Perturbation induced formation of a 3D-network of microcrystals producing soft materials†
David
Bardelang
*a,
Michel
Giorgi
b,
Virginie
Hornebecq
c,
Anatoli
Stepanov
d,
Egon
Rizzato
a,
Md. Badruz
Zaman
e,
Gordon
Chan
f,
Olivier
Ouari
*a and
Paul
Tordo
a
aEquipe SREP, Institut de Chimie Radicalaire, ICR, UMR 7273, case 521, Aix-Marseille Université et CNRS, Avenue Escadrille Normandie-Niemen 13397, Marseille Cedex 20, France. E-mail: david.bardelang@univ-amu.fr; Fax: (+33) 491 288 758; Tel: (+33) 491 288 610
bSpectropole, FR 1739, Aix-Marseille Université, Avenue Escadrille Normandie-Niemen 13 397, Marseille Cedex 20, France
cEquipe S2P, Laboratoire MADIREL, UMR 7246, Aix-Marseille Université et CNRS, Centre de Saint-Jérôme F-13397, Marseille Cedex 20, France
dIM2NP, UMR 6242, Aix-Marseille Université et CNRS, case 142, Avenue Escadrille Normandie-Niemen 13397, Marseille Cedex 20, France
eSteacie Institute for Molecular Sciences, NRC Canada, 100 Sussex Drive, Ottawa, Ontario K1A0R6 Canada and Center of Excellence for Research in Engineering Materials, Faculty of Engineering, King Saud University, Riyadh, 11421, Saudi Arabia
fG. Chan, Institute for Research in Construction, NRC Canada, 1200 Montreal Road, Ottawa, Ontario K1A0R6, Canada
First published on 13th April 2012
Abstract
Toluene and 1-chloronaphthalene immobilization was observed when perturbing stimuli such as agitation or ultrasound are applied during the cooling of a hot supersaturated solution of the rigid dinitroxide bTbk. X-Ray diffraction and cryo-SEM on gels and solid samples (crystal, powder) confirmed the pivotal role of clathrate type microcrystals in the solvent immobilization process.
A topic of increasing interest in materials science and engineering is the study of supramolecular gels.1 These soft materials are versatile objects that can be used as platforms for sensors,2 templates for the construction of ordered nano- and microstructures,3 for tissue engineering4 and as drug carriers.5 Nowadays gels have found large applications in the fields of cosmetics, pharmaceutics and food engineering.6 Supramolecular organogels are soft materials composed of a solvent trapped within an interweaved three-dimensional network of self-assembled structures, most often nano- or microfibers.7 Usually, a material is considered as a gel if a whole volume of liquid is immobilized by a gelator as tested by the ‘tube inversion’ method. In the case of low molecular weight organo gelators (LMOGs), the formation of supramolecular architectures relies on a self-assembly process of gelator molecules through weak forces (i.e., hydrogen bonds, π stacking, van der Waals interactions). A robust solvent entrapped 3D-network is formed from the branching of the growing fibers through structural mismatches.
Recently, some authors have pointed out that crystal engineering and rheological studies can be interesting strategies to improve our understanding of the intermolecular interactions;8,9 but most of the LMOGs are serendipitously found and the design of new gelator molecules is still challenging10 because of the need of successive and hierarchical self-assembly events, the solvent dependency and the limited understanding of the gelation mechanism at the molecular level. During the cooling of hot gelator solutions, crystallization, which could have resulted in precipitation of crystals, is aborted and 1D- fibers are formed and evolved in self-assembled 3D- networks within which the solvent molecules are entrapped. Most of the gelation processes are triggered by heat (followed by a cooling step). However, LMOGs, which can respond to other non classical stimuli such as ultrasound,11 agitation,12 enzymes,13 ion recognition,14 have recently appeared and offer new possibilities in chemistry and life sciences. For example, anion-switchable supramolecular gels can be obtained to control the crystal growth of pharmaceuticals14b and intracellular enzymatic activated progelator triggered gelation causing cell death.13c In fact, a mechanical perturbation, previously considered as unsuitable for getting ordered architectures,15 can initiate,16 modify17,18 or accelerate19 the self-assembly events affording supramolecular fibrils and gels. But there are still very few examples of gel formation triggered by agitation.12
In this report, we describe the formation of gel-like materials in toluene and 1-chloronaphthalene by the dinitroxide bTbk (bisTEMPO-bis-ketal‡;Scheme 1 and Fig. 1). The TEMPO-based biradical bTbk was designed as a polarizing agent for dynamic nuclear polarization (DNP)/solid state NMR applications and high enhancement factors were obtained due to its rigid structure where the two TEMPO moieties are constrained nearly orthogonally.20
 | ||
| Fig. 1 Gel-like materials obtained using the dinitroxide bTbk. | ||
 | ||
| Scheme 1 Molecular structure of the bTbk dinitroxide. | ||
During solubility studies, it appeared that bTbk self-assembles depending on the applied stimulus either in the solid state (heat/cooling: large crystals) or in the gel state (heat/perturbation/cooling: solvent immobilization). A closer look at the architectures obtained from the two experiments reveals a common crystalline structure displaying an open framework and 1D channels. The structural assembly in the gellified state has been determined at the molecular level by a combination of different techniques, i.e., X-ray diffraction on powders, gels and single crystals and cryo-SEM (Fig. 2), and this level of information has only been rarely obtained for LMOGs.
