Negishi reaction in BODIPY dyes. Unprecedented alkylation by palladium-catalyzed C–C coupling in boron dipyrromethene derivatives†
Gonzalo Duran-Sampedroa,
Eduardo Palaoa,
Antonia R. Agarrabeitiaa,
Santiago de la Moyaa,
Noël Boensb and
María J. Ortiz*a
aDepartment of Organic Chemistry I, Complutense University of Madrid, Ciudad Universitaria s/n, 28040, Madrid, Spain. E-mail: mjortiz@ucm.es; Fax: +34 91 394 4103; Tel: +34 91 394 4309
bDepartment of Chemistry, Katholieke Universiteit Leuven, Celestijnenlaan 200f – bus 02404, 3001 Leuven, Belgium
First published on 14th April 2014
Abstract
Negishi reactions of 3-halogen and 3,5-dihalogen substituted BODIPYs with different organozinc reagents are reported as the first examples of this valuable palladium-catalyzed C–C coupling reaction into the family of the BODIPY dyes. It is demonstrated that the Negishi coupling is especially useful for obtaining interesting alkylated BODIPYs, including synthetically-valuable asymmetrically-3,5-disubstituted BODIPYs.
BODIPY (boron dipyrrin or boron dipyrromethene) dyes constitute one of the most important families of luminophores, due to their easily tunable absorption and emission properties.1 These systems are highly interesting for the development of valuable photonic applications, such as chemosensors and probes, biological labels, laser dyes, potential photodynamic therapy agents, and a plethora of photonic devices, including solar light harvesting antennas or solar cells.2 Additionally, chiral BODIPYs exhibiting some particular chiroptical properties (e.g., a clearly bisignated dichroic signal in the visible region) have been recently highlighted as interesting dyes for the development of useful technologies (e.g., CPL-based sensing).3 There is, therefore, an understandable interest in the synthesis of new BODIPY derivatives, not only for improving useful photonic properties, but also for revealing the key structural factors ruling them.
BODIPY dyes can be obtained by using two general methodologies: (1) functionalization of pyrroles which are used as precursors of the desired BODIPY after final boron complexation (pre-functionalization),1a,c,4 and (2) functionalization of the BODIPY core (post-functionalization).1a–c,4b–d,5 The post-functionalization methodology is highly attractive for expanding the diversity of the BODIPY family, especially for some typologies which are difficult to obtain directly by pre-functionalization.2e,6 However, functionalizing the BODIPY core is not trivial, and in many cases critical problems arise concerning the control of the BODIPY reactivity (lack of reactivity, uncontrolled reactivity, etc.).
Many of the most important BODIPY functionalization reactions are based on the use of halogen-substituted BODIPYs. Significantly, 3-halo and 3,5-dihaloBODIPYs have been extensively used, because they can be easily prepared by controlled electrophilic aromatic substitution (SEAr) reactions in dipyrromethane precursors7 and, afterwards, submitted to nucleophilic substitution with alcohols, amines or enolates to give rise to the corresponding substituted BODIPYs.3,7a–c,8 Moreover, 3-halo and 3,5-dihaloBODIPYs have been also used as convenient precursors of interesting carbon-substituted BODIPYs through palladium-catalyzed C–C coupling reactions. Thus, Dehaen et al. have reported the use of the Stille, Suzuki, Heck and Sonogashira reactions for the preparation of valuable aryl, alkenyl and alkynyl BODIPYs, with fluorescence spanning the visible spectrum, from a 3,5-dichloroBODIPY.9 On the other hand, Ravikanth et al. have recently reported the preparation of several symmetric and asymmetric BODIPY derivatives, with interesting photophysical and electrochemical properties, by Sonogashira and Suzuki reactions of the corresponding 3,5-dihaloBODIPYs precursor.10
It should be noted that, to the best of our knowledge, only aryl, alkenyl or alkynyl derivatives were obtained by applying the above-mentioned palladium-catalyzed C–C couplings in BODIPYs.1a–c,9−11 Strikingly, alkyl BODIPYs were not reported by using those reactions, despite these derivatives are very interesting dyes for many different technological applications (e.g., biological labelling and molecular probing), being mainly prepared through to complex pre-functionalization routes instead.12
On the other hand, Negishi reaction in BODIPYs is unprecedented, although it would allow the preparation of alkyl derivatives, as the Suzuki one would, but with the valuable advantage of a high functional-group compatibility (including the labile BODIPY BF2 group), due to the nature of the Negishi-required organozinc reagents.13
The above mentioned facts prompted us to essay the workability of the Negishi reaction in the BODIPY family, specially directed to the synthesis of alkyl BODIPYs.
