascular NO/cGMP and the amelioration of renal functions.136 The anticoagulant activity of CTXA was investigated by Yoo et al.,29 who revealed that CTXA could inhibit the generation of cell-based thrombin, activated factor X (FXa) and thrombin and exhibited thrombolytic activity by decreasing the ratio of plasminogen activator inhibitor type 1 (PAI-1) to tissue-type plasminogen activator (t-PA).Park et al.53 revealed that xanthones bearing 6,7 vicinal dihydroxy groups on the A ring, including CTXA, cudratricusxanthone F, cudraxanthones D, L and M, macluraxanthone B, and 1,3,6,7-tetrahydroxy-2-(3-methylbut-2-enyl)-8-(2-methylbut-3-en-2-yl)-9H-xanthen-9-one, displayed nanomolar inhibitory activity (IC50: 80–270 nM) against neuraminidase. Cudraflavanone A, which bears a C-8 hydrated prenyl group, also displayed high neuraminidase-inhibiting activity, with an IC50 of 380 nM.137 This implied that these xanthones and flavonoids may be potential antiviral agents in the future.
The above descriptions indicated that many compounds have a variety of activities, in particular CTXA, which is a major and important component with a wide range of activities. Recently, pharmacokinetic studies of representative constituents of C. tricuspidata have also attracted attention. The in vitro metabolic profiling of CTXA in human liver microsomes has been recently investigated, which revealed that eight identified metabolites of CTXA were involved with cytochrome P450 enzymes (CYPs) and uridine 5′-diphospho-glucuronosyltransferase enzymes (UGTs).138 In a follow-up study, CTXA has been demonstrated to exhibit reversible competitive inhibition of CYP1A2 and CYP2C9 and non-competitive inhibition of CYP2C8 in human liver microsomes, which has begun to shed light on the in vivo metabolism of CTXA.139 Cudratricusxanthone B, as another example, has also been investigated for its pharmacokinetics by a fast and sensitive HPLC-MS/MS method, but its oral bioavailability (OB) remains unclear and merits future investigation.140 Therefore, it is suggested that the pharmacokinetics of this plant should be studied systematically.
5 Conclusions
This review provides an up-to-date and comprehensive summary concerning the botany, traditional uses, phytochemistry and pharmacology of the traditional folk medicine C. tricuspidata. As a medicinal plant, C. tricuspidata has been used to treat rheumatism, bruising, scabies, hepatitis, jaundice, gonorrhea, dysmenorrhea and amenorrhea in East Asia for thousands of years. During the last few decades, C. tricuspidata-derived extracts and compounds have attracted much attention for their promising biological activities, including anti-inflammatory, antioxidant, antitumor, hepatoprotective, immunomodulatory, neuroprotective, antiobesity, antimicrobial, antiatherosclerotic, skin-protecting, and antidiabetic activities. Obviously, some pharmacological activities are not related to the traditional uses of this species but provide valuable hints for new areas of application. Xanthones and flavonoids are the two major classes of constituent that contribute either directly or indirectly to the biological effects of C. tricuspidata, followed by minor classes, including organic acids, polysaccharides, phenylpropanoids, and others. Findings and knowledge regarding the phytochemistry and pharmacology of C. tricuspidata have established a basis for further research on, and development of, this medicinal plant and its active components. Notably, the unique structures isolated from C. tricuspidata have aroused interest in research on the chemical and biogenic synthesis of these bioactive compounds that is suitable for large-scale preparation and molecular modification. This should be beneficial for the development and application of natural compounds from C. tricuspidata and their synthetic analogues. As a rich source of medicines and functional foods, quality control of C. tricuspidata is crucial to ensure both safety and efficacy. It is suggested that current advances, including mass spectrometry-based chemical profiling and DNA barcoding, should be used to authenticate, differentiate, and evaluate the quality of C. tricuspidata. Importantly, a common international criterion should be established with the ultimate goal of ensuring the effectiveness and safety and maximizing the medicinal benefits of C. tricuspidata.As recent insights into the pharmacological mechanisms of C. tricuspidata are limited to in vitro bioassays of a limited number of molecules, it is essential and urgent to investigate the mechanisms of the bioactive extracts/isolates in appropriate animal models. To the best of our knowledge, few relevant data from clinical trials of C. tricuspidata (only in Chinese clinics) have been reported, and most clinical trials used a relatively small sample size and insufficient information. It is suggested that the efficacy of C. tricuspidata should be assessed in the future by combining its pharmacological effects, mechanisms of action and clinical applications. Detailed studies of the pharmacokinetics and toxicological properties and preclinical and clinical trials of C. tricuspidata are also eagerly awaited. More knowledge should be accumulated concerning the bioavailability, metabolism and toxicity of C. tricuspidata, which will be valuable for understanding its dosage efficacy and in vivo effects. It should be noted that the interaction between the bioactive constituents of C. tricuspidata and the human microbiota is an underappreciated aspect, as the gut microbiota plays a vital role in the pathogenesis and progression of obesity, diabetes and related metabolic disorders.141 Current findings have demonstrated that C. tricuspidata may serve as a good source of prebiotics that promote the growth of probiotic bacteria and improve the antioxidant activity of dairy products, which is of great interest for the development of functional foods.
Acknowledgements
We would like to thank Drs Xue-qing Zhang (Ocean University of China), Cheng Qu (Nanjing University of Chinese Medicine) and master Miao-yin Zhang (The Johns Hopkins University) for critically reading a previous version of this manuscript. We apologize to authors whose relevant work was not included in this review owing to space constraints. This work was supported by the National High Technology Research and Development Program of China (863 Program) (No. 2013AA093001), The Scientific and Technological Innovation Project Financially Supported by Qingdao National Laboratory for Marine Science and Technology (No. 2015ASKJ02), and the Taishan Scholars Program, China.Notes and references
- D. C. Kim, C. S. Yoon, T. H. Quang, W. Ko, J. S. Kim, H. Oh and Y. C. Kim, Int. J. Mol. Sci., 2016, 17, 255 CrossRef PubMed
.
- B. S. Jung and M. K. Shin, Encyclopedia of Illustrated Korean Natural Drugs, Young Lim Press, Seoul, 1990 Search PubMed
.
- L. Reich, Uncommon Fruits for Every Garden, Timber Press, Portland, 2004 Search PubMed
.
- T. Fujimoto, Y. Hano and T. Nomura, Planta Med., 1984, 50, 218–221 CrossRef CAS PubMed
.
- Chinese Pharmacopoeia Commission, Pharmacopoeia of the People's Republic of China, China Medical Science and Technology Press, Beijing, 1997, vol. I, p. 467 Search PubMed
.
- Nanjing University of Traditional Chinese Medicine, Dictionary of Chinese Herbal Medicine, Science and Technology Press, Shanghai, 2006 Search PubMed
.
- Jiangsu New Medical College, Encyclopedia of Chinese Medicinal Substances, Shanghai People′s Publisher, Shanghai, China, 1977 Search PubMed
.
- H. H. Ding, D. H. Chen, L. F. Zhu, Q. Wu and X. H. Zhou, Zhongchengyao, 2001, 23, 151–152 Search PubMed
.
- T. J. Kim, H. J. Han, Y. Lim, M. C. Song, J. Kim, J. T. Hong, H. S. Yoo, M. Y. Pyo, B. Y. Hwang, M. K. Lee and Y. P. Yun, J. Cardiovasc. Pharmacol., 2009, 53, 341–348 CrossRef CAS PubMed
.
- S. Nam, H. W. Jang and T. Shibamoto, J. Agric. Food Chem., 2012, 60, 9097–9105 CrossRef CAS PubMed
.
- Y. Z. Yu, Y. C. Deng, X. Qin, M. Zhang, L. F. Li and Z. W. Kong, Agrochemicals, 2009, 48, 224–226 Search PubMed
.
- J. Y. Jeong, Y. H. Jo, K. Y. Lee, S. Do, B. Y. Hwang and M. K. Lee, Bioorg. Med. Chem. Lett., 2014, 24, 2329–2333 CrossRef CAS PubMed
.
- Y. T. Hou, Tezhong Jingji Dongzhiwu, 2001, 7, 25 Search PubMed
.
- O. K. Kim, D. E. Nam, W. J. Jun and J. M. Lee, J. Food Biochem., 2015, 39, 508–516 CrossRef
.
- S. H. Chang, E. J. Jung, D. G. Lim, B. Oyungerel, K. II Lim, E. Her, W. S. Choi, M. H. Jun, K. D. Choi, D. J. Han and S. C. Kim, J. Pharm. Pharmacol., 2008, 60, 1221–1226 CrossRef CAS PubMed
.
- I. K. Lee, C. J. Kim, K. S. Song, H. M. Kim, H. Koshino, M. Uramoto and I. D. Yoo, Phytochemistry, 1996, 41, 213–216 CrossRef CAS PubMed
.
- Y. S. Zou, A. J. Hou, G. F. Zhu, Y. F. Chen, H. D. Sun and Q. S. Zhao, Bioorg. Med. Chem., 2004, 12, 1947–1953 CrossRef CAS PubMed
.
- Y. S. Kim, Y. Lee, J. Kim, E. Sohn, C. S. Kim, Y. M. Lee, K. Jo, S. Shin, Y. Song, J. H. Kim and J. S. Kim, J. Evidence-Based Complementary Altern. Med., 2012, 277–293 Search PubMed
.
- D. H. Kim, S. Lee, Y. W. Chung, B. M. Kim, H. Kim, K. Kim and K. M. Yang, BioMed Res. Int., 2016, 2016, 8432759 Search PubMed
.