 | ||
| Fig. 2 Thermoreversible solvent immobilization triggered by a perturbing stimulus upon cooling. Cryo-SEM micrographs of frozen (images recorded at −190 °C) and metallized (Au/Pt alloy) bTbk gel-like material obtained with ultrasound (a), manual stirring (b), magnetic stirring (c) and circularly shaking (d). | ||
When saturated hot toluene solutions of bTbk were submitted to ultrasound during their cooling to room temperature, gel-like materials were obtained (Fig. 2a). The critical gelation concentration was found to be ∼ 80 mM in toluene (4% weight). If no stimulus is applied during the cooling phase, or if the stimulus time was insufficient, large orange crystals formed within a few minutes. If the sonication time is too intense (longer than 45 s) gel-like suspensions were obtained, irrelevant of the bTbk concentration (see Table S1†). Interestingly, gel-like materials also formed when a mechanical stimulus (circular stirring with a spatula or a magnetic stirrer) was applied upon cooling of the bTbk toluene solutions (Fig. 2b and 2c).12 The obtained gels are robust enough to sustain the magnetic stirrer. Also, circular shaking of the vial produced a gel-like material (Fig. 2d), indicating that shear forces are important for gelation.17,18 The time stability of the gels was concentration dependant ranging from minutes to several hours/days. At low concentrations of bTbk, near the critical gelation concentration, the obtained gels are weak and tend to collapse after a few minutes but as the concentration increases, the gels are much more stable. We believe that at high concentrations, the number of branching points between the microcrystals increases and can thus strengthen the gels. At concentrations above the critical gelation concentration, the process was highly reproducible and thermally reversible, provided a perturbation is applied during the cooling. Therefore, the obtained gels can be turned back to solutions upon heating and back again to gels if a perturbation is applied during the cooling and this cycle can be repeated several times without any lose of solvent immobilization ability. A range of solvents was screened and gelified liquids were additionally observed in 1-chloronaphthalene (Table S2†). At first sight, the fact that aromatic solvents are the only ones to be immobilized is surprising. This is well-known that gelators are very sensitive in terms of solubility to the solvents to be gelified. Clearly in our case bTbk is highly soluble in most of the non gelifying solvents investigated (> 320 mM) and also tends to precipitate in ethyl acetate, diethyl ether or hexane. It seems that bTbk is a good gelator for solvents in which it has a reduced solubility (between 80 and 160 mM). To the best of our knowledge only one example of a nitroxide organogelator has been reported and the nitroxide moiety was part of a steroid.21
Without perturbations, bTbk crystallizes as large crystals in toluene and in a large variety of solvents in the same trigonal R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c space group with 1D corrugated channels along crystallographic axis c (Fig. 3a).22 The framework is only composed of the bTbk molecules while the solvent is included in large pockets (∼ 11.4 × 11.4 × 12.2 Å) separated by approximately 10 Å of a quite constricted tube. Based on the generally accepted definition,23 four strong (C-H⋯O: 2.43 Å, 172°) and eight weak (C-H⋯O: ∼ 2.62 Å, 152°) non classical CH⋯ON hydrogen bonds per bTbk are responsible for the network assembly (Fig. 3b). The nitroxide group has a strong H-bond acceptor character due to the three electron πN–O bond, and a significant negative charge is displayed onto the O-atom.
c space group with 1D corrugated channels along crystallographic axis c (Fig. 3a).22 The framework is only composed of the bTbk molecules while the solvent is included in large pockets (∼ 11.4 × 11.4 × 12.2 Å) separated by approximately 10 Å of a quite constricted tube. Based on the generally accepted definition,23 four strong (C-H⋯O: 2.43 Å, 172°) and eight weak (C-H⋯O: ∼ 2.62 Å, 152°) non classical CH⋯ON hydrogen bonds per bTbk are responsible for the network assembly (Fig. 3b). The nitroxide group has a strong H-bond acceptor character due to the three electron πN–O bond, and a significant negative charge is displayed onto the O-atom.
 | ||
| Fig. 3 (a) X ray crystal structure§ of bTbk crystallized from toluene (hydrogens removed for clarity) viewed down crystallographic axis c (the toluene molecules are disordered over six sites inside the channels). (b) Multiple CH⋯ON interactions surrounding 1 bTbk molecule. | ||
This property is illustrated by the strong dipolar moment (3.14 D) associated to the nitroxide moiety.24 Characterization of the crystal structure was pivotal because of the central role of isostructural bTbk microcrystals in the gel-like materials (see Fig. 5a). These results are noteworthy because in all these cases (i) a perturbation is compulsory to observe the gelation-kind process, otherwise large crystals which phase separate were always obtained and (ii) small faceted microcrystals were always observed when studying the gel microstructures by microscopy (Fig. 2). The cryo-SEM technique was employed because the bicontinuous texture of the gel is preserved and it enables imaging of the underlying architecture, which was not possible by SEM due to the collapse of the open framework during the evaporation of the solvent (clathrate microcrystals). In the vast majority of cases in LMOGs, it is virtually impossible to determine the single-crystal structure of a gel fiber because they are too small for diffraction measurements and also due to significant degrees of disorder.6,7,25 However, bTbk self-assembly appears to be an example at the limit of the supramolecular gel field and we were interested in understanding how the gels could self-sustain when the vials were turned upside down since microcrystals are expected to phase separate and precipitate in toluene. Images of the cryogenized samples at higher magnification showed an amorphous looking phase linking together the side faces or the tips of microcrystals (see blue circles in Fig. 4). Therefore, entangled networks of rodlike, stuck microcrystals (length ∼ 50-100 μm, diameter ∼ 1-15 μm) were observed on frozen and metallized gels by cryo-SEM. This amorphous looking phase presumably acts as a cement to maintain the crystals together in a three dimensional network thus accounting for gelation. However, different morphologies are observed when circular shaking of the vials was applied as the perturbing stimulus and microtubes were produced (Fig. 2d). Usually, the orientation parameter in a gel is weak and the distribution of the 1D self-assembled species is random. It has been reported that, in certain cases such as when external stimuli are applied during gelation, this orientation parameter can become stronger.9b Such a phenomenon can be at the origin of the microtube formation. In the case of ultrasound stimulus, a mixture of two stuck phases (spherulites and rodlike crystals) is present in the sample (Fig. 2a). Ultrasound appears to have the stronger effect on the crystallization process, aborting the crystal growth and is likely promoting the occurrence of an amorphous phase, which could explain the formation of the microcrystalline gel state.
 | ||
| Fig. 4 Cryo-SEM micrographs of a frozen and metallized (Au/Pt alloy) gel sample obtained with bTbk in toluene by manual stirring upon cooling of a hot saturated solution. Note the sticky phase (blue circles), which likely maintains the gel state by sticking the microcrystals together. | ||
X-Ray diffraction (XRD) experiments on gel and powder samples have been performed to investigate the degree of crystallinity in these samples. The XRD patterns of toluene gels confirmed the presence of the same crystalline open framework structure as observed for large bTbk crystals (Fig. 5a). These patterns showed sharp reflections assigned to the channel structure which is in good agreement with the pictures obtained by cryo-SEM. Besides the existence of a 3D network of stuck microcrystals, the fact that bTbk tend to crystallize according to 1D channels could have also a role on gel formation. In several reports, 1D self-assembling structures have been pointed as an important requisite for gel formation.26
 | ||
| Fig. 5 Theoretical XRD pattern (a, top) of the crystalline open framework structure of bTbk and the experimental one (bottom) of a bTbk gel of toluene. EPR spectrum of (b) bTbk in toluene (0.5 mM, 298 K), (c) a bTbk gel (298 K), (d) a bTbk/toluene solvate single crystal of the open framework structure. | ||
Owing to the paramagnetic nature of bTbk, we used X-band EPR spectroscopy to further characterize the gel and the crystals. The X band liquid-state EPR spectrum of 0.5 mM bTbk in toluene (Fig. 5b) shows an EPR spectrum typically observed for nitroxide radicals in solution. The spectrum consists of three lines separated by the isotropic hyperfine coupling due to the interaction with a 14N nucleus and an isotropic hyperfine coupling of 1.51 mT (Fig. 5b). In a previous study,27 it has been determined that the electron–electron spin exchange coupling is negligible compared to the hyperfine coupling. The analysis of the EPR spectrum of the bTbk/toluene gel (Fig. 5c) showed the superimposition of two signals: a three-line spectrum (aN = 1.51 mT) corresponding to bTbk in solution and a broad one-line spectrum assigned to bTbk microcrystals. This result is in agreement with the data obtained by cryo-SEM and XRD on the gels.
The reason for this unexpected gelation process could stand in the simultaneous occurrences for the gelator of two divergent processes, which are (i) a strong tendency to self-assemble according to a predetermined scheme, i.e., the open framework, and (ii) the introduction of a perturbation (either mechanical or ultrasound) that tend to abort the 1D crystal growing and triggers the occurrence of structural mismatches, i.e., micro-crystals branching leading to a 3D network.28 Interestingly, no gel formation was observed when the amino (2) or the thioketal (3) analogues of bTbk (Scheme 2) were assayed.
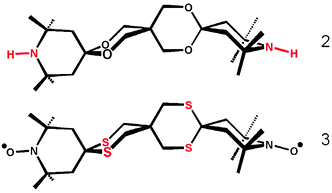 | ||
| Scheme 2 Molecular structure of the bTbk amine and thioether analogues. | ||
Unfortunately, it was not possible to get single crystals of the amine analogue but the structure of analogue 3 showed a layer structure with no void space amenable for guest inclusion.29 As suggested by Terech et al.,30 this gelation feature underpinning rigid objects (crystalline fibers, rigid rods, semirigid fibers) is indeed more general and in this present work constitutes another example of such kind of soft materials. The observed solid-like behavior may well be an example of arrested crystallization in which bTbk phase separate.31 This differs from spinodal decomposition in that the observed gelation seems to require nucleation of bTbk microcrystals first, followed by crystal expansion and cross-linking (amorphous phase occurrence triggered by the perturbing stimulus) even though phase separation finally occurs uniformly throughout the entire material as in spinodal decomposition. The present mechanism may be very subtle in that perturbations deviate the expected thermodynamic nucleation of bTbk to afford a kinetically trapped self-assembly with a degree of order inferior to that of a crytal.11a
In conclusion, we report that soft materials can be obtained from toluene solutions containing dinitroxide bTbk when ultrasound or stirring type stimuli are applied. These perturbations seems to hamper the bTbk propension to crystallize into large crystals. The XRD, EPR and cryo-SEM studies pointed out a common crystalline microphase (open framework) of the gel-like materials, which are composed of networks of branched microcrystals whose architecture was isostructural to bTbk single crystals that grew up upon cooling in the absence of mechanical perturbations. We report a case where it seems that frustration of crystallization of macroscopic crystals led to solvent entrapped 3D networks of stuck microcrystals triggered by an external stimulus. Besides its high percentage (4%) for solvent immobilization and the highly crystalline feature of the obtained soft materials, we ask the question whether bTbk should still be classified as an organogelator or as a compound self-assembling to a new family of gel-like materials.
