Open Access Article
Open Access ArticleSupplying silicon reduces cadmium accumulation in pak choi by decreasing soil Cd bioavailability and altering the microbial community†
Rui
Jing‡
ac,
Yao
Yu‡
b,
Xuerong
Di
a,
Xu
Qin
a,
Lijie
Zhao
a,
Xuefeng
Liang
 a,
Yuebing
Sun
a and
Qingqing
Huang
a,
Yuebing
Sun
a and
Qingqing
Huang
 *a
*a
aKey Laboratory of Original Agro-Environmental Pollution Prevention and Control, Agro-Environmental Protection Institute, Ministry of Agriculture and Rural Affairs, Tianjin 300191, China. E-mail: huangqingqing@caas.cn
bSchool of Resources and Environmental Engineering, Hefei University of Technology, Hefei 230009, China
cCollege of Resources and Environment, Northeast Agricultural University, Harbin 150030, China
First published on 20th March 2025
Abstract
Silicon-containing materials have been widely used in Cd-contaminated soil remediation. However, the immobilization effects of sodium silicate on Cd migration and transformation in an acidic soil–vegetable system have not been thoroughly studied. Herein, a pot experiment was performed to investigate the effects of sodium silicate application on pak choi growth, oxidative status, Cd uptake and accumulation in pak choi, soil Cd bioavailability and fractions, and soil bacterial communities. The results showed that sodium silicate application significantly increased soil pH (0.29–1.61 units) and induced the transformation of the Cd fraction from an exchangeable fraction (Exc-Cd) into an iron and manganese oxide-bound fraction (OX-Cd) and organic matter-bound fraction (OM-Cd), decreasing Cd bioavailability by 13.7–20.8% in Cd-contaminated acidic soil. As a result, sodium silicate application significantly alleviated Cd toxicity, enhanced pak choi growth, and reduced Cd concentration in roots by 23.5–89.0% and in shoots by 58.5–81.0%, with Cd concentration in the edible part at a Si application rate equal to or greater than 0.4 g Si per kg soil falling below the safety limits for Cd as defined in China's food safety standard (GB 2762-2022). In addition, sodium silicate application significantly increased soil bacterial richness (Ace index and Chao1) and diversity (Shannon and Simpson index) and altered the soil microbial structure. These findings suggested that sodium silicate has great potential as an environmentally friendly amendment to immobilize Cd-contaminated acidic soil and reduce Cd accumulation in vegetables.
Environmental significanceSoil contamination with Cd has become a major environmental issue for food security and sustainable agriculture in China. Recently, silicon-containing materials have been widely used to remediate Cd-contaminated soil. Pak choi (Brassica rapa ssp. chinensis) is one of the most consumed leafy vegetables in China, and pak choi generally accumulates more Cd than other types of vegetables, making it a significant contributor to dietary Cd intake. However, up to now, the mechanisms regarding how Si affects Cd migration and transformation in the soil–pak choi system are still poorly understood. |
1 Introduction
Cadmium (Cd) is one of the most toxic and widespread pollutants in agricultural soil, and as a non-essential element, it compromises various biological functions in humans and plants.1 Soil Cd can readily be absorbed by plant roots and accumulate in edible parts, eventually entering the food chain and posing risks to human health.2 Vegetables, as a primary agricultural product, are an essential component of the human diet worldwide, providing essential nutrients like dietary fiber, protein, minerals, and vitamins.3 However, vegetables also absorb and accumulate various nonessential elements such as heavy metals, and substantial evidence has indicate that vegetables can accumulate Cd over a wide range of concentrations and consumption of such Cd-contaminated vegetables is a significant health risk to humans.4–6 Currently, Cd pollution in acidic soil in some areas of southern China poses a serious threat to vegetable production.7 Thus, to ensure food safety and protect human health, effective strategies need to be developed to remediate Cd-contaminated agricultural soil.Functional metal antagonist fertilizers like silicon (Si) fertilizer and selenium (Se) fertilizer have recently attracted attention as effective Cd mitigation strategies for crops.8,9 Among these, Si is considered a “multitalented quasi-essential” element for plant growth and has received a lot of attention for its ability to reduce Cd toxicity and accumulation in numerous plant species, including wheat,10 rice,11,12 and soybean.13 In soil, Si can immobilize Cd by increasing soil pH and creating silicate complexes with Cd, thereby reducing soil Cd bioavailability.14–16 Furthermore, once absorbed by plant root systems from soil, Si can improve plant tolerance to Cd stresses by activating their antioxidative system.10 In addition, Si in plants can increase Cd retention in roots by enhancing cell wall Cd adsorption capacity to trap more Cd in root cell walls and sequestering more Cd into the vacuole, finally reducing root-to-shoot Cd translocation.10 Moreover, Si can decrease Cd accumulation in plants by reducing the expression level of genes involved in Cd absorption and transportation.17 So far, numerous studies have been conducted to study the effect of Si on the alleviation of Cd stress in crops, as well as the mechanism underlying this effect. However, the majority of these studies focused on grain crops such as rice and wheat, while the effects of Si on Cd accumulation in plants differ based on species, with few studies on vegetables. Pak choi (Brassica rapa ssp. chinensis), one of the most popular leafy vegetables consumed in China, generally accumulates more Cd than other types of vegetables, making it an important source of Cd in the diet.6,18 However, the mechanisms regarding how Si affects Cd migration, transformation, and accumulation in the soil–pak choi system remain poorly understood. Furthermore, the majority of these studies that have assessed Si as a tool to mitigate plant uptake of Cd have used soils either amended with Cd salts or soils with very high Cd concentrations, which have limited relevance to mild and moderate Cd-contaminated soil. Additionally, soil microorganisms are sensitive to environmental changes and can be used as bio-indicators to assess soil health.19 Moreover, on the one hand, soil Cd can damage microbial activity by interfering with the function and structure of the microbial community, whereas soil microorganisms can detoxify heavy metals by immobilizing or transforming them.20 However, the impact of Si on Cd migration and transformation, and the soil microbial community in the soil–vegetable system is currently poorly understood and needs to be investigated further.
In this study, we chose pak choi as our research material to determine whether Si fertilizer applied to soil can reduce Cd concentrations in pak choi grown in a moderate Cd-contaminated soil. The main aims of the study were the following: (1) to investigate the effects of exogenous Si application on pak choi growth, oxidative status, Cd uptake and accumulation in pak choi, Cd bioavailability and fractions in soil, and the soil bacterial community, and (2) to explore the underlying mechanisms involving Si for Cd migration and transformation in the soil–vegetable system. This study will provide theoretical and technological guidelines for safe pak choi production in Cd-contaminated soil.
2 Materials and methods
2.1 Soil characterization
The soil used in the current study was surface tillage soil (0–20 cm) collected from a moderate Cd-contaminated paddy field in Chenzhou (25°49′36′′ N, 112°35′10′′ E), Hunan Province, that was polluted from historical smelting and mining. The following were the chemical properties of the tested soil as determined by the methodology of Bao (2000):21 the soil pH, 6.13; organic matter, 20.1 g kg−1; cation exchange capacity, 25.3 cmol kg−1; total nitrogen, 1.25 g kg−1; available phosphorus, 9.34 mg kg−1; available potassium, 183 mg kg−1; total Cd, 1.83 mg kg−1. Text S1† presents the detailed soil physicochemical analysis. Based on the Soil Environmental Quality Standards of China (GB 15618-2018), the concentration of Cd in the tested soil was 4.57 times greater than the threshold value of 0.4 mg kg−1 (5.5 < pH < 6.5). After the visible plant residues and stones were removed, the collected soils were air-dried for 14 days, ground thoroughly, and then filtered through a 2 mm sieve for further use.2.2 Experimental design
A pot experiment was conducted in a greenhouse situated in the Nankai District of Tianjin, China. Exogenous Si was applied in the form of solid sodium silicate (Na2SiO3·9H2O, analytically pure, Macklin Inc., Shanghai, China), with five different doses of Si added into soil including 0.00 g Si per kg soil (no addition of Si, SiCK), 0.20 g Si per kg soil (Si0.2), 0.40 g Si per kg soil (Si0.4), 0.80 g Si per kg soil (Si0.8), and 1.0 g Si per kg soil (Si1.0). Each treatment was repeated four times, with each pot containing 2 kg of soil. The basic information of sodium silicate were as follows: the soil pH, 12.53; total Cd, 0.05 mg kg−1; total Cr, 2.7 mg kg−1; total Pb, 0.8 mg kg−1; total As, 0.29 mg kg−1; and total Hg, 0.003 mg kg−1. Based on the conventional vegetable fertilization practices, 0.15 g P2O5 per kg soil for phosphorus (P) in the form of CaH2PO4·H2O (analytically pure, Macklin Inc., Shanghai, China), 0.2 g K2O per kg soil for potassium (K) in the form of KCl (analytically pure, Macklin Inc., Shanghai, China), and 0.2 g N per kg soil for nitrogen(N) in the form of CO(NH2)2 (analytically pure, Macklin Inc., Shanghai, China) were added as basal fertilizers in the pot experiment. Both exogenous Si and base fertilizers were thoroughly mixed with the tested soil in each pot 14 d prior to pak choi sowing.Pak choi (Brassica rapa subsp. chinensis) seeds were immersed in 30% H2O2 for 30 min before rinsing with deionized water. Then, twenty seeds were initially scattered on the soil surface of each pot, and the seedlings were reduced to five when they reached 5 cm in height. In the period of pak choi growth, deionized water was added to maintain soil moisture at an approximate 75% of its water holding capacity. The growing conditions of pak choi were as follows: a 14 h natural sunlight period per day supplemented with sodium vapor lamps to maintain light intensity >350 μmol m−2 s−1; 28 °C and 20 °C daytime and nighttime temperatures, respectively; and 60–70% relative humidity. Furthermore, to account for possible spatial variations in temperature and light, the pots were randomly placed in a greenhouse.
2.3 Plant sampling and analysis
Pak choi was harvested 45 days after sowing, split into roots and shoots (edible part), rinsed with deionized water, and weighed (fresh weight). Fresh shoots and roots were then frozen in liquid nitrogen, ground, and stored at −80 °C for further analysis.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min. The whole procedure was performed at 4 °C. The contents of MDA and H2O2 in plant tissues were expressed as nanomoles per gram of fresh weight (nmol g−1 FW) and micromoles per gram of fresh weight (μmol g−1 FW), respectively. The antioxidant activities were denoted as per milligram protein (U mg−1 protein) or per gram protein (U g−1 protein).
000 rpm for 10 min. The whole procedure was performed at 4 °C. The contents of MDA and H2O2 in plant tissues were expressed as nanomoles per gram of fresh weight (nmol g−1 FW) and micromoles per gram of fresh weight (μmol g−1 FW), respectively. The antioxidant activities were denoted as per milligram protein (U mg−1 protein) or per gram protein (U g−1 protein).
2.4 Soil sampling and analysis
Following the pak choi harvest, rhizosphere soil samples were collected from each pot.22 The rhizosphere soil samples were separated into two parts: one was frozen in liquid nitrogen and stored at −80 °C for microbial analysis, while the other was air-dried at room temperature and sieved to determine soil pH, available Cd, and sequential Cd extraction.23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5.24 Soil Cd fractionation was classified into five fractions: the exchangeable fraction (Exc-Cd; extracted with 1.0 M of MgCl2, pH = 7), carbonate-bound fraction (CB–Cd; extracted by 1.0 M CH3COONa, pH = 5), iron and manganese oxide-bound fraction (OX-Cd; extracted by 0.04 M NH2OH·HCl in 25% CH3COOH solution), organic matter-bound fraction (OM-Cd; extracted by 0.02 M HNO3 and 30% H2O2), and residual fraction (Res-Cd; digested with HNO3–HF–HClO4). ICP-MS was used to determine the total Cd content as well as that of each fraction. Furthermore, the mobility factor (MF) was determined as a ratio of Cd concentrations in Exc-Cd and CB-Cd to the sum of all fractions to assess Cd mobility in soil. The details of the above analysis methods are described in Text S1.†
2.5.24 Soil Cd fractionation was classified into five fractions: the exchangeable fraction (Exc-Cd; extracted with 1.0 M of MgCl2, pH = 7), carbonate-bound fraction (CB–Cd; extracted by 1.0 M CH3COONa, pH = 5), iron and manganese oxide-bound fraction (OX-Cd; extracted by 0.04 M NH2OH·HCl in 25% CH3COOH solution), organic matter-bound fraction (OM-Cd; extracted by 0.02 M HNO3 and 30% H2O2), and residual fraction (Res-Cd; digested with HNO3–HF–HClO4). ICP-MS was used to determine the total Cd content as well as that of each fraction. Furthermore, the mobility factor (MF) was determined as a ratio of Cd concentrations in Exc-Cd and CB-Cd to the sum of all fractions to assess Cd mobility in soil. The details of the above analysis methods are described in Text S1.†
The sequencing reads were preprocessed with Quantitative Insights Into Microbial Ecology (QIIME, version 1.8.0) software, which included quality filtering and merging. The sequences were then divided into Operational Taxonomic Units (OTUs) based on a 97% similarity level using Upares (version 1.8.0). Uchime was used to identify and delete chimeric sequences. The OTU sequences were then analyzed using the Mothur, QIIME, and R programs for alpha, taxonomy, and beta diversity.
2.5 Statistical analysis
All data for this research were analyzed and exhibited in the form of mean value ± standard deviation (SD; n = 4) utilizing Microsoft Excel 2010. The statistical analysis was performed utilizing SAS v. 9.4 (SAS Institute Inc., USA) with the least significant difference test applied to multiple comparisons when significant differences between treatments were identified (p < 0.05). The principal component analysis, correlation analysis, and figure plotting were all performed with Origin 2021b. Moreover, the direct and indirect effects of related parameters (Si application, soil pH, and Cd in the first four fractions (Exc-Cd, CB-Cd, OX-Cd, and OM-Cd)) on Cd concentrations in pak choi tissues based on hypothesized relationships were examined using a structural equation model (SEM), which was carried out using the Lavaan Package (version 0.6-9) in R (version 4.1.1, R Core Team, Austria 2015). Correlation analysis was used to analyze the relationships of microbial diversity indices and the richness index with soil properties. Furthermore, to examine the association between the soil bacterial community and soil parameters, a redundancy analysis (RDA) was conducted using Canoco 5.0.3 Results
3.1 Plant biomass and Cd accumulation in pak choi tissues
The biomass of pak choi roots and shoots significantly increased with increasing Si dosage (when less than 0.8 g kg−1); nevertheless, the fresh weights of both parts significantly declined by 23.0% and 21.6% at the highest Si dose compared to those with 0.8 g kg−1 of Si (Fig. S1†).As indicated in Fig. 1, Cd concentration in pak choi tissues greatly decreased with the increasing Si dosage with the reductions being more pronounced in the roots (Fig. 1). In comparison to the no Si control, the addition of sodium silicate decreased Cd concentration in roots by 23.5–89.0% and in shoots by 58.5–81.0%. The Si1.0 treatment resulted in the lowest Cd concentration in both roots and shoots (0.36 mg kg−1 fresh weight (FW) for roots and 0.11 mg kg−1 FW for shoots) were both observed. Moreover, the application of Si at 0.4 g Si per kg soil effectively reduced the concentration of Cd in the edible part to the statutory safety limits of 0.2 mg kg−1 (GB 2762-2022).
3.2 Oxidative stress and antioxidative activity in pak choi
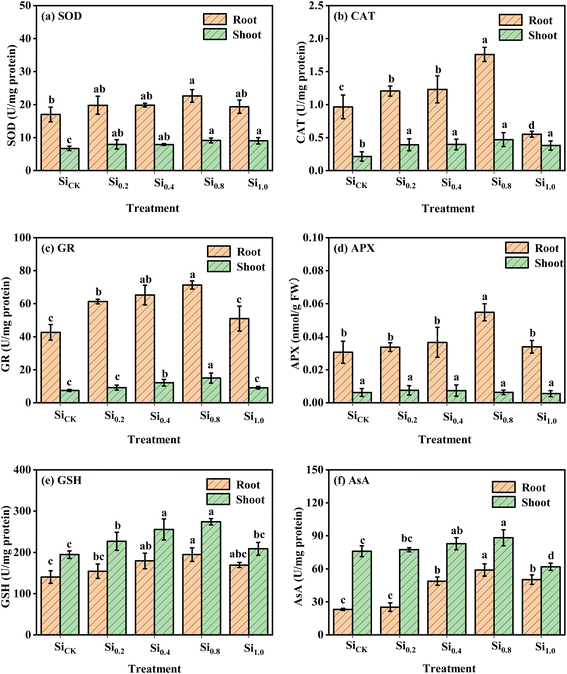
In general, soil sodium silicate application significantly reduced the concentration of MDA and H2O2 in roots and shoots as compared to the no Si control (Fig. S2†), and further reducing effects were observed when higher doses of Si were applied. The lowest MDA and H2O2 concentrations in roots and shoots were observed under the Si0.8 treatment; MDA and H2O2 concentrations in the Si0.8 treatment were 36.9% and 17.7% in roots and 47.8% and 51.0% in shoots, respectively, lower than those of the control, but they were increased by the highest Si treatment (Si1.0) as compared to the Si0.8 treatment, for which they remained below the control levels.Except for APX activity in pak choi shoots, soil treatment of sodium silicate had a significant influence on the activity of all the tested antioxidative enzymes as well as the concentration of GSH and AsA, The application of Si significantly enhanced SOD activity by 14.0–33.1% in roots and by 17.4–36.0% in shoots as compared to the no Si control (Fig. 2a). The addition of Si significantly increased CAT in roots, with the exception of the highest Si treatment (Si1.0) as compared to the no Si control. The Si0.8 treatment exhibited the greatest CAT activity in roots, approximately 1.80-fold higher than that of the no Si control (Fig. 2b). Similar to SOD activity, the application of Si significantly increased shoot CAT activity by 78.2–120% relative to the no Si control, but the Si application rate has no significant effects (Fig. 2b). The GR activity greatly increased with increasing Si application rates from 0.0 to 0.8 g kg−1, and it reached the maximum under the Si0.8 treatment (67.4% in roots and 101% in shoots higher than those of the no Si control (Fig. 2c)). The variation in APX activity was very minor; the only significant difference was observed in shoots at 0.8 g kg−1 of Si compared to the control (Fig. 2d). At the same time, adding Si significantly increased the GSH and AsA concentration in pak choi at Si application rates less than 0.8 g kg−1; the highest GSH and AsA concentrations in pak choi observed after the Si0.8 treatment were 38.5% and 155% in roots and 41.0% and 16.1% in shoots, respectively, higher than that of the no Si control (Fig. 2e and f). However, the concentration of GSH and AsA in pak choi decreased after the highest Si treatment (Si1.0) as compared to the Si0.8 treatment.
3.3 Soil pH, Cd fractions and mobility
Soil application of sodium silicate significantly increased soil pH by approximately 0.29–1.61 units relative to the no Si control treatment, and soil pH increased as the Si application rate increased (Fig. 3a). In terms of Cd fractions in soil, Cd in the exchangeable fraction (Exc-Cd) was the most enriched Cd fraction, followed by Res-Cd (residual fraction), OX-Cd (manganese oxide-bound fraction), CB-Cd (carbonate-bound fraction), and finally OM-Cd (organic matter-bound fraction), regardless of Si application (Fig. 3b). The application of Si had a significant impact on soil Cd fractions, with the exception of Res-Cd. The Exc-Cd fraction had a notably reduction of 5.2–12%, whereas the CB-Cd fraction, OX-Cd fraction, and OM fraction showed a rise of 9.5–21.0%, 5.3–66%, and 6.2–52%, respectively. Additionally, soil Si treatment resulted in a 13.7–20.8% decrease in soil Cd mobility relative to the no Si control (Fig. 3c).3.4 Correlation analyses of plant biomass, Cd concentration in pak choi, and soil properties
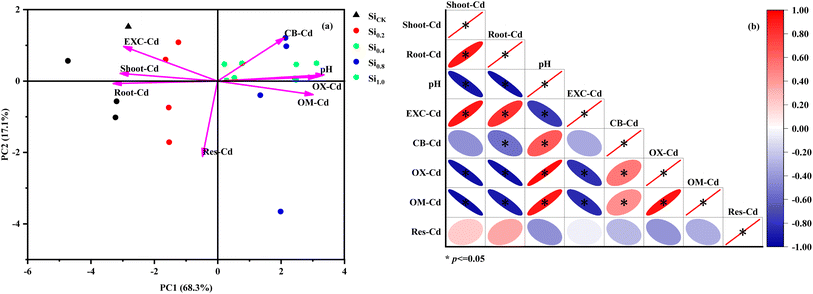
As illustrated in Fig. 4, Cd concentrations in roots and shoots were correlated positively with Exc-Cd, but negatively with soil pH, CB-Cd, OX-Cd, and OM-Cd (Fig. 4a). Additionally, the relationship between plant biomass and Cd concentration in plants, as well as soil properties, revealed that root fresh weight was positively correlated with root-Cd, root SOD, root GR, root MDA, soil pH, and soil Exc-Cd, but negatively correlated with soil pH, root MDA, and root GR (Fig. S3a†), and the shoot findings were consistent with the root results (Fig. S3b†). Furthermore, the principal component analysis revealed that the first principal component accounted for 64.8% of the total variance and the second for 13.2%. Soil pH, CB-Cd, OX-Cd and OM-Cd all showed positive loadings on PC1, whereas Cd in pak choi tissues and Exc-Cd had relatively higher negative loadings (Fig. 4b). Furthermore, the eigen values for each factor were as follows: shoot-Cd 5.46, root-Cd 1.36, soil pH 0.811, Exc-Cd 0.164, CB-Cd 0.092, OX-Cd 0.060, OM-Cd 0.032, and Res-Cd 0.0128, respectively. Moreover, as compared to the no Si control, treatments with higher doses of Si were found to be more displaced to the right along PC1, with the Si1.0 treatments having the highest score, indicating that soil Si application altered soil Cd availability by increasing soil pH and redistributing soil Cd speciation.Additionally, a structural equation model (SEM) was constructed to analyze the pathways that explain the effects of soil Si application on Cd concentration in pak choi. The SEM built in this study was well-fitted (χ2 = 12.226, df = 13, p = 0.509, CFI = 1.000, and GFI = 0.999), and the fitted models explained 99.9% of the variance in pak choi Cd concentration (Fig. 5). According to the path coefficient, soil Si application showed no significant direct effects on Cd accumulation in pak choi, but it did have an indirect impact by significantly affecting soil pH and Cd fractions. As shown in Fig. 5a, soil Si application had significantly positive and direct impacts on the soil pH, CB-Cd, OX-Cd, and OM-Cd, but had negative and direct effects on Exc-Cd. Furthermore, soil pH and CB-Cd had a negative and direct influence on root Cd concentration, but Exc-Cd and OX-Cd had a positive effect. Overall, Si application was the most effective in suppressing Cd accumulation in pak choi, with the highest standardized total effects (direct plus indirect effects reached −0.933), followed by soil pH (direct effects reached −0.676), OX-Cd, CB-Cd, and Exc-Cd (Fig. 7B).
3.5 Soil bacterial community and composition
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 604
604![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 high-quality sequence reads were obtained and clustered into OTUs at a threshold of 97% sequence identity. All samples exhibited coverage indices more than 0.994, indicating that the majority of bacterial communities were detected with high data reliability, and that further sequencing would no longer create new OTUs (Table S1†). Soil Si application significantly increased the richness (expressed by Chao1 and Ace indices) and diversity indices (expressed by Shannon and Simpson indices), but the Si application dose has no significant effects (Table S1†).
800 high-quality sequence reads were obtained and clustered into OTUs at a threshold of 97% sequence identity. All samples exhibited coverage indices more than 0.994, indicating that the majority of bacterial communities were detected with high data reliability, and that further sequencing would no longer create new OTUs (Table S1†). Soil Si application significantly increased the richness (expressed by Chao1 and Ace indices) and diversity indices (expressed by Shannon and Simpson indices), but the Si application dose has no significant effects (Table S1†).
4 Discussion
The objective of this study was to investigate the effects of Si fertilizer on Cd migration, transformation, and accumulation in the soil–pak choi system, as well as to explore the underlying mechanisms. At the same time, the effects of silicon fertilizer addition on soil environmental quality were discussed from the perspective of microorganisms. The findings of the present study demonstrated that Si application could greatly immobilize Cd, decrease its bioavailability, and subsequently reduce Cd accumulation in pak choi tissues. Furthermore, the unique mechanisms of exogenous Si on soil Cd migration and transformation in the soil–vegetable system were discussed and primarily elucidated in the following sections.4.1 Si redistributed Cd fractions and reduced its bioavailability in Cd-contaminated acidic soil
Heavy metal fractions in soil exhibit different mobility and bioavailability characteristics. It has been demonstrated that the transformation of heavy metals from labile to stable fractions is a critical step in immobilization remediation.25,26 In the current study, soil sodium silicate application significantly reduced the proportions of Exc-Cd (Fig. 3b and c), which is easily absorbed by plants, while increasing the proportions of OM-Cd and OX-Cd, which are difficultly absorbed by plants. Consistent with these findings, Ma et al. discovered that Si-rich materials significantly reduced the mobility of Cd,27 indicating that Si application effectively immobilized Cd and hence reduced Cd concentrations in the rice tissues. The primary mechanisms of exogenous Si changing the fraction and bioavailability of soil Cd are as following: first, Si application altered the soil properties (mainly pH), which subsequently altered the soil Cd fraction and the bioavailability. In this study, exogenous Si in the form of sodium silicate was alkaline (pH value of 12.53), with the ability to neutralize soil acidity and enhance soil pH (Fig. 3a). Furthermore, following application, the dissolution of sodium silicate releases SiO42− ions, which can react with water molecules to generate silicic acid (H4SiO4) and four OH− ions, neutralizing H+ ions and increasing soil pH.28 Similarly, numerous research studies have reported that Si-rich materials application increased soil pH.8 It is well established that soil pH is the most critical factor influencing the mobility and bioavailability of heavy metals in polluted soil.29 An elevation in soil pH can result in an increase in negatively charged surface sorption sites, the generation of hydroxyl species of metal cations, and the precipitation of Cd2+ as Cd(OH)2 or CdCO3, ultimately leading to lower Cd mobility and bioavailability in soils.30,31In addition, it has been reported that a high pH led to co-precipitation between iron oxides with heavy metals, which promoted the heavy metal adsorption on soil and reduced heavy metal bioavailability.3 This is supported by the present results that soil sodium silicate application significantly increased the fractions of OX-Cd (Fig. 3b). Furthermore, the application of Si caused the redistribution of Cd fractions in soil by forming Si–Cd complexes, which facilitated the transformation of Cd from easily exchangeable fractions to more stable fractions. Xiao15 discovered that Si application promotes the transformation of soil Cd and Pb from acid soluble and reducible fractions to oxidizable and residual fractions, decreasing their bioavailability and rice absorption. Previous research recorded that Cd2+ in soil could react with silicic acid to form a Si–Cd complex, which the plant root cannot absorb directly.16,19 Moreover, researchers have used different spectroscopic techniques to confirm the formation of the Si–Cd complex in soil with Si application.16 Additionally, silicate in soil could polymerize and link to iron oxide surfaces, then complex with ferrosilicon to produce a large number of negatively charged functional groups, such as silanol, reducing the bioavailability of Cd2+ by providing many adsorption sites.32 However, the micro-mechanism of Cd transfer and transformation in soil by sodium silicate application is not well established and requires further investigation. Based on the findings and discussion presented above, the following conclusions could be drawn: in Cd-contaminated acidic soil, sodium silicate application reduced soil Cd bioavailability by increasing soil pH and redistributing the Cd fraction from exchangeable fractions to more stable fractions, resulting in a lower Cd availability to pak choi roots. However, due to the complex conditions of wild fields, the actual performance of soil amendments on the field scale may be inconsistent with their effects in pot experiments, and thus more investigation is needed to evaluate the performance of sodium silicate in the field-scale actual environment.
4.2 Si reduced Cd accumulation and alleviated Cd toxicity in pak choi tissues
Research has been extensively performed to minimize Cd uptake by plants through Si application. In this study, soil sodium silicate application significantly reduced Cd accumulation in pak choi (Fig. 1). Similar findings have been reported in wheat10 and rice.12 A possible explanation for these findings is that sodium silicate application reduced soil Cd bioavailability by increasing soil pH and redistributing Cd fractions from exchangeable fractions to more stable fractions. This was verified by the SEM results, which demonstrated that Si application had an indirect impact on Cd concentration in pak choi via its strong effect on soil pH and Cd fractions (Fig. 5). Furthermore, standardized total effects derived from SEM further indicated that Cd concentration in pak choi was mainly driven by soil pH, followed by OX-Cd, CB-Cd, and Exc-Cd. The above findings indicate that the predominated mechanisms involving sodium silicate for Cd migration and transformation in the soil–pak choi system is by regulating the soil pH value. Moreover, the reduction in Cd concentrations in plants caused by Si application is not just related to its immobilization effects on soil Cd bioavailability, but could also result from its reducing effect on root uptake. Previous studies33 discovered that applying silicates at a lower rate had no effect on soil pH but greatly reduced Cd uptake and transport in Cd-stressed maize. Furthermore, Si has been shown to suppress the expression of genes involved in Cd absorption and transportation (OsHMA2 and OsNramp5).17 Zhou et al. similarly observed that Si application inhibited Cd uptake in wheat growing in Cd-contaminated soils by upregulating the expression of efflux transporters (TaTM20 and TaHMA3) and downregulating the expression of influx transporters (TaNramp5).10Generally, plant biomass reflects plant growth conditions under abiotic stress, and it is also a significant indicator for accessing plant tolerance to Cd toxicity.34 The present study found that soil sodium silicate application greatly increased the biomass of pak choi (Fig. S1†), indicating that sodium silicate application could alleviate Cd toxicity in pak choi. Comparable findings have been reported in Chinese cabbage,35 wheat,10 soybean,13 and rice.17 The underlying mechanisms could be described as follows. Firstly, exogenous addition of Si alleviated Cd toxicity in pak choi by reducing Cd accumulation through increasing soil pH and redistributing Cd fractions, as minimizing Cd uptake and accumulation is critical to decrease Cd toxicity in plants. Secondly, exogenous Si improved plant defense capacity against oxidative damage provoked by the heavy metal through enhancing the activity of the antioxidant system.10,13 And in this research, the results of correlations among the biomass, MDA and H2O2 content, antioxidant enzyme activity, and antioxidant content confirmed the above notion (Fig. S3†). In general, Cd stress causes the generation of reactive oxygen species (ROS), which inhibits plant development and reduces biomass.13 To scavenge excess ROS and decrease oxidative damage, plants have developed defense systems include enzymatic and non-enzymatic antioxidant mechanisms.36 In general, Cd stress generates reactive oxygen species (ROS), inhibiting plant development and reducing biomass.13 Plants have developed defense systems to scavenge excess ROS and reduce oxidative damage, including enzymatic and non-enzymatic antioxidant mechanisms. In the present study, soil sodium silicate application increased antioxidant enzyme activities (SOD, CAT, GR, and APX) as well as antioxidant concentration (GSH and AsA) to maintain a higher antioxidant capacity, thereby alleviating Cd-induced oxidative stress as evidenced by lower MDA and H2O2 contents in pak choi tissues (Fig. 2, S2 and S3†). These findings all showed that soil sodium silicate application could increase the antioxidant capacity of pak choi and alleviate Cd toxicity.
4.3 Effects of Si application on the soil environment
In the study, sodium silicate application effectively immobilized Cd significantly by increasing soil pH and redistributing the Cd fraction from labile fractions to stable fractions, and consequently reduced Cd accumulation in pak choi. However, as an additive to soil, the application of soil amendments may pose a risk of heavy metal accumulation in soil. For sodium silicate itself, heavy metal levels in sodium silicate are much lower than the ecological index of As, Cd, Pb, Cr, and Hg in fertilizers (GB/T 23349-2009). Furthermore, sodium silicate mainly composed of Na, Si, and a small amount of the Fe element, would not cause heavy metal pollution in soil. Therefore, possible harm from the sodium silicate application to the soil environment is very low. However, it is worth noting that although sodium silicate effectively immobilized Cd in contaminated soil, excessive application of sodium silicate may result in soil alkalization and soil hardness. Furthermore, even though the highest dose of sodium silicate application resulted in the lowest Cd concentration in pak choi tissues, Si application at 0.4 g kg−1 (Si0.4) was successful in reducing Cd concentration in the edible part to the safety limits for Cd. Moreover, the Si0.8 treatment produced the highest fresh weights of pak choi, as well as the highest concentrations of MDA and H2O2 concentration in pak choi tissues, which were all recorded in the Si0.8 treatment. Herein, the application dosage should be considered for practical application in the field, and further long-term trials are required to determine the optimal dosage.Besides, it is well recognized that Cd contamination affects soil microbial biological activity; as indicators of soil environment quality, soil microbes are vital for material cycling, energy flow, and nutrient conversion. The microbial community in the current study differed noticeably between Si treatments and control samples; additionally, sodium silicate application significantly increased soil bacterial richness (Chao1 and Ace indices) and diversity (Shannon and Simpson indices). Rhizosphere-associated microorganisms are important in reducing Cd toxicity and promoting plant growth,37 and the rhizosphere of plants cultivated in Cd-contaminated soils has been identified to contain the majority of the prevalent bacteria at the phylum level (e.g., Proteobacteria, Actinobacteria, and Bacteroidetes).38 According to a previous study,39Proteobacteria and Actinobacteria are the most active bacteria in soils contaminated with heavy metals, and the current study found that their relative abundances accounted for more than 60% (Fig. 6). Furthermore, sodium silicate application reduced Actinobacteria abundance while increasing Bacteroidetes, Acidobacteria, Verrucomicrobia, and Planctomycetes abundance. Previous research has shown that Actinobacteria are tolerant of Cd whereas Proteobacteria, Bacteroidetes, and Verrucomicrobia are susceptible to it.40,41 Furthermore, soil pH and Cd in the first four fractions were the primary factors determining the variation of bacterial communities (Fig. 7). Similarly, previous studies38 discovered that the environment index and Cd faction were largely related to the microbial community. Among soil properties, soil pH is a key factor influencing the microbial community and activity. Soil pH can affect the precipitation-dissolution balance and soil Cd bioavailability of Cd in soil, resulting in a shift in abundances of Cd-sensitive/tolerant bacteria, and consequently considerably influencing microbial abundance and diversity.40 Furthermore, based on the current findings, more in-depth research in the future is needed to investigate the effect of sodium silicate on Cd migration and transformation in the soil–pak choi system via microbial activity using the soil microbiome and metabolomics technology. Taken together, these findings revealed that the fluctuations in the bacterial community were driven by variation of soil treatment with sodium silicate, and all of the above results indicate that soil immobilization with sodium silicate helped maintain soil functionality in Cd-contaminated acidic soil. Furthermore, long-term effectiveness and persistence of sodium silicate application for remediating Cd-contaminated acidic soil, as well as concerns about the effects on soil health and crop yields require evaluation on the field-scale.
5 Conclusions
The current investigation demonstrated that sodium silicate application significantly increased soil pH and induced the transformation of the Cd fraction from Exc-Cd to OM-Cd and OX-Cd, resulting in a lower Cd bioavailability in Cd-contaminated acidic soil. As a result, effective immobilization of Cd in polluted soil with sodium silicate application significantly reduced Cd toxicity and accumulation in pak choi. Meanwhile, soil bacterial richness and diversity were significantly increased by sodium silicate application, and the microbial community differed noticeably between Si treatments and no Si control. Furthermore, soil pH and Cd in the first four fractions were the primary factors determining the variation of bacterial communities, and soil pH was the most important factor controlling pak choi Cd accumulation after exogenous Si treatment, according to a structural equation model (SEM). These findings suggested that sodium silicate has great potential as an environmentally friendly amendment to immobilize Cd-contaminated acidic soil if applied at an appropriate dosage. Further research is required to evaluate the environmental safety and long-term stability of sodium silicate application in the field, thereby supporting large-scale soil remediation with sodium silicate.Data availability
Data will be made available on request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (41601343) and Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS–CSGLCA–202302).References
- E. Kobayashi, Y. Suwazono, M. Dochi, R. Honda and T. Kido, Influence of Consumption of Cadmium-Polluted Rice or Jinzu River Water on Occurrence of Renal Tubular Dysfunction and/or Itai-itai Disease, Biol. Trace Elem. Res., 2009, 127, 257–268 CrossRef CAS PubMed.
- P. Wang, H. Chen, P. M. Kopittke and F.-J. Zhao, Cadmium contamination in agricultural soils of China and the impact on food safety, Environ. Pollut., 2019, 249, 1038–1048 CrossRef CAS PubMed.
- X. Liu, S. Gu, S. Yang, J. Deng and J. Xu, Heavy metals in soil-vegetable system around E-waste site and the health risk assessment, Sci. Total Environ., 2021, 779, 146438 CrossRef CAS PubMed.
- R. L. Mnisi, P. P. Ndibewu, L. D. Mafu and G. C. Bwembya, Bioaccessibility and risk assessment of essential and non-essential elements in vegetables commonly consumed in Swaziland, Ecotoxicol. Environ. Saf., 2017, 144, 396–401 CrossRef CAS PubMed.
- H. Fang, W. Li, S. Tu, Y. Ding, R. Wang, C. Rensing, Y. Li and R. Feng, Differences in cadmium absorption by 71 leaf vegetable varieties from different families and genera and their health risk assessment, Ecotoxicol. Environ. Saf., 2019, 184, 109593 CrossRef CAS.
- L. Huang, Q. Wang, Q. Zhou, L. Ma, Y. Wu, Q. Liu, S. Wang and Y. Feng, Cadmium uptake from soil and transport by leafy vegetables: A meta-analysis, Environ. Pollut., 2020, 264, 114677 CrossRef CAS PubMed.
- H. Chen, X. Yang, P. Wang, Z. Wang, M. Li and F.-J. Zhao, Dietary cadmium intake from rice and vegetables and potential health risk: A case study in Xiangtan, southern China, Sci. Total Environ., 2018, 639, 271–277 CrossRef CAS.
- K. Zhao, Y. Yang, H. Peng, L. Zhang, Y. Zhou, J. Zhang, C. Du, J. Liu, X. Lin, N. Wang, H. Huang and L. Luo, Silicon fertilizers, humic acid and their impact on physicochemical properties, availability and distribution of heavy metals in soil and soil aggregates, Sci. Total Environ., 2022, 822, 153483 CrossRef CAS PubMed.
- P. Zeng, J. Liu, H. Zhou, B. Wei, J. Gu, Y. Liao, B. Liao and X. Luo, Co-application of combined amendment (limestone and sepiolite) and Si fertilizer reduces rice Cd uptake and transport through Cd immobilization and Si–Cd antagonism, Chemosphere, 2023, 316, 137859 CrossRef CAS.
- J. Zhou, C. Zhang, B. Du, H. Cui, X. Fan, D. Zhou and J. Zhou, Soil and foliar applications of silicon and selenium effects on cadmium accumulation and plant growth by modulation of antioxidant system and Cd translocation: Comparison of soft vs. durum wheat varieties, J. Hazard. Mater., 2021, 402, 123546 CrossRef CAS.
- Y. Zhao, M. Liu, L. Guo, D. Yang, N. He, B. Ying and Y. Wang, Influence of silicon on cadmium availability and cadmium uptake by rice in acid and alkaline paddy soils, J. Soils Sediments, 2020, 20, 2343–2353 CrossRef CAS.
- Y. Cai, B. Pan, B. Liu, K. Cai, J. Tian and W. Wang, The Cd sequestration effects of rice roots affected by different Si management in Cd-contaminated paddy soil, Sci. Total Environ., 2022, 849, 157718 CrossRef CAS PubMed.
- Q. Zhou, Z. Cai, P. Xian, Y. Yang, Y. Cheng, T. Lian, Q. Ma and H. Nian, Silicon-enhanced tolerance to cadmium toxicity in soybean by enhancing antioxidant defense capacity and changing cadmium distribution and transport, Ecotoxicol. Environ. Saf., 2022, 241, 113766 Search PubMed.
- Y. Wang, K. Zhang, L. Lu, X. Xiao and B. Chen, Novel insights into effects of silicon-rich biochar (Sichar) amendment on cadmium uptake, translocation and accumulation in rice plants, Environ. Pollut., 2020, 265, 114772 CrossRef CAS PubMed.
- Z. Xiao, M. Peng, Y. Mei, L. Tan and Y. Liang, Effect of organosilicone and mineral silicon fertilizers on chemical forms of cadmium and lead in soil and their accumulation in rice, Environ. Pollut., 2021, 283, 117107 CrossRef CAS PubMed.
- L. Guo, A. Chen, C. Li, Y. Wang, D. Yang, N. He and M. Liu, Solution chemistry mechanisms of exogenous silicon influencing the speciation and bioavailability of cadmium in alkaline paddy soil, J. Hazard. Mater., 2022, 438, 129526 CrossRef CAS PubMed.
- J. Feng Shao, J. Che, N. Yamaji, R. Fang Shen and J. Feng Ma, Silicon reduces cadmium accumulation by suppressing expression of transporter genes involved in cadmium uptake and translocation in rice, J. Exp. Bot., 2017, 68, 5641–5651 CrossRef PubMed.
- B. Wang, C. Chu, H. Wei, L. Zhang, Z. Ahmad, S. Wu and B. Xie, Ameliorative effects of silicon fertilizer on soil bacterial community and pakchoi (Brassica chinensis L.) grown on soil contaminated with multiple heavy metals, Environ. Pollut., 2020, 267, 115411 CrossRef CAS PubMed.
- D. Bhaduri, D. Sihi, A. Bhowmik, B. C. Verma, S. Munda and B. Dari, A review on effective soil health bio-indicators for ecosystem restoration and sustainability, Front. Microbiol., 2022, 13, 938481 CrossRef.
- S. Tiwari and C. Lata, Heavy Metal Stress, Signaling, and Tolerance Due to Plant-Associated Microbes: An Overview, Front. Plant Sci., 2018, 9, 452 CrossRef PubMed.
- S. D. Bao, Soil and Agricultural Chemistry Analysis, China Agricultural Press, 2000 Search PubMed.
- D. Zhu, Y. Niu, K. Fan, F. Zhang, Y. Wang, G. Wang and S. Zheng, Selenium-oxidizing Agrobacterium sp. T3F4 steadily colonizes in soil promoting selenium uptake by pakchoi (Brassica campestris), Sci. Total Environ., 2021, 791, 148294 CrossRef CAS PubMed.
- R. S. Lu, The Analysis Method of Soil Agricultural Chemistry, China Agricultural Science and Technology Press, 2000 Search PubMed.
- A. Tessier, P. G. C. Campbell and M. Bisson, Sequential extraction procedure for the speciation of particulate trace metals, Anal. Chem., 1979, 51, 844–851 CrossRef CAS.
- F. Guo, C. Ding, Z. Zhou, G. Huang and X. Wang, Stability of immobilization remediation of several amendments on cadmium contaminated soils as affected by simulated soil acidification, Ecotoxicol. Environ. Saf., 2018, 161, 164–172 CrossRef CAS PubMed.
- X. Liang, N. Li, L. He, Y. Xu, Q. Huang, Z. Xie and F. Yang, Inhibition of Cd accumulation in winter wheat (Triticum aestivum L.) grown in alkaline soil using mercapto-modified attapulgite, Sci. Total Environ., 2019, 688, 818–826 CrossRef CAS PubMed.
- C. Ma, K. Ci, J. Zhu, Z. Sun, Z. Liu, X. Li, Y. Zhu, C. Tang, P. Wang and Z. Liu, Impacts of exogenous mineral silicon on cadmium migration and transformation in the soil-rice system and on soil health, Sci. Total Environ., 2021, 759, 143501 CrossRef CAS PubMed.
- J. F. Ng, O. H. Ahmed, M. B. Jalloh, L. Omar, Y. M. Kwan, A. A. Musah and K. H. Poong, Soil Nutrient Retention and pH Buffering Capacity Are Enhanced by Calciprill and Sodium Silicate, Agronomy, 2022, 12, 219 CrossRef CAS.
- H.-Y. Yu, C. Liu, J. Zhu, F. Li, D.-M. Deng, Q. Wang and C. Liu, Cadmium availability in rice paddy fields from a mining area: The effects of soil properties highlighting iron fractions and pH value, Environ. Pollut., 2016, 209, 38–45 CrossRef CAS PubMed.
- Y. Sun, Y. Xu, Y. Xu, L. Wang, X. Liang and Y. Li, Reliability and stability of immobilization remediation of Cd polluted soils using sepiolite under pot and field trials, Environ. Pollut., 2016, 208, 739–746 CrossRef CAS.
- Y. Yang, Y. Li, M. Wang, W. Chen and Y. Dai, Limestone dosage response of cadmium phytoavailability minimization in rice: A trade-off relationship between soil pH and amorphous manganese content, J. Hazard. Mater., 2021, 403, 123664 CAS.
- M. Stein, A. Georgiadis, J. Ingwersen and T. Rennert, Does silica addition affect translocation and leaching of cadmium and copper in soil?, Environ. Pollut., 2021, 288, 117738 CrossRef CAS.
- Y. Liang, J. W. C. Wong and L. Wei, Silicon-mediated enhancement of cadmium tolerance in maize (Zea mays L.) grown in cadmium contaminated soil, Chemosphere, 2005, 58, 475–483 CrossRef CAS.
- Z.-W. Zhang, Y.-Y. Dong, L.-Y. Feng, Z.-L. Deng, Q. Xu, Q. Tao, C.-Q. Wang, Y.-E. Chen, M. Yuan and S. Yuan, Selenium Enhances Cadmium Accumulation Capability in Two Mustard Family Species—Brassica napus and B. juncea, Plants, 2020, 9, 904 CrossRef CAS.
- Z. Wu, S. Liu, J. Zhao, F. Wang, Y. Du, S. Zou, H. Li, D. Wen and Y. Huang, Comparative responses to silicon and selenium in relation to antioxidant enzyme system and the glutathione-ascorbate cycle in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress, Environ. Exp. Bot., 2017, 133, 1–11 CrossRef CAS.
- O. Sytar, A. Kumar, D. Latowski, P. Kuczynska, K. Strzałka and M. N. V. Prasad, Heavy metal-induced oxidative damage, defense reactions, and detoxification mechanisms in plants, Acta Physiol. Plant., 2013, 35, 985–999 CrossRef CAS.
- S. R. Manoj, C. Karthik, K. Kadirvelu, P. I. Arulselvi, T. Shanmugasundaram, B. Bruno and M. Rajkumar, Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review, J. Environ. Manage., 2020, 254, 109779 CrossRef CAS PubMed.
- X. Li, B. Li, Y. Zheng, L. Luo, X. Qin, Y. Yang and J. Xu, Physiological and rhizospheric response characteristics to cadmium of a newly identified cadmium accumulator Coreopsis grandiflora Hogg. (Asteraceae), Ecotoxicol. Environ. Saf., 2022, 241, 113739 CrossRef CAS PubMed.
- R. Margesin, G. A. Płaza and S. Kasenbacher, Characterization of bacterial communities at heavy-metal-contaminated sites, Chemosphere, 2011, 82, 1583–1588 CrossRef CAS PubMed.
- L. Y. Luo, L. L. Xie, D. C. Jin, B. B. Mi, D. H. Wang, X. F. Li, X. Z. Dai, X. X. Zou, Z. Zhang, Y. Q. Ma and F. Liu, Bacterial community response to cadmium contamination of agricultural paddy soil, Appl. Soil Ecol., 2019, 139, 100–106 CrossRef.
- Q. Liu, Z. Chen, J. Tang, J. Luo, F. Huang, P. Wang and R. Xiao, Cd and Pb immobilisation with iron oxide/lignin composite and the bacterial community response in soil, Sci. Total Environ., 2022, 802, 149922 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4em00583j |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |