Molecular organic conductors with triiodide/hole dual channels as efficient electrolytes for solid-state dye sensitized solar cells†
Yujian
Huang
ab,
Xiaowen
Zhou
a,
Shibi
Fang
a and
Yuan
Lin
*a
aCAS Key Laboratory of Photochemistry, Center for Molecular Science, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: linyuan @ iccas.ac.cn; Fax: +86-10-8261-7315; Tel: +86-10-8261-5031
bGraduate School of Chinese Academy of Sciences, Beijing 100049, China. Fax: +86-10-8261-7315; Tel: +86-10-8261-5031
First published on 8th May 2012
Abstract
Two new solid organic conductors, which can provide dual channels for triiodide/hole transportation, were used as electrolytes for solid-state dye sensitized solar cells (SDSCs), resulting in a high energy conversion efficiency of 3.82%. Hole transport in these SDSCs has a minor contribution to photocurrent due to the oxidation potentials of triphenylamine.
Since O'Regan and Gratzel's pioneering work in 1991,1 research on dye sensitized solar cells (DSCs) has spread rapidly around the world because of their facile fabrication procedure, light weight, cheap cost and an increasing public concern about the shortage of energy supplies.2 The electrolyte, as one of the critical components in DSCs, regenerates dye, following electron injection, and is responsible for charge transport between the photoanode and the counter electrode. So far, the most popular and efficient electrolyte system is that of I−/I3−.1,3 Besides iodide-containing electrolytes, organic hole transport materials as solid-state electrolytes have also been explored for improving the long-term stability of the cells because of their attractive diversity and good hole conductivity, such as 2,2′,7,7′-tetrakis(N,N-di-p-methoxyphenyl-amine)9,9′-spirobifluorene(spiro-MeOTAD),4 tetraphenylbenzidines5 and so on. The combination of iodide/triiodide and hole transport in solid-state electrolytes is expected to provide dual channels for triiodide/hole transportation. It utilizes both the iodide/iodine redox couple and hole transport, which offers ample opportunities for new discoveries and further development of these intriguing systems. However, to the best of our knowledge, the use of electrolytes with such dual channels in DSCs has scarcely appeared in the literature until it was clearly proposed in 2010 by Loh et al.6
In view of the potential importance of the new electrolytes with triiodide/hole dual channels, two solid-state molecular organic conductors containing imidazolium iodide groups and triphenylamine units were designed, synthesized and applied in SDSCs, which can provide iodide and play a role as a hole conducting layer simultaneously. In addition, as imidazolium-based ionic liquids are the most used and efficient electrolytes for high-performance DSCs,7,8 these solid-state conductors retain the advantage of the imidazolium structure and widen their application. In this communication, we tried to identify the different effects of hole and tri-iodide transports in the dual channels electrolyte system. A definite but inferior hole transport has been observed, and the explanation of it was considered in terms of the oxidation potential, while efficiencies of 3.03% and 3.82% have been achieved for mono-iodide and tri-iodide coordinated triphenylamine-imidazolium systems, demonstrating the effect of the ionic/hole dual transport in SDSCs based on such an electrolyte system.
It is believed that introduction of alkoxy groups can decrease the glass transition temperature (Tg) value of the conductors and thus enhance their penetration into the mesoporous TiO2 layer. Besides, the coordination interactions between alkoxy groups and Li+ ions (cations typically used in DSCs) can result in faster charge transfer and higher conductivity. For these reasons, the triethylene glycol monomethyl ether (TEG) group is attached to the imidazolium cation part in our synthesized molecular conductors.
Molecular structures of solid molecular conductors TPA-IM-I and TPA(IM-I)3 are given in Fig. 1. For comparison, TPA-IM-Cl was synthesized to investigate the hole transport in the triphenylamine units. A deep penetration of the electrolytes into the porous TiO2 films occurs in light of the smaller size of the ionic conductors compared to that of the polymer electrolytes, which can facilitate the reduction of the oxidized dye molecules and be beneficial to high photocurrent generation.9
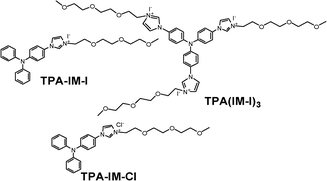 | ||
| Fig. 1 Molecular structures of organic conductors. | ||
Solid-state electrolytes were composed of the synthesized conductors 4-tert-butylpyridine, Li[(CF3SO2)2N] and methyl-3-methyl imidazolium tetrafluoroborate. N3 was chosen as the sensitizer. Preparation of electrolyte and the cell assembly are displayed in the ESI†. The photocurrent–voltage (J–V) performance of the assembled SDSCs was measured with a computer-programmed Keithley 2611 Source Meter under illumination with simulated sunlight (AM 1.5, 100 mW cm−2) supplied by a Newport solar simulator (69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 911); the active area was 0.25 cm2.10
911); the active area was 0.25 cm2.10
Lithium ions have often been used as the cation to iodide in liquid electrolyte cells, and LiTFSI (Li(CF3SO2)2N) greatly improves the performance of SDSCs when added to the spiro-MeOTAD matrix by increasing its conductivity.11 In this study, the binding affinity of the triethylene glycol monomethylether (TEG) group toward lithium ions was investigated by 1H-NMR spectroscopy.12 The results indicate a good association between the TEG group and the lithium ions (Fig. S2, ESI†). As lithium cations have been shown to increase photocurrents,13 the association enhances the electrolyte electrical properties and thus device performance.
Fig. 2 shows the J–V characteristics of the SDSCs using TPA-IM-I, TPA-IM-I with iodine, TPA(IM-I)3 and TPA(IM-I)3 with iodine, and the IPCE spectra of TPA-IM-I and TPA(IM-I)3-based SDSCs. The characteristic photovoltaic parameters of short-circuit current density (Jsc), open-circuit voltage (Voc), fill factor (FF) and photovoltaic conversion efficiency (η) are listed in Table 1.
 | ||
| Fig. 2 (a) J–V characteristics of the SDSCs containing TPA-IM-I or TPA(IM-I)3 with and without I2. (b) IPCE curves of the devices containing TPA-IM-I or TPA(IM-I)3. | ||
| Electrolytes | V oc/V | J sc/mA cm−2 | FF | η (%) |
|---|---|---|---|---|
| TPA-IM-I | 0.575 | 4.77 | 0.702 | 1.92 |
| TPA-IM-I with I2 | 0.550 | 7.71 | 0.716 | 3.03 |
| TPA(IM-I)3 | 0.635 | 7.51 | 0.635 | 2.99 |
| TPA(IM-I)3 with I2 | 0.585 | 9.61 | 0.680 | 3.82 |
Without iodine, SDSCs employing TPA-IM-I and TPA(IM-I)3 show attractive conversion efficiencies. Wang et al. have reported an iodine-free electrolyte in all-solid-state dye-sensitized solar cells with an overall conversion efficiency of 5.29%.14 In such SDSCs with electrolytes containing iodide but no iodine, I3− is formed at the TiO2/electrolyte interface and then diffuses to the counter electrode to capture electrons. Diffusion of I3− in the electrolyte often becomes a limiting process in an ionic liquid electrolyte based DSC, especially when the iodine concentration and consequently the triiodide concentration in the electrolyte are low.15 In most cases, the addition of I2 can increase the concentration of I3−, which is advantageous for the photocurrent. As shown in Table 1, the photocurrent increases with the addition of iodine, which indicates an improved diffusion property of I3−. SDSCs using TPA(IM-I)3 with iodine as the solid-state electrolyte achieved the best photovoltaic performance. The Jsc is 28% higher after the addition of iodine, leading to a η of 3.82%. An increase in concentration of I3− also enhances the recombination of electrons and increases the dark current, resulting in a decrease in the Voc.
Clearly, the cell performance of the SDSCs using TPA(IM-I)3 is better than that using TPA-IM-I. As depicted in Fig. 2(b), SDSCs with TPA(IM-I)3 exhibit higher IPCEs in the whole spectral response region compared to the TPA-IM-I counterpart. These observations reveal that the molecular structure of the solid-state conductor plays an important role in the photoelectrical performance of the SDSCs. The weak interaction force between imidazolium cation and iodide anion is in favor of the dissociation of I−. The rigid branching structure of TPA(IM-I)3 shows a huge steric hindrance, improves the freedom of iodide and provides more iodide for the regeneration of oxidized dye. Inspired by the unique structure, the SDSCs made from TPA(IM-I)3 exhibit the highest Jsc. Consequently, it is not difficult to understand that the TPA(IM-I)3-based SDSCs present the highest conversion efficiency.
To find the contribution of hole transport to the photocurrent, completely iodine-free SDSCs were fabricated by using TPA-IM-Cl as the hole conducting material. Table 2 shows detailed solar cell parameters obtained for TPA-IM-Cl-based SDSCs. However, they achieved a low conversion efficiency, although this demonstrates that their function is based on a hole transport mechanism in the triphenylamine units. In these electrolyte systems, the charge is transported through a hopping mechanism and dye is regenerated by hole injection into the hole conductor.
| Electrolytes | V oc/V | J sc/mA cm−2 | FF | η (%) |
|---|---|---|---|---|
| TPA-IM-Cl | 0.575 | 0.259 | 0.307 | 0.034 |
| TPA-IM-Cl with N(p-C6H4Br)3SbCl6 | 0.425 | 0.272 | 0.413 | 0.047 |
| TPA-IM-Cl with I2 | 0.505 | 0.694 | 0.665 | 0.233 |
One of the plausible explanations of the differences in TPA-IM-Cl-based SDSCs here, in comparison to the conventional SDSCs based on organic hole conductors, is the conductivity of the electrolytes. An important strategy for the performance improvement of spiro-MeTAD-based SDSCs is the addition of N(p-C6H4Br)3SbCl6, which is suggested for improvement in the hole transport rate and consequently the energy conversion efficiency.4 Considering the triphenylamine unit of TPA-IM-Cl, we chose N(p-C6H4Br)3SbCl6 as a dopant. However, as shown in Table 2, the performance is only enhanced slightly after the addition of N(p-C6H4Br)3SbCl6. Because this discrepancy is minor, we have investigated the reproducibility of these results and excluded this possibility of experimental error. Moreover, enough lithium salt is included in the electrolyte. Therefore, we can exclude the possibility that the conductivity of the electrolyte is responsible for the low energy conversion efficiency of TPA-IM-Cl-based SDSCs.
The low performance of TPA-IM-Cl-based SDSCs indicates that the hole transport in our molecular organic conductor based electrolytes does not work efficiently. Furthermore, the device using TPA-IM-Cl with iodine outperformed the device employing TPA-IM-Cl with N(p-C6H4Br)3SbCl6. In the “TPA-IM-Cl with I2” device, the hole transport mechanism in TPA-IM-Cl is crucial to the formation of iodide because the regeneration of dye is initiated by the hole conductor. Iodide is formed by the reduction of iodine at the counter electrode and diffuses to regenerate the dye cation. These observations imply that in our electrolyte systems, hole transport has only a minor contribution to photocurrent, while ion transport plays a main role.
In order to determine the important factors that limit the hole transport in these SDSCs, we investigated the electrochemical properties of these conductors using cyclic voltammetry (CV). CV was carried out in a three-electrode system using 0.1 M lithium perchlorate as supporting electrolyte. (4-Imidazol-1-yl-phenyl)-diphenyl-amine was used for comparison.
The cyclic voltammograms are shown in Fig. 3. The I3−/I− couple is characterized by two oxidation waves: the wave at about 0.2 V vs. SCE corresponds to the oxidation of I− to I3−, whereas the second wave at about 0.6 V vs. SCE was ascribed to the oxidation of I3− to I2.16 Anodic peak current densities of TPA (IM-I)3 are much higher than those of TPA-IM-I, which is ascribed to the higher iodide concentration in TPA (IM-I)3. The oxidation potentials of triphenylamine units in these conductors show (4-imidazol-1-yl-phenyl)-diphenyl-amine < TPA-IM-I < TPA-IM-Cl < TPA(IM-I)3.
 | ||
| Fig. 3 Cyclic voltammograms of molecular conductors and (4-imidazol-1-yl-phenyl)-diphenyl-amine with a concentration of 0.001 M in acetonitrile, with 0.1 M LiClO4 as supporting electrolyte and scan rate of 10 mV s–1. | ||
The electron-withdrawing nature of the halogen anions leads to a positive shift in the oxidation potential of the triphenylamine units. The oxidation potential of the triphenylamine group in TPA(IM-I)3, which has three iodide anions, is more positive than that for TPA-IM-I. The oxidation potential of the triphenylamine in TPA-IM-Cl is more positive compared with that of TPA-IM-I, because the electron-withdrawing effect of Cl− is higher than that of I−. All the oxidation potentials for triphenylamine units in these conductors are higher than the oxidation potential for N3, which is 0.85 V vs. SCE,17 and their positive shifts result in the unbeneficial regeneration of dye. Therefore, we propose that energy level alignment at the dye/electrolyte interface is the most important factor that limits the hole transport in these SDSCs. The electron-withdrawing effect of the halogen anions decreases the contribution of hole transport to the photocurrent.
The charge transfer in molecular organic conductor based SDSCs was investigated by electrochemical impedance spectra (EIS). Typically, there are three arcs in the impedance spectrum of a SDSC. The high frequency arc and the middle frequency one correspond to the charge transfer process at the counter electrode/electrolyte interface and the TiO2/dye/electrolyte interface, respectively. The diameter of the high frequency arc (the inset of Fig. 4) is very small, suggesting a low charge transfer resistance at the counter electrode/electrolyte. From Fig. 4(a), one can see that SDSCs employing TPA-IM-Cl without iodine have a significantly large middle frequency arc, while SDSCs with iodine exhibit a small diameter of the middle frequency arc, indicating that the retarded charge transfer at the TiO2/dye/electrolyte interface is remarkably improved by the addition of iodine. From Fig. 4(b), low charge transfer resistances for SDSCs employing different conductors with iodine can be seen, and the sequence of the resistance is consistent with the above expressed photovoltaic performances, proving that ionic transport works efficiently and plays a main role as compared with hole transport.
 | ||
| Fig. 4 Nyquist plot of SDSCs containing TPA-IM-Cl with and without iodine (a) or containing molecular organic conductors with iodine (b). Inset is the magnification of the high frequency region. All of the devices were measured under open-circuit voltage conditions at 1 sun. | ||
The thermo stability of conductors was studied by thermogravimetric analysis (TGA). The TGA curves indicate that there is no weight loss for temperature at least up to 180 °C (Fig. S3, ESI†). This type of high thermal stability is beneficial for fabricating durable DSCs. Even though they were fabricated without sealing materials, the SDSCs kept 85% of their efficiencies after one month in ambient conditions. The stability is probably related to the high thermal stability of these conductors in the electrolytes.
In summary, two new solid organic conductors containing TEG moieties, TPA-IM-I and TPA(IM-I)3, which can provide dual channels for hole/triiodide transportation, were synthesized and applied successfully as the electrolyte for SDSCs. These conductors exhibited high thermal stability, and enhanced solubility of Li[(CF3SO2)2N] in SDSCs. SDSCs using TPA(IM-I)3 with iodine obtained a η of 3.82%. CV and electrochemical impedance spectra (EIS) reveal that hole transport in these SDSCs has a minor contribution to photocurrent. The electron-withdrawing effect on the oxidation potentials of triphenylamine units leads to less efficient regeneration of dye in these systems. With lower oxidation potentials of triphenylamine units in these conductors, a higher efficiency might be expected. We hope that the findings presented in this work will motivate researchers to develop new electrolytes towards high efficiency and good stability of SDSCs.
Acknowledgements
The authors gratefully acknowledge financial support of National Natural Science Foundation of China (50973116).References
- B. O’Regan and M. Gratzel, Nature, 1991, 353, 737–740 CrossRef.
- A. Hagfeldt and M. Gratzel, Acc. Chem. Res., 2000, 33, 269–277 CrossRef CAS.
- Y. Bai, Y. M. Cao, J. Zhang, M. Wang, R. Z. Li, P. Wang, S. M. Zakeeruddin and M. Gratzel, Nat. Mater., 2008, 7, 626–630 CrossRef CAS.
- U. Bach, D. Lupo, P. Comte, J. E. Moser, F. Weissortel, J. Salbeck, H. Spreitzer and M. Gratzel, Nature, 1998, 395, 583–585 CrossRef CAS.
- C. S. Karthikeyan and M. Thelakkat, Inorg. Chim. Acta, 2008, 361, 635–655 CrossRef CAS.
- K. P. Loh, A. Midya, Z. B. Xie, J. X. Yang, Z. K. Chen, D. J. Blackwood, J. Wang and S. Adams, Chem. Commun., 2010, 46, 2091–2093 RSC.
- D. Chen, Q. Zhang, G. Wang, H. Zhang and J. Li, Electrochem. Commun., 2007, 9, 2755–2759 CrossRef CAS.
- Y. Zhao, J. Zhai, J. L. He, X. Chen, L. Chen, L. B. Zhang, Y. X. Tian, L. Jiang and D. B. Zhu, Chem. Mater., 2008, 20, 6022–6028 CrossRef CAS.
- H. Wang, X. Zhang, F. Gong, G. Zhou and Z. S. Wang, Adv. Mater., 2012, 24, 121–124 CrossRef CAS.
- N. Fu, X. Xiao, X. Zhou, J. Zhang and Y. Lin, J. Phys. Chem. C, 2012, 116, 2850–2857 CAS.
- H. J. Snaith and M. Graetzel, Appl. Phys. Lett., 2006, 89 Search PubMed.
- S. Lu, S. D. Lepore, S. Y. Li, D. Mondal, P. C. Cohn, A. K. Bhunia and V. W. Pike, J. Org. Chem., 2009, 74, 5290–5296 CrossRef CAS.
- S. A. Haque, Y. Tachibana, R. L. Willis, J. E. Moser, M. Gratzel, D. R. Klug and J. R. Durrant, J. Phys. Chem. B., 2000, 104, 538–547 CrossRef CAS.
- G. Q. Wang, L. A. Wang, S. P. Zhuo, S. B. Fang and Y. A. Lin, Chem. Commun., 2011, 47, 2700–2702 RSC.
- G. Namutdinova, S. Sensfuss, M. Schroedner, A. Hinsch, R. Sastrawan, D. Gerhard, S. Himmler and P. Wasserscheid, Solid State Ionics, 2006, 177, 3141–3146 CrossRef.
- V. A. Macagno, M. C. Giordano and A. J. Arvia, Electrochim. Acta, 1969, 14, 335–357 CrossRef CAS.
- J. N. Clifford, E. Palomares, M. K. Nazeeruddin, M. Gratzel, J. Nelson, X. Li, N. J. Long and J. R. Durrant, J. Am. Chem. Soc., 2004, 126, 5225–5233 CrossRef CAS.
Footnote |
| † Electronic Supplementary Information (ESI) available: Experimental details,1H-NMR spectra and TGA curves. See DOI: 10.1039/c2ra20629c/ |
| This journal is © The Royal Society of Chemistry 2012 |