- O. K. Kim, D. E. Nam, W. J. Jun and J. M. Lee, Food Nutr. Res., 2015, 59, 29165 CrossRef PubMed
.
- H. U. Son and S. H. Lee, Biomed. Rep., 2013, 1, 624–628 CAS
.
- G. S. Jeong, D. S. Lee and Y. C. Kim, Int. Immunopharmacol., 2009, 9, 241–246 CrossRef CAS PubMed
.
- K. H. Park, Y. D. Park, J. M. Han, K. R. Im, B. W. Lee, Y. Jeong, T. S. Jeong and W. S. Lee, Bioorg. Med. Chem. Lett., 2006, 16, 5580–5583 CrossRef CAS PubMed
.
- L. Kuang, L. Wang, Q. Wang, Q. Zhao, B. Du, D. Li, J. Luo, M. Y. Liu, A. J. Hou and M. Qian, Biochem. Pharmacol., 2011, 81, 1192–1200 CrossRef CAS PubMed
.
- R. B. An, D. H. Sohn and Y. C. Kim, Biol. Pharm. Bull., 2006, 29, 838–840 CAS
.
- Y. H. Tian, H. C. Kim, J. M. Cui and Y. C. Kim, Arch. Pharm. Res., 2005, 28, 44–48 CrossRef CAS PubMed
.
- J. Kwon, N. T. Hiep, D. W. Kim, B. Y. Hwang, H. J. Lee, W. Mar and D. Lee, J. Nat. Prod., 2014, 77, 1893–1901 CrossRef CAS PubMed
.
- N. T. Hiep, J. Kwon, D. W. Kim, B. Y. Hwang, H. J. Lee, W. Mar and D. Lee, Phytochemistry, 2015, 111, 141–148 CrossRef CAS PubMed
.
- H. Yoo, S. K. Ku, W. Lee, S. Kwak, Y. D. Baek, B. W. Min, G. S. Jeong and J. S. Bae, Arch. Pharmacal Res., 2014, 37, 1069–1078 CrossRef CAS PubMed
.
- I. K. Lee, K. S. Song, C. J. Kim, H. M. Kim, G. T. Oh and I. D. Yoo, Agric. Chem. Biotechnol., 1994, 37, 105–109 CAS
.
- X. H. Han, S. S. Hong, Q. Jin, D. Lee, H. K. Kim, J. Lee, S. H. Kwon, D. Lee, C. K. Lee, M. K. Lee and B. Y. Hwang, J. Nat. Prod., 2009, 72, 164–167 CrossRef CAS PubMed
.
- G. M. Uddin, H. J. Lee, J. S. Jeon, D. Chung and Y. C. Kim, Nat. Prod. Sci., 2011, 17, 206–211 CAS
.
- J. Lee, S. J. Lee and K. T. Lim, Food Chem. Toxicol., 2012, 50, 2109–2117 CrossRef CAS PubMed
.
- H. Y. Joo and K. T. Lim, Environ. Toxicol. Pharmacol., 2009, 27, 247–252 CrossRef CAS PubMed
.
- H. Y. Joo and K. T. Lim, Korean J. Food Sci. Technol., 2009, 41, 93–99 Search PubMed
.
- P. S. Oh and K. T. Lim, J. Appl. Toxicol., 2009, 29, 496–504 CrossRef CAS PubMed
.
- Editorial Board of Flora of China, Flora of China, Science Publishing House, Beijing, 2003, vol. 5, pp. 35–36 Search PubMed
.
- W. Y. Xiong, J. Z. Wang and T. D. Shi, Woody Medicine Plants of China, Shanghai: Shanghai Science and Education Press, Shanghai, 1993, pp. 85−88 Search PubMed
.
- L. Shi, Asian J. Exp. Biol. Sci., 2010, 1, 311–314 Search PubMed
.
- M. Aleksandar, Journal of Mountain Agriculture on the Balkans, 2016, 19, 134–147 Search PubMed
.
- H. J. Choi, C. T. Kim, Y. D. Min and M. J. Rang, J. Med. Plants Res., 2013, 14, 7–14 Search PubMed
.
- Z. Q. Chen, Ben Cao Shi Yi, People's Medical Publishing House, Beijing, 1994, vol. II Search PubMed
.
- Z. S. Kou, Ben Cao Yan Yi, China Medical Science Press, Beijing, 2005 Search PubMed
.
- Z. M. Mi, Ben Cao Hui Yan, Shanghai Science & Technology Publishing House Press, Shanghai, 2005, p. 630 Search PubMed
.
- B. D. Xiao, Lingnan Caiyao Lu, Guangdong Science Publishing House Press, Guangdong, 2009 Search PubMed
.
- N. H. Li, Chinese Medicinal Herbs of Hong Kong, The Commercial Press, Hong Kong, 2002 Search PubMed
.
- H. S. Guan and S. G. Wang, Chinese Marine Materia Medica, Science and Technology Press, Shanghai, China, 2009, p. 371 Search PubMed
.
- Donguibogam Committee, Translated Donguibogam, Bubinmunwha Press, Seoul, 1999 Search PubMed
.
- B. W. Lee, S. W. Gal, K. M. Park and K. H. Park, J. Nat. Prod., 2005, 68, 456–458 CrossRef CAS PubMed
.
- J. H. Hwang, S. S. Hong, X. H. Han, J. S. Hwang, D. Lee, H. Lee, Y. P. Yun, Y. Kim, J. S. Ro and B. Y. Hwang, J. Nat. Prod., 2007, 70, 1207–1209 CrossRef CAS PubMed
.
- G. S. Jeong, R. B. An, H. O. Pae, H. T. Chung, K. H. Yoon, D. G. Kang, H. S. Lee and Y. C. Kim, Planta Med., 2008, 74, 1368–1373 CrossRef CAS PubMed
.
- E. J. Seo, M. J. Curtis-Long, B. W. Lee, H. Y. Kim, Y. B. Ryu, T. Jeong, W. S. Lee and K. H. Park, Bioorg. Med. Chem. Lett., 2007, 17, 6421–6424 CrossRef CAS PubMed
.
- Y. B. Ryu, M. J. Curtis-Long, J. W. Lee, J. H. Kim, J. Y. Kim, K. Y. Kang, W. S. Lee and K. H. Park, Bioorg. Med. Chem., 2009, 17, 2744–2750 CrossRef CAS PubMed
.
- P. J. O'Brien, Chem.-Biol. Interact., 1991, 80, 1–41 CrossRef
.
- Y. S. Zou, A. J. Hou and G. F. Zhu, Chem. Biodiversity, 2005, 2, 131–138 CAS
.
- B. W. Lee, J. H. Lee, S. T. Lee, H. S. Lee, W. S. Lee, T. S. Jeong and K. H. Park, Bioorg. Med. Chem. Lett., 2005, 15, 5548–5552 CrossRef CAS PubMed
.
- M. B. Wang, J. M. Huang and A. J. Hou, Fudan University Journal of Medical Sciences, 2006, 33, 559–562 CAS
.
- L. Chen, Y. Duan, C. Li, Y. Wang, X. Tong, Y. Dai and X. Yao, Magn. Reson. Chem., 2013, 51, 842–846 CrossRef CAS PubMed
.
- I. K. Lee, C. J. Kim, K. S. Song, H. M. Kim, I. D. Yoo, H. Koshino, Y. Esumi and M. Uramoto, J. Nat. Prod., 1995, 58, 1614–1617 CrossRef CAS
.
- J. Hošek, M. Bartos, S. Chudík, S. Dall'Acqua, G. Innocenti, M. Kartal, L. Kokoška, P. Kollăr, Z. Kutil, P. Landa, R. Marek, V. Závalová, M. Žemlička and K. Šmejkal, J. Nat. Prod., 2011, 74, 614–619 CrossRef PubMed
.
- H. J. Lee, Q. S. Auh, Y. M. Lee, S. K. Kang, S. W. Chang, D. S. Lee, Y. C. Kim and E. C. Kim, Planta Med., 2013, 79, 1298–1306 CrossRef CAS PubMed
.
- D. S. Lee, W. Ko, D. C. Kim, Y. C. Kim and G. S. Jeong, Molecules, 2014, 19, 10818–10831 CrossRef PubMed
.
- B. W. Lee, N. S. Kang and K. H. Park, J. Korean Soc. Appl. Biol. Chem., 2004, 47, 270–273 CAS
.
- Y. Z. Yin, R. D. Chen, D. W. Zhang, L. R. Qiao, J. H. Li, R. S. Wang, X. Liu, L. Yang, D. Xie, J. H. Zou, C. M. Wang and J. G. Dai, J. Mol. Catal. B: Enzym., 2013, 89, 28–34 CrossRef CAS
.
- R. S. Wang, R. D. Chen, J. H. Li, X. Liu, K. B. Xie, D. W. Chen, Y. Z. Yin, X. Y. Tao, D. Xie, J. H. Zou, L. Yang and J. G. Dai, J. Biol. Chem., 2014, 289, 35815–35825 CrossRef CAS PubMed
.
- Y. H. Jo, S. B. Kim, Q. Liu, S. G. Do, B. Y. Hwang and M. K. Lee, PLoS One, 2017, 12, e0172069 Search PubMed
.
- X. X. Huang, H. F. Lai and Z. W. Li, Her. Med., 2013, 32, 664–666 CAS
.
- B. Li, M. Wang, Y. N. Tan, M. M. Tong and Y. J. Zhai, Zhongguo Zhongyao Zazhi, 2013, 38, 167–170 CAS
.
- J. S. Jeon, S. M. Kim, H. J. Lee, B. H. Um, H. K. Kim and C. Y. Kim, J. Liq. Chromatogr. Relat. Technol., 2012, 35, 1607–1615 CAS
.
- G. T. Jung, I. O. Ju, S. R. Choi, D. H. You and J. J. Noh, Korean Journal of Food Preservation, 2013, 20, 330–335 CrossRef
.
- L. Shi and Y. Fu, Acta Biochim. Biophys. Sin., 2011, 43, 418–424 CrossRef CAS PubMed
.
- L. Shi, K. Chen, Q. Dong, J. Fang and K. Ding, Front. Chem. China, 2008, 3, 209–212 CrossRef
.
- L. Shi, Y. L. Fu and K. S. Chen, Fitoterapia, 2007, 78, 298–301 CrossRef CAS PubMed
.
- L. Shi, Q. Dong and K. Ding, Food Chem., 2014, 152, 291–296 CrossRef CAS PubMed
.
- Z. P. Zheng, H. Y. Tan, J. Chen and M. Wang, Fitoterapia, 2013, 84, 242–247 CrossRef CAS PubMed
.
- V. K. Bajpai, A. Sharma and K. H. Baek, Food Control, 2013, 32, 582–590 CrossRef CAS
.
- E. N. Novruzov and U. M. Agamirov, Chem. Nat. Compd., 2002, 38, 468–469 CrossRef CAS
.
- E. J. Cho, T. Yokozawa, D. Y. Rhyu, S. C. Kim, N. Shibahara and J. C. Park, Phytomedicine, 2003, 10, 544–551 CrossRef CAS PubMed
.
- H. S. Han, S. Y. Kim, D. J. Lim and W. K. Whang, Asian Journal of Beauty and Cosmetology, 2016, 14, 317–328 CrossRef
.
- T. J. Kim, H. J. Han, S. S. Hong, J. H. Hwang, B. Y. Hawang, H. S. Yoo, Y. R. Jin, J. J. Lee, J. Y. Yu, K. H. Lee, B. W. Kang and Y. P. Yun, Biol. Pharm. Bull., 2007, 30, 805–809 CAS
.
- H. J. Han, T. J. Kim, Y. R. Jin, S. S. Hong, J. H. Hwang, B. Y. Hwang, K. H. Lee, T. K. Park and Y. P. Yun, Planta Med., 2007, 73, 1163–1168 CrossRef CAS PubMed
.
- J. E. Kim and K. W. Lee, Int. J. Mol. Sci., 2015, 16, 25096–25107 CrossRef CAS PubMed
.
- G. Yang, K. Lee, M. Lee, I. Ham and H. Y. Choi, BMC Complementary Altern. Med., 2012, 12, 250 CrossRef CAS
.
- W. G. Seo, H. O. Pae, G. S. Oh, K. Y. Chai, Y. G. Yun, T. O. Kwon and H. T. Chung, Gen. Pharmacol. Vasc. Syst., 2000, 35, 21–28 CrossRef CAS
.
- E. G. Lee, H. J. Yun, S. I. Lee and W. H. Yoo, Korean J. Intern. Med., 2010, 25, 93–100 CrossRef PubMed
.
- E. G. Lee, S. Lee, H. J. Chae, S. J. Park, Y. C. Lee and W. H. Yoo, Biol. Res., 2010, 43, 225–231 Search PubMed
.
- H. L. T. Anh, D. T. Tuan, D. T. Trang, B. H. Tai, N. X. Nhiem, P. H. Yen, P. V. Kiem, C. V. Minh, T. M. Duc, H. K. Kang, Y. C. Kim and Y. H. Kim, J. Asian Nat. Prod. Res., 2016, 1–9 Search PubMed
.
- Y. H. Jo, S. B. Kim, Q. Liu, B. Y. Hwang and M. K. Lee, Arch. Pharm., 2017, 350, e1600263 CrossRef PubMed
.
- S. K. Ku, M. S. Han, G. S. Jeong and J. S. Bae, Anim. Cells Syst., 2014, 18, 9–16 CrossRef CAS
.
- S. J. Galli, M. Tsai and A. M. Piliponsky, Nature, 2008, 454, 445–454 CrossRef CAS PubMed
.
- T. Lee, J. Kwon, D. Lee and W. Mar, J. Agric. Food Chem., 2015, 63, 5459–5467 CrossRef CAS PubMed
.
- J. U. Shim and K. T. Lim, Inflammation, 2009, 32, 211–217 CrossRef CAS PubMed
.
- J. Lee and K. T. Lim, Naunyn-Schmiedeberg's Arch. Pharmacol., 2010, 382, 51–61 CrossRef CAS PubMed
.
- C. H. Park and K. T. Lim, Immunol. Invest., 2010, 39, 171–185 CrossRef CAS PubMed
.
- P. S. Oh and K. T. Lim, J. Cell. Biochem., 2010, 109, 124–131 CAS
.
- P. S. Oh, K. Lim and K. T. Lim, Cell Biochem. Funct., 2010, 28, 352–359 CrossRef CAS PubMed
.