Herein we report the coupling reaction of 3-bromo, 3,5-dibromo and 3,5-dichloroBODIPYs 1–3 with different organozinc reagents ([R–Zn], Scheme 1), and demonstrate its versatility for obtaining carbon-substituted BODIPYs, including alkylated and asymmetric derivatives.
HaloBODIPYs 1–3 (Scheme 1) were obtained straightforwardly by previously described pre-functionalization routes based on SEAr reactions.7a–d Highly accessible [R–Zn] and common Pd(PPh3)2Cl2 were used for the Negishi reactions tested. The results obtained are shown in Table 1.
| Entry | Halo-BODIPY | [Zn–R] (reaction conditions)a | Major product (R/Y or R/R) | Yieldb (%) |
|---|---|---|---|---|
| a See reaction conditions in ESI.b Isolated yield.c 4f/4g = 2/1, determined by 1H NMR (see ESI). | ||||
| 1 | 1 | ZnEt2 (N) | 4a (Et/H) | 64 |
| 2 | 2 | ZnEt2 (N) | 5a (Et/Et) | 86 |
| 3 | 2 | ZnEt2 (C) | 4b (Et/Br) | 61 |
| 4 | 3 | ZnEt2 (N) | 5a (Et/Et) | 73 |
| 5 | 3 | ZnEt2 (C) | 4c (Et/Cl) | 75 |
| 6 | 3 | ZnMe2 (N) | 5b (Me/Me) | 80 |
| 7 | 3 | ZnMe2 (C) | 4d (Me/Cl) | 77 |
| 8 | 3 | BuZnBr (N) | 5c (Bu/Bu) | 52 |
| 9 | 3 | BuZnBr (C) | 4e (Bu/Cl) | 62 |
| 10 | 3 | Zn(iPr)2 (C) | 4f (iPr/Cl)/4g (Pr/Cl)c | 70c |
| 11 | 3 | BnZnBr (N) | 5d (Bn/Bn) | 20 |
| 12 | 3 | BnZnBr (C) | 4h (Bn/Cl) | 18 |
| 13 | 3 | PhZnBr (N) | 5e (Ph/Ph) | 56 |
| 14 | 3 | PhZnBr (C) | 4i (Ph/Cl) | 70 |
| 15 | 3 | PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CZnBr (N) CZnBr (N) |
5f (PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C/PhC C/PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) C) |
54 |
| 16 | 3 | PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CZnBr (C) CZnBr (C) |
4j (PhC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C/Cl) C/Cl) |
56 |
| 17 | 3 | TMSC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CZnBr (N) CZnBr (N) |
5g (TMSC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C/TMSC C/TMSC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) C) |
70 |
| 18 | 4d | BuZnBr (N) | 5h (Me/Bu) | 65 |
Negishi reactions were conducted under standard reaction conditions to reach the highest level of C–C coupling (normal conditions, N), or under controlled conditions (C, mainly by controlling the stoichioimetry and the reaction time) to reach the highest level of mono-coupling when 3,5-dibromoBODIPY 2 or 3,5-dichloroBODIPY 3 are used as starting halogenated BODIPYs (see Experimental details in ESI†).
Most of the Negishi alkylations tested took place satisfactorily with high yields (Table 1, entries 1–10), demonstrating that reaction control for mono-coupling of 3,5-dihaloBODIPY 2 and 3 (entries 3, 5, 7 and 9) is possible. No significant differences in reactivity were found between starting 3,5-dibrominated and 3,5-dichlorinated BODIPYs (entries 2–5). For BODIPY isopropylation (entry 10), the expected isopropyl to propyl isomerization was detected, which could be avoided by using an appropriated, more sophisticated palladium catalyst (Pd-PEPPSI-IPentCl).14 In contrast, benzylations took place with low yields (entries 11 and 12), although conversion of starting 3,5-dihaloBODIPY was almost complete. This can be accounted for by the high reactivity (methylene acidity) of the obtained benzylated 5d and 4h. Nonetheless, this reactive property could be used in the future for the easy preparation of new BODIPY derivatives, following the carbanion based BODIPY post-functionalization methodology described by Ziessel et al.5b It must be noted that, according to our knowledge, 5d and 4h are the first benzylated BODIPYs described up to date.
Arylations and alkynylations by Negishi reaction (entries 13–17 in Table 1) were also conducted for the comparison with other related palladium-catalyzed C–C coupling reactions. Thus, the yields in the preparation of phenylated 5e and 4i by Negishi reactions (entries 13 and 14) were only slightly higher than those reported previously by Stille reactions by Dehaen et al.9 (56 vs. 50%, and 70 vs. 63%, respectively), but avoiding the use of the more toxic organotin reagent required for the latter. On the other hand, the yields in the preparation of phenylethynylBODIPYs 5f and 4j by Negishi reactions (entries 15 and 16) were similar to those obtained previously by Sonogashira reactions9 (54 vs. 57%, and 56 vs. 59%, respectively). Finally, the yield in preparing (trimethylsilyl)ethynylated 5g from 3 by Negishi reaction (entry 17) was higher than that the reported by Ravikanth et al.,10 starting from 2 and using the Sonogashira reaction (70 vs. 60%).
An interesting application of the Negishi coupling is the preparation of asymmetrically substituted 3,5-dialkylBODIPYs. As an example, methylated chloroBODIPY 4d (entry 7 in Table 1) was used as intermediate in the preparation of asymmetrically dialkylated (5-methyl and 3-butyl) 5h (entry 18 in Table 1), which was obtained with high overall yield (50%), from readily available 3,5-dichloroBODIPY 3.
Finally, the study of the photophysical properties for the novel alkylated BODIPYs was also conducted (Fig. 1 and S1 in ESI†).
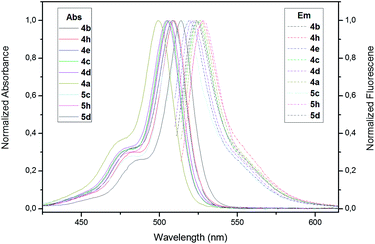 | ||
| Fig. 1 A selection of the normalized visible absorption spectra and corresponding fluorescence emission spectra of the new compounds in AcOEt. | ||
The narrow absorption and emission spectra of the dyes in ethyl acetate (AcOEt) solution are in full accord with those of classic BODIPY dyes,1,15 with absorption and emission maxima around 500 and 520 nm, respectively. As found for common difluoroboron dipyrrins, the Stokes shifts are quite small.15 The fluorescence quantum yields (Φ) are lower than the measured for commercial PM546, which is used as reference (Table 2). The rather low Φ values can be accounted for by the enhanced deactivation of the singlet excited state, due to the rotational mobility of the p-tolyl group at the 8-position.16
| BODIPY | λabs(max) (nm) | λem(max) (nm) | Δν (cm−1) | Φ |
|---|---|---|---|---|
| a Absorption (λabs) and fluorescence emission (λem) wavelength at the maximum, Stokes shift (Δν) and fluorescence quantum yield (Φ). | ||||
| PM546 | 494 | 504 | 400 | 0.85 |
| 4a | 500 | 516 | 620 | 0.02 |
| 4b | 509 | 528 | 707 | 0.07 |
| 4c | 505 | 522 | 645 | 0.14 |
| 4d | 505 | 519 | 534 | 0.11 |
| 4e | 506 | 523 | 642 | 0.12 |
| 4h | 509 | 523 | 526 | 0.17 |
| 5c | 509 | 525 | 599 | 0.12 |
| 5d | 514 | 524 | 371 | 0.13 |
| 5h | 506 | 521 | 569 | 0.13 |
In summary, we report the first examples of the Negishi C–C coupling reaction in BODIPYs (3-halo and 3,5-dihaloBODIPYs), highlighting its workability for obtaining alkylated BODIPY dyes, including synthetically-valuable asymmetrically-3,5-disubstituted derivatives. We are convinced that the well-known functional group compatibility of the organozinc reagents augurs a promising future for the Negishi reaction when applied to the preparation of functionalized BODIPY dyes (e.g., useful ω-substituted alkyl BODIPYs for biomolecular probing).
Funding from the MINECO of Spain (MAT2010-20646-C04-02) is gratefully acknowledged. G.D.-S. thanks the MICINN of Spain for a predoctoral scholarship (FPI).
Notes and references
- (a) A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS PubMed; (b) R. Ziessel, G. Ulrich and A. Harriman, New J. Chem., 2007, 31, 496–501 RSC; (c) G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS PubMed; (d) A. C. Benniston and G. Copley, Phys. Chem. Chem. Phys., 2009, 11, 4124–4131 RSC.
- (a) M. Benstead, G. H. Mehl and R. W. Boyle, Tetrahedron, 2011, 67, 3573–3601 CrossRef CAS PubMed; (b) N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC; (c) S. G. Awuah and Y. You, RSC Adv., 2012, 2, 11169–11183 RSC; (d) A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77–88 RSC; (e) G. Durán-Sampedro, A. R. Agarrabeitia, L. Cerdán, M. E. Pérez-Ojeda, A. Costela, I. García-Moreno, I. Esnal, J. Bañuelos, I. López Arbeloa and M. J. Ortiz, Adv. Funct. Mater., 2013, 23, 4195–4205 CrossRef.
- (a) E. M. Sánchez-Carnerero, F. Moreno, B. L. Maroto, A. R. Agarrabeitia, J. Bañuelos, T. Arbeloa, I. López-Arbeloa, M. J. Ortiz and S. de la Moya, Chem. Commun., 2013, 49, 11641–11643 RSC; (b) E. M. Sánchez-Carnerero, F. Moreno, B. L. Maroto, A. R. Agarrabeitia, M. J. Ortiz, B. G. Vo, G. Muller and S. de la Moya, J. Am. Chem. Soc., 2014, 136, 3346–3349 CrossRef PubMed.
- (a) J. Bañuelos-Prieto, A. R. Agarrabeitia, I. Garcia-Moreno, I. Lopez-Arbeloa, A. Costela, L. Infantes, M. E. Perez-Ojeda, M. Palacios-Cuesta and M. J. Ortiz, Chem.–Eur. J., 2010, 16, 14094–14105 CrossRef PubMed; (b) C. Yu, L. Jiao, H. Yin, J. Zhou, W. Pang, Y. Wu, Z. Wang, G. Yang and E. Hao, Eur. J. Org. Chem., 2011, 5460–5468 CrossRef CAS; (c) V. Leen, D. Miscoria, S. Yin, A. Filarowski, J. M. Ngongo, M. Van der Auweraer, N. Boens and W. Dehaen, J. Org. Chem., 2011, 76, 8168–8176 CrossRef CAS PubMed; (d) V. Leen, T. Leemans, N. Boens and W. Dehaen, Eur. J. Org. Chem., 2011, 4386–4396 CrossRef CAS; (e) V. Leen, P. Yuan, L. Wang, N. Boens and W. Dehaen, Org. Lett., 2012, 14, 6150–6153 CrossRef CAS PubMed.
- (a) R. Guliyev, A. Coskun and E. U. Akkaya, J. Am. Chem. Soc., 2009, 131, 9007–9013 CrossRef CAS PubMed; (b) G. Ulrich, R. Ziessel and A. Haefele, J. Org. Chem., 2012, 77, 4298–4311 CrossRef CAS PubMed; (c) Z. Kostereli, T. Ozdemir, O. Buyukcakir and E. U. Akkaya, Org. Lett., 2012, 14, 3636–3639 CrossRef CAS PubMed; (d) B. Verbelen, V. Leen, L. Wang, N. Boens and W. Dehaen, Chem. Commun., 2012, 48, 9129–9131 RSC; (e) E. Palao, A. R. Agarrabeitia, J. Bañuelos-Prieto, T. Arbeloa Lopez, I. Lopez-Arbeloa, D. Armesto and M. J. Ortiz, Org. Lett., 2013, 16, 4454–4457 CrossRef PubMed.
- (a) C. Tahtaoui, C. Thomas, F. Rohmer, P. Klotz, G. Duportail, Y. Mély, D. Bonnet and M. Hibert, J. Org. Chem., 2007, 72, 269–272 CrossRef CAS PubMed; (b) T. Lundrigan and A. Thompson, J. Org. Chem., 2013, 78, 757–761 CrossRef CAS PubMed; (c) R. Ziessel, G. Ulrich, A. Haefele and A. Harriman, J. Am. Chem. Soc., 2013, 135, 11330–11344 CrossRef CAS PubMed.
- (a) M. Baruah, W. Qin, N. Basarić, W. M. De Borggraeve and N. Boens, J. Org. Chem., 2005, 70, 4152–4157 CrossRef CAS PubMed; (b) T. Rohand, M. Baruah, W. Qin, N. Boens and W. Dehaen, Chem. Commun., 2006, 266–268 RSC; (c) L. Li, B. Nguyen and K. Burgess, Bioorg. Med. Chem. Lett., 2008, 18, 3112–3116 CrossRef CAS PubMed; (d) V. Lakshmi and M. Ravikanth, Dalton Trans., 2012, 41, 5903–5911 RSC.
- (a) E. Fron, E. Coutiño-Gonzalez, L. Pandey, M. Sliwa, M. Van der Auweraer, F. C. De Schryver, J. Thomas, Z. Dong, V. Leen, M. Smet, W. Dehaen and T. Vosch, New J. Chem., 2009, 33, 1490–1496 RSC; (b) D. W. Domaille, L. Zheng and C. J. Chang, J. Am. Chem. Soc., 2010, 132, 1194–1195 CrossRef CAS PubMed; (c) L. Jiao, W. Pang, J. Zhou, Y. Wei, X. Mu, G. Bai and E. Hao, J. Org. Chem., 2011, 76, 9988–9996 CrossRef CAS PubMed; (d) X. Li, S. Huang and Y. Hu, Org. Biomol. Chem., 2012, 10, 2369–2372 RSC; (e) M. J. Ortiz, A. R. Agarrabeitia, G. Duran-Sampedro, J. Bañuelos Prieto, T. Arbeloa Lopez, W. A. Massad, H. A. Montejano, N. A. García and I. Lopez Arbeloa, Tetrahedron, 2012, 68, 1153–1162 CrossRef CAS PubMed.
- (a) T. Rohand, W. Qin, N. Boens and W. Dehaen, Eur. J. Org. Chem., 2006, 4658–4663 CrossRef CAS; (b) S. Yin, V. Leen, S. V. Snick, N. Boens and W. Dehaen, Chem. Commun., 2010, 46, 6329–6331 RSC.
- (a) M. R. Rao, S. M. Mobin and M. Ravikanth, Tetrahedron, 2010, 66, 1728–1734 CrossRef CAS PubMed; (b) V. Lakshmi and M. Ravikanth, Dyes Pigm., 2013, 96, 665–671 CrossRef CAS PubMed.
- Selected examples: (a) M. J. Ortiz, I. Garcia-Moreno, A. R. Agarrabeitia, G. Duran-Sampedro, A. Costela, R. Sastre, F. Lopez Arbeloa, J. Bañuelos Prieto and I. Lopez Arbeloa, Phys. Chem. Chem. Phys., 2010, 12, 7804–7811 RSC; (b) S. Rihn, M. Erden, A. De Nicola, P. Retailleu and R. Ziessel, Org. Lett., 2011, 13, 1916–1919 CrossRef CAS PubMed; (c) H.-Y. Lin, W.-C. Huang, Y.-C. Chen, H.-H. Chou, C.-Y. Hsu, J. T. Lin and H.-W. Lin, Chem. Commun., 2012, 48, 8913–8915 RSC; (d) L. Wang, J.-W. Wang, A.-J. Cui, X.-X. Cai, Y. Wan, Q. Chen, M.-Y. He and W. Zhang, RSC Adv., 2013, 3, 9219–9222 RSC.
- (a) V. Hornillos, E. Carrillo, L. Rivas, F. Amat-Guerri and A. U. Acuña, Bioorg. Med. Chem. Lett., 2008, 18, 6336–6339 CrossRef CAS PubMed; (b) C. Spies, A.-M. Huynh, V. Huch and G. Jung, J. Phys. Chem. C, 2013, 117, 18163–18169 CrossRef CAS; (c) A. M. Hansen, A. L. Sewell, R. H. Pedersen, C.-L. Long, N. Gadegaard and R. Marquez, Tetrahedron, 2013, 69, 8527–8533 CrossRef CAS PubMed.
- (a) X.-F. Wu, P. Anabarasan, H. Neumann and M. Beller, Angew. Chem., Int. Ed., 2010, 49, 9047–9050 CrossRef CAS PubMed; (b) R. Jana, T. P. Pathak and M. S. Sigman, Chem. Rev., 2011, 111, 1417–1492 CrossRef CAS PubMed; (c) H. Li, C. C. C. Johansson Seechurn and T. J. Colacot, ACS Catal., 2012, 2, 1147–1164 CrossRef CAS.
- M. Pompeo, R. D. J. Froese, N. Hadei and M. G. Organ, Angew. Chem., Int. Ed., 2012, 51, 11354–11357 CrossRef CAS PubMed.
- Selected examples: (a) E. Vos de Wael, J. A. Pardoen, J. A. van Koeveringe and J. Lugtenburg, Recl. Trav. Chim. Pays-Bas, 1977, 96, 306–309 CrossRef CAS; (b) J. Karolin, L. B.-A. Johansson, L. Strandberg and T. Ny, J. Am. Chem. Soc., 1994, 116, 7801–7806 CrossRef CAS; (c) M. Kollmannsberger, K. Rurack, U. Resch-Genger and J. Daub, J. Phys. Chem. A, 1998, 102, 10211–10220 CrossRef CAS; (d) K. Rurack, M. Kollmannsberger and J. Daub, Angew. Chem., Int. Ed, 2001, 40, 385–387 CrossRef CAS; (e) A. Costela, I. García-Moreno, C. Gomez, R. Sastre, F. Amat-Guerri, M. Liras, F. López Arbeloa, J. Bañuelos Prieto and I. López Arbeloa, J. Phys. Chem. A, 2002, 106, 7736–7742 CrossRef CAS; (f) W. Qin, M. Baruah, A. Stefan, M. Van der Auweraer and N. Boens, ChemPhysChem, 2005, 6, 2343–2351 CrossRef CAS PubMed; (g) Z. Dost, S. Atilgan and E. U. Akkaya, Tetrahedron, 2006, 62, 8484–8488 CrossRef CAS PubMed; (h) Z. Ekmekci, M. D. Yilmaz and E. U. Akkaya, Org. Lett., 2008, 10, 461–464 CrossRef CAS PubMed; (i) L. Li, J. Han, B. Nguyen and K. Burgess, J. Org. Chem., 2008, 73, 1963–1970 CrossRef CAS PubMed; (j) L. Jiao, C. Yu, J. Li, Z. Wang, M. Wu and E. Hao, J. Org. Chem., 2009, 74, 7525–7528 CrossRef CAS PubMed; (k) L. Jiao, W. Pang, J. Zhou, Y. Wei, X. Mu, G. Bai and E. Hao, J. Org. Chem., 2011, 76, 9988–9996 CrossRef CAS PubMed.
- H. L. Kee, C. Kirmaier, L. Yu, P. Thamyongkit, W. J. Youngblood, M. E. Calder, L. Ramos, B. C. Noll, D. F. Bocian, W. R. Scheidt, R. R. Birge, J. S. Lindsey and D. Holten, J. Phys. Chem. B, 2005, 109, 20433–20443 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, Fig. S1, as well as 1H and 13C NMR spectra of new compounds. See DOI: 10.1039/c4ra00651h |
| This journal is © The Royal Society of Chemistry 2014 |