Acknowledgements
We thank CNRS and the National Research Council of Canada, and also the referees for very interesting comments and suggestions. Md. B. Z. acknowledges the VP Program, King Saud University, Saudi Arabia.References
- (a) J. A. Foster and J. W. Steed, Angew. Chem., Int. Ed., 2010, 49, 6718–6724 CrossRef CAS; (b) A. R. Hirst, B. Escuder, J. F. Miravet and D. K. Smith, Angew. Chem., Int. Ed., 2008, 47, 8002–8018 CrossRef CAS; (c) D. Bardelang, F. Camerel, R. Ziessel, M. Schmutz and M. J. Hannon, J. Mater. Chem., 2008, 18, 489–494 RSC; (d) Z. Yang and B. Xu, J. Mater. Chem., 2007, 17, 2385–2393 RSC.
- P. Mukhopadhyay, Y. Iwashita, M. Shirakawa, S.-I. Kawano, N. Fujita and S. Shinkai, Angew. Chem., Int. Ed., 2006, 45, 1592–1595 CrossRef CAS.
- (a) K. Sada, M. Takeuchi, N. Fujita, M. Numata and S. Shinkai, Chem. Soc. Rev., 2007, 36, 415–435 RSC; (b) N. Fujita, Y. Sakamoto, M. Shirakawa, M. Ojima, A. Fujii, M. Ozaki and S. Shinkai, J. Am. Chem. Soc., 2007, 129, 4134–4135 CrossRef CAS; (c) K. J. C. van Bommel, A. Friggeri and S. Shinkai, Angew. Chem., Int. Ed., 2003, 42, 980–999 CrossRef CAS.
- (a) J. Boekhoven, A. M. Brizard, K. N. K. Kowlgi, G. J. M. Koper, R. Eelkema and J. H. van Esch, Angew. Chem., Int. Ed., 2010, 49, 4825–4828 CrossRef CAS; (b) L. A. Estroff and A. D. Hamilton, Chem. Rev., 2004, 104, 1201–1217 CrossRef CAS; (c) K. Y. Lee and D. J. Mooney, Chem. Rev., 2001, 101, 1869–1879 CrossRef CAS.
- (a) G. Liang, Z. Yang, R. Zhang, L. Li, Y. Fan, Y. Kuang, Y. Gao, T. Wang, W. W. Lu and B. Xu, Langmuir, 2009, 25, 8419–8422 CrossRef CAS; (b) J. C. Tiller, Angew. Chem., Int. Ed., 2003, 42, 3072–3075 CrossRef CAS; (c) B. Xing, C.-W. Yu, K.-H. Chow, P.-L. Ho, D. Fu and B. Xu, J. Am. Chem. Soc., 2002, 124, 14846–14847 CrossRef CAS.
- (a) M. George and R. G. Weiss, Acc. Chem. Res., 2006, 39, 489–497 CrossRef CAS; (b) N. M. Sangeetha and U. Maitra, Chem. Soc. Rev., 2005, 34, 821–836 RSC.
- (a) T. Ishi-i and S. Shinkai, Top. Curr. Chem., 2005, 258, 119–160 CrossRef CAS; (b) P. Terech and R. G. Weiss, Chem. Rev., 1997, 97, 3133–3159 CrossRef CAS.
- P. Dastidar, Chem. Soc. Rev., 2008, 37, 2699–2715 RSC.
- (a) P. Terech, S. Dourdain, S. Bhat and U. Maitra, J. Phys. Chem. B, 2009, 113, 8252–8267 CrossRef CAS; (b) P. Terech, Langmuir, 2009, 25, 8370–8372 CrossRef CAS.
- J. H. van Esch and B. Feringa, Angew. Chem., Int. Ed., 2000, 39, 2263–2266 CrossRef CAS.
- (a) D. Bardelang, Soft Matter, 2009, 5, 1969–1971 RSC; (b) G. Cravotto and P. Cintas, Chem. Soc. Rev., 2009, 38, 2684–2697 RSC; (c) D. Bardelang, F. Camerel, J. C. Margeson, D. M. Leek, M. Schmutz, Md. B. Zaman, K. Yu, D. V. Soldatov, R. Ziessel, C. I. Ratcliffe and J. A. Ripmeester, J. Am. Chem. Soc., 2008, 130, 3313–3315 CrossRef CAS.
- (a) M.-O. M. Piepenbrock, N. Clarke and J. W. Steed, Soft Matter, 2010, 6, 3541–3547 RSC; (b) Z. Xie, A. Zhang, L. Ye, X. Wang and Z.-G. Feng, J. Mater. Chem., 2009, 19, 6100–6102 RSC.
- (a) Z. Yang, G. Liang and B. Xu, Acc. Chem. Res., 2008, 41, 315–326 CrossRef CAS; (b) Z. Yang, P.-L. Ho, G. Liang, K. H. Chow, Q. Wang, Y. Cao, Z. Guo and B. Xu, J. Am. Chem. Soc., 2007, 129, 266–267 CrossRef CAS; (c) Z. Yang, K. Xu, Z. Guo, Z. Guo and B. Xu, Adv. Mater., 2007, 19, 3152–3156 CrossRef CAS.
- (a) M.-O. M. Piepenbrock, G. O. Lloyd, N. Clarke and J. W. Steed, Chem. Rev., 2010, 110, 1960–2004 CrossRef CAS; (b) J. A. Foster, M.-O. M. Piepenbrock, G. O. Lloyd, N. Clarke, J. A. K. Howard and J. W. Steed, Nat. Chem., 2010, 2, 1037–1043 CrossRef CAS; (c) H. Maeda, Chem.–Eur. J., 2008, 14, 11274–11282 CrossRef CAS.
- J. M. J. Paulusse, D. J. M. van Beek and R. P. Sijbesma, J. Am. Chem. Soc., 2007, 129, 2392–2397 CrossRef CAS.
- T. Naota and H. Koori, J. Am. Chem. Soc., 2005, 127, 9324–9325 CrossRef CAS.
- In the case of fibrils and fibers, Tycko and colleagues have reported that agitation of solutions containing amyloid peptides can dramatically influence their self-assembly into stuck, straight fibrils: W. Qiang, W.-M. Yau and R. Tycko, J. Am. Chem. Soc., 2011, 133, 4018–4029 CrossRef CAS.
- Otto and colleagues observed that the same type of stimulus applied to other peptides enables the self-assembling pathway to deviate from expected outcomes affording unexpected self-assembled structures of well-defined composition: J. M. A. Carnall, C. A. Waudby, A. M. Belenguer, M. C. A Stuart, J. J.-P. Peyralans and S. Otto, Science, 2010, 327, 1502–1506 CrossRef CAS.
- N. Komiya, T. Muraoka, M. Iida, M. Miyanaga, K. Takahashi and T. Naota, J. Am. Chem. Soc., 2011, 133, 16054–16061 CrossRef CAS.
- (a) A. J. Rossini, A. Zagdoun, M. Lelli, J. Canivet, S. Aguado, O. Ouari, P. Tordo, M. Rosay, W. E. Maas, C. Copéret, D. Farrusseng, L. Emsley and A. Lesage, Angew. Chem., Int. Ed., 2012, 51, 123–127 CrossRef CAS; (b) A. Zagdoun, A. J. Rossini, D. Gajan, A. Bourdolle, O. Ouari, M. Rosay, W. E. Maas, P. Tordo, M. Lelli, L. Emsley, A. Lesage and C. Copéret, Chem. Commun., 2012, 48, 654–656 RSC; (c) E. Salnikov, M. Rosay, S. Pawsey, O. Ouari, P. Tordo and B. Bechinger, J. Am. Chem. Soc., 2010, 132, 5940–5941 CrossRef CAS; (d) Y. Matsuki, T. Maly, O. Ouari, H. Karoui, F. Le Moigne, E. Rizzato, S. Lyubenova, J. Herzfeld, T. Prisner, P. Tordo and R. G. Griffin, Angew. Chem., Int. Ed., 2009, 48, 4996–5000 CrossRef CAS.
- (a) P. Terech and S. Friol, Tetrahedron, 2007, 63, 7366–7374 CrossRef CAS; (b) P. Térech, F. Volino and R. Ramasseul, C. R. Acad. Sci., 1981, 292, 41–43 Search PubMed; (c) R. Ramasseul and A. Rassat, Tetrahedron Lett., 1974, 15, 2413–2414 CrossRef.
- A comprehensive crystallographic study will be reported elsewhere.
- (a) R. K. Castellano, Curr. Org. Chem., 2004, 8, 845–865 CrossRef CAS; (b) T. Steiner, Angew. Chem., Int. Ed., 2002, 41, 48–76 CrossRef CAS; (c) G. R. Desiraju, Acc. Chem. Res., 1996, 29, 441–449 CrossRef CAS; (d) R. Taylor and O. Kennard, J. Am. Chem. Soc., 1982, 104, 5063–5070 CrossRef CAS.
- E. G. Rozantsev and E. N. Gur'yanova, Russ. Chem. Bull., 1966, 15, 936–939 CrossRef.
- As outlined by Terech and Weiss (see Ref. 7b) “In essence, gelation occurs when normal crystallization has gone awry”. Still there are some exceptions, see for example ref. 8.
- K. N. King and A. J. McNeil, Chem. Commun., 2010, 46, 3511–3513 RSC.
- M. Gafurov, S. Lyubenova, V. Denysenkov, O. Ouari, H. Karoui, F. Le Moigne, P. Tordo and T. Prisner, Appl. Magn. Reson., 2009, 37, 505–514 CrossRef CAS.
- A similar behavior has recently been observed for organic polymers, see for example: J. M. Harner and D. A. Hoagland, J. Phys. Chem. B, 2010, 114, 3411–3418 CrossRef CAS.
- E. L. Dane, B. Corzilius, E. Rizzato, P. Stocker, T. Maly, A. A. Smith, R. G. Griffin, O. Ouari, P. Tordo and T. M. Swager, J. Org. Chem., 2012, 77, 1789–97 CrossRef CAS.
- (a) P. Terech, N. M. Sangeetha and U. Maitra, J. Phys. Chem. B, 2006, 110, 15224–15233 CrossRef CAS; (b) P. Terech, C. Scherer, P. Lindner and R. Ramasseul, Langmuir, 2003, 19, 10648–10653 CrossRef CAS; (c) P. Terech, A. Coutin and A. M. Giroud-Godquin, J. Phys. Chem. B, 1997, 101, 6810–6818 CrossRef CAS.
- P. J. Lu, E. Zaccarelli, F. Ciulla, A. B. Schofield, F. Sciortino and D. A. Weitz, Nature, 2008, 453, 499–503 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: effect of concentration and sonication time on supramolecular gelation, single crystal XRD data and additional cryo-SEM images. CCDC reference number 794601. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c2ra20208e |
| ‡ BisTEMPO-bis-ketal is for 2,2,4,4,14,14,16,16-octamethyl-7,11,18,21-tetraoxo-3,15 dinitroxyltrispiro[5.2.2.512.29.26] henicosane, TEMPO for 2,2,6,6-tetramethylpiperidine-N-oxyl. |
§ The crystals were measured on a Bruker-Nonius KappaCCD diffractometer with the Mo(Kα) radiation at 213 K. Data reductions were performed with denzo-SMN, structures solved by direct methods and refined with Shelxl97. For the open framework crystals, more than 30 attempts were made with different bTbk/solvent combinations but we were able to locate the highly disordered guest in the case of toluene only. A molecule of toluene with partial occupancy of 0.33 was then determined in the asymmetric unit and constrained during the refinement cycles. A more detailed crystallographic study will be reported elsewhere. CCDC number 794601.† Crystal size 0.50 × 0.15 × 0.20, C41.49H68N3O9, M = 752.89, trigonal, space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, a = 25.104(4), c = 30.000(3), V = 16373.3(17) Å3, T = 213(2)K, Z = 36, ρcalc = 0.919 Mg m−3, 2θMax = 25.96°, 192 parameters, 7 restrains, residual electron density max. 0.880, min. −0.262 eÅ−3. Final R indices (I > 2σ(I)): R1 = 0.0810, wR2 = 0.2304 (28637 reflections total, 3318 unique, 2403 (I > 2σ(I))). c, a = 25.104(4), c = 30.000(3), V = 16373.3(17) Å3, T = 213(2)K, Z = 36, ρcalc = 0.919 Mg m−3, 2θMax = 25.96°, 192 parameters, 7 restrains, residual electron density max. 0.880, min. −0.262 eÅ−3. Final R indices (I > 2σ(I)): R1 = 0.0810, wR2 = 0.2304 (28637 reflections total, 3318 unique, 2403 (I > 2σ(I))). |
| This journal is © The Royal Society of Chemistry 2012 |