- C. H. Jeong, G. N. Choi, J. H. Kim, J. H. Kwak, H. J. Heo, K. H. Shim, B. R. Cho, Y. Bae and J. S. Choi, Prev. Nutr. Food Sci., 2009, 14, 283–289 CrossRef CAS
.
- J. Y. Kim, J. H. Chung, I. Hwang, Y. S. Kwan, J. K. Chai, K. H. Lee, T. H. Han and J. H. Moon, Korean J. Hortic. Sci. Technol., 2009, 27, 489–496 CAS
.
- Y. Lee, J. Oh, H. Lee, N. K. Lee, D. Y. Jeong and Y. S. Jeong, Biotechnol. Bioprocess Eng., 2015, 20, 861–870 CrossRef CAS
.
- Y. Lee, J. Oh and Y. S. Jeong, Food Sci. Biotechnol., 2015, 24, 1817–1821 CrossRef CAS
.
- N. S. Oh, J. Y. Lee, S. Oh, J. Y. Joung, S. G. Kim, Y. K. Shin, K. W. Lee, S. H. Kim and Y. Kim, J. Dairy Sci., 2016, 99, 1–12 CrossRef PubMed
.
- N. S. Oh, J. Y. Lee, J. Y. Joung, K. S. Kim, Y. K. Shin, K. W. Lee, S. H. Kim, S. Oh and Y. Kim, Appl. Microbiol. Biotechnol., 2016, 100, 5919–5932 CrossRef CAS PubMed
.
- S. H. Nile and D. H. Kim, Nat. Prod. Commun., 2015, 10, 1839–1842 Search PubMed
.
- G. R. Shin, S. Lee, S. Lee, S. Do, E. Shin and C. H. Lee, Ind. Crops Prod., 2015, 70, 322–331 CrossRef CAS
.
- J. H. Lee, B. W. Lee, J. H. Kim, W. D. Seo, K. C. Jang and K. H. Park, J. Appl. Biol. Chem., 2005, 48, 193–197 CAS
.
- W. G. Seo, H. O. Pae, G. S. Oh, K. Y. Chai, Y. G. Yun, H. T. Chung, K. K. Jang and T. O. Kwon, Am. J. Chin. Med., 2001, 29, 313–320 CrossRef CAS PubMed
.
- S. B. Kwon, M. J. Kim, J. M. Yang, H. P. Lee, J. T. Hong, H. S. Jeong, E. S. Kim and D. Y. Yoon, PLoS One, 2016, 11, e0150235 Search PubMed
.
- Z. Zhang, H. J. Wu, N. H. Pi, T. Zhang, W. Y. Yuan, A. J. Hou and J. H. Liu, World Clin. Drugs, 2009, 30, 601–605 CAS
.
- Y. H. Rho, S. H. Yoon, E. K. Kim, J. Y. Kang, B. W. Lee, K. H. Park and Y. S. Bae, Nat. Prod. Res., 2007, 21, 616–624 CrossRef CAS PubMed
.
- Y. H. Rho, B. W. Lee, K. H. Park and Y. S. Bae, Anti-Cancer Drugs, 2007, 18, 1023–1028 CrossRef CAS PubMed
.
- S. H. Kim, S. H. Yoon, B. W. Lee, K. H. Park, Y. H. Kim and Y. S. Bae, Oncol. Res., 2005, 15, 327–332 CAS
.
- H. J. Lee, S. S. Jue, S. K. Kang, W. J. Bae, Y. C. Kim and E. C. Kim, Am. J. Chin. Med., 2015, 43, 1–14 CrossRef PubMed
.
- M. R. Shin, H. J. Lee, S. K. Kang, Q. S. Auh, Y. M. Lee, Y. C. Kim and E. C. Kim, BioMed Res. Int., 2014, 2014, 934691 Search PubMed
.
- S. M. Jeon, D. S. Lee and G. S. Jeong, J. Ethnopharmacol., 2016, 194, 57–62 CrossRef CAS PubMed
.
- Y. J. Choi, H. M. Kim and H. D. Kim, Arch. Pharmacal Res., 2009, 32, 59–63 CrossRef CAS PubMed
.
- Y. H. Jo, B. Shin, Q. Liu, K. Y. Lee, D. C. Oh, B. Y. Hwang and M. K. Lee, J. Nat. Prod., 2014, 77, 2361–2366 CrossRef CAS PubMed
.
- C. H. Jeong, G. N. Choi, J. H. Kim, J. H. Kwak, H. R. Jeong, D. O. Kim and H. J. Heo, Food Sci. Biotechnol., 2010, 19, 1113–1117 CrossRef
.
- X. H. Han, S. S. Hong, J. S. Hwang, S. H. Jeong, J. H. Jeong, J. H. Hwang, M. H. Lee, M. K. Lee, D. Lee, J. S. Ro and B. Y. Hwang, Arch. Pharmacal Res., 2005, 28, 1324–1327 CrossRef CAS
.
- D. W. Kim, J. Kwon, S. J. Sim, D. Lee and W. Mar, J. Funct. Foods, 2017, 29, 104–114 CrossRef CAS
.
- D. W. Kim, K. T. Lee, J. Kwon, H. J. Lee, D. Lee and W. Mar, Life Sci., 2015, 130, 25–30 CrossRef CAS PubMed
.
- C.
S. Yoon, D. C. Kim, T. H. Quang, J. Seo, D. G. Kang, H. S. Lee, H. Oh and Y. C. Kim, Molecules, 2016, 21, 1–12 CrossRef PubMed
.
- B. H. Kim, J. Kwon, D. Lee and W. Ma, Planta Med. Lett., 2015, 2, e15–e18 CrossRef
.
- J. Y. Lim, B. Y. Hwang, K. W. Hwang and S. Y. Park, Phytother. Res., 2012, 26, 1948–1956 CrossRef CAS PubMed
.
- Y. H. Jo, S. B. Kim, Q. Liu, J. W. Lee, B. Y. Hwang and M. K. Lee, Bioorg. Med. Chem. Lett., 2015, 25, 3455–3457 CrossRef CAS PubMed
.
- T. H. Quang, N. T. T. Ngan, C. S. Yoon, K. H. Cho, D. G. Kang, H. S. Lee, Y. C. Kim and H. Oh, Molecules, 2015, 20, 11173–11183 CrossRef CAS PubMed
.
- D. H. Suh, E. S. Jung, H. M. Park, S. H. Kim, S. Lee, Y. H. Jo, M. K. Lee, G. Jung, S. G. Do and C. H. Lee, PLoS One, 2016, 11, e0149022 Search PubMed
.
- Y. H. Jo, K. M. Choi, Q. Liu, S. B. Kim, H. J. Ji, M. Kim, S. K. Shin, S. G. Do, E. Shin, G. Jung, H. S. Yoo, B. Y. Hwang and M. K. Lee, Nutrients, 2015, 7, 10480–10490 CrossRef CAS PubMed
.
- K. B. Lee, K. S. Song, E. H. Moon, J. Lee and Y. C. Yoo, J. Korean Soc. Appl. Biol. Chem., 2009, 52, 234–240 CrossRef
.
- P. S. Oh and K. T. Lim, Naunyn-Schmiedeberg's Arch. Pharmacol., 2009, 380, 115–124 CrossRef CAS PubMed
.
- W. Palinski, M. E. Rosenfeld, S. Ylä-Herttuala, G. C. Gurtner, S. S. Socher, S. W. Butler, S. Parthasarathy, T. E. Carew, D. Steinberg and J. L. Witztum, Proc. Natl. Acad. Sci. U. S. A., 1989, 86, 1372–1376 CrossRef CAS
.
- Y. S. Park, S. H. Kim, S. Y. Kim, G. M. Koh, J. H. Suh and J. S. Kang, Pharmacol. Pharm., 2016, 7, 358–367 CrossRef
.
- H. Lee, H. Ha, J. K. Lee, C. Seo, N. Lee, D. Jung, S. Park and H. K. Shin, Phytother. Res., 2012, 26, 594–599 CrossRef PubMed
.
- S. T. Hu, Z. P. Zheng, X. C. Zhang, F. Chen and M. F. Wang, J. Funct. Foods, 2015, 13, 375–383 CrossRef CAS
.
- O. K. Kim, W. J. Jun and J. M. Lee, Nutrients, 2016, 8, 60 CrossRef PubMed
.
- D. S. Lee and G. S. Jeong, Int. Immunopharmacol., 2014, 21, 26–33 CrossRef CAS PubMed
.
- D. G. Kang, T. Y. Hur, G. M. Lee, H. Oh, T. O. Kwon, E. J. Sohn and H. S. Lee, Life Sci., 2002, 70, 2599–2609 CrossRef CAS PubMed
.
- Y. B. Ryu, M. J. Curtis-Long, J. W. Lee, H. W. Ryu, J. Y. Kim, W. S. Lee and K. H. Park, Bioorg. Med. Chem. Lett., 2009, 19, 4912–4915 CrossRef CAS PubMed
.
- J. Sim, E. Choi, G. S. Jeong and S. Lee, Biopharm. Drug Dispos., 2015, 36, 325–336 CrossRef CAS PubMed
.
- J. Sim, E. Choi, Y. M. Lee, G. S. Jeong and S. Lee, Food Chem. Toxicol., 2015, 81, 171–175 CrossRef CAS PubMed
.
- N. H. Pi, J. Chen, J. M. Huang and A. J. Hou, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2010, 878, 1953–1958 CrossRef PubMed
.
- J. Xu, H. B. Chen and S. L. Li, Med. Res. Rev., 2016, 1–46 Search PubMed
.
- C. T. Cao, C. L. Sun, W. B. Bai, J. X. Sun, G. Q. Li, S. Y. Ou and Y. Yang, Shipin Kexue, 2015, 36, 48–51 CAS
.
- C. Lei, C. C. Liu, E. H. Pi and A. J. Hou, Helv. Chim. Acta, 2014, 97, 1683–1688 CrossRef CAS
.
- T. Nomura, Y. Hano and T. Fujimoto, Heterocycles, 1983, 20, 213–218 CrossRef CAS
.
- J. Kwon, N. T. Hiep, D. W. Kim, S. Hong, Y. Guo, B. Y. Hwang, H. J. Lee, W. Mar and D. Lee, J. Nat. Prod., 2016, 79, 1938–1951 CrossRef CAS PubMed
.
- Y. Hano, Y. Matsumoto, J. Y. Sun and T. Nomura, Planta Med., 1990, 56, 399–402 CrossRef CAS PubMed
.
- Y. Hano, Y. Matsumoto, J. Y. Sun and T. Nomura, Planta Med., 1990, 56, 478–481 CrossRef CAS PubMed
.
- Y. Hano, Y. Matsumoto, K. Shinohara, J. Y. Sun and T. Nomura, Planta Med., 1991, 57, 172–175 CrossRef CAS PubMed
.
- Z. P. Zheng, J. Y. Liang and L. H. Hu, J. Integr. Plant Biol., 2006, 48, 996–1000 CrossRef CAS
.
- T. Fujimoto, Y. Hano, T. Nomura and J. Uzawa, Planta Med., 1984, 50, 161–163 CrossRef CAS PubMed
.
- Y. Hano, Y. Matsumoto, K. Shinohara, J. Y. Sun and T. Nomura, Heterocycles, 1990, 31, 1339–1344 CrossRef CAS
.
- W. C. Wu, Y. J. Zhai and Z. Y. Li, Zhongyaocai, 2010, 33, 913–915 CAS
.
- Y. Z. Yin, R. S. Wang, R. D. Chen, L. R. Qiao, L. Yang, C. M. Wang and J. G. Dai, Zhongguo Zhongyao Zazhi, 2012, 37, 3734–3737 CAS
.
- H. S. Young, J. H. Park, H. J. Park and J. S. Choi, Arch. Pharmacal Res., 1989, 12, 39–41 CrossRef CAS
.
- G. M. Zhang, X. Y. Xu and J. F. Xi, Zhongchengyao, 2008, 30, 771–773 CAS
.
- Y. J. Lee, S. Kim, S. J. Lee, I. Ham and W. K. Whang, Arch. Pharmacal Res., 2009, 32, 195–200 CrossRef CAS PubMed
.
- B. W. Lee, J. H. Lee, S. W. Gal, Y. H. Moon and K. H. Park, Biosci., Biotechnol., Biochem., 2006, 70, 427–432 CrossRef CAS PubMed
.
- T. Fujimoto and T. Nomura, Planta Med., 1985, 51, 190–193 CrossRef
.
- W. G. Shan, L. L. Shi, Y. M. Ying, X. R. Hou and Z. J. Zhan, J. Chem. Res., 2013, 37, 285–286 CrossRef CAS
.
- Y. Guan, Z. Yin, L. Guo, X. Huang, W. Ye and W. Shen, Zhongguo Zhongyao Zazhi, 2009, 34, 1108–1110 CAS
.
- A. K. Ibrahim, A. A. Mohamed and T. Mukul, World J. Pharm. Res., 2014, 3, 981–997 Search PubMed
.
- Y. Kang, J. U. Choi, E. A. Lee and H. R. Park, Food Sci. Biotechnol., 2013, 22, 1449–1452 Search PubMed
.
- H. J. Lee, S. T. Cho, T. G. Lee, U. J. Kim, E. C. Ma and D. H. Lee, Repub. Korean Kongkae Taeho Kongbo, 2015, 5, 33 Search PubMed
.
- C. P. Li, X. J. Chang, L. Fang, J. B. Yao, R. W. Wang, Z. J. Zhan, Y. M. Ying and W. G. Shan, Chem. Nat. Compd., 2016, 52, 202–204 CrossRef CAS
.
- A. P. Wang, M. C. Liu and S. J. Yang, Chin. J. Exp. Tradit. Med. Formulae, 2011, 17, 113–115 CAS
.
- C. H. Miu, Z. B. Gu and G. J. Yang, Zhongchengyao, 2002, 24, 211–212 Search PubMed
.
- Y. H. Zhang, W. W. Ren and S. W. Wan, Yiyao Gongye, 1980, 3, 15 Search PubMed
.
- N. T. Hiep, J. Kwon, D. W. Kim, S. Hong, Y. Guo, B. Y. Hwang, N. Kim, W. Mar and D. Lee, Tetrahedron, 2017, 73, 2747–2759 CrossRef CAS
.
Footnote |
| † These authors have contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |