Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Membrane technology for water reuse in decentralised non-sewered sanitation systems: comparison of pressure driven (reverse osmosis) and thermally driven processes (membrane distillation and pervaporation)†
E.
Mercer
 ab,
C.
Davey
a,
Y. Bajón
Fernández
a,
S.
Septien
b,
S.
Tyrrel
a,
E.
Cartmell
c,
M.
Pidou
a and
E. J.
McAdam
ab,
C.
Davey
a,
Y. Bajón
Fernández
a,
S.
Septien
b,
S.
Tyrrel
a,
E.
Cartmell
c,
M.
Pidou
a and
E. J.
McAdam
 *a
*a
aCranfield Water Science Institute, Vincent Building, Cranfield University, Bedfordshire, UK. E-mail: e.mcadam@cranfield.ac.uk
bWASH R&D Centre, School of Engineering, University of KwaZulu-Natal, Durban, 4041, South Africa
cScottish Water, Castle House, Carnegie Campus, Dunfermline, UK
First published on 19th August 2024
Abstract
Membrane processes are an established barrier technology for water reclamation from wastewater. Applied at a household scale to improve sanitation practice, membrane technology can disrupt the source–receptor pathway, alleviate water scarcity through eliminating flush water and recover clean water for reuse. However, blackwater comprises a distinct composition compared to municipal wastewater, and there is only limited understanding on whether membrane selectivity is sufficient to produce water of sufficient quality for reuse. In this study, pressure driven and thermally driven membranes are evaluated for their potential to treat blackwater, by relating selectivity to relevant water quality standards (ISO 30500) and the transmission of volatile organic compounds (VOCs) that are primarily associated with faecal odour, and thus constitute a critical challenge to water reuse. Both pressure driven (reverse osmosis) and thermally driven (membrane distillation and pervaporation) membranes were able to produce water that conformed to category B of the ISO 30500 standard for the majority of determinants. A critical limiting factor was in the selectivity for ammonia and odorous VOCs which were generally poorly removed by reverse osmosis and membrane distillation. The high ammonia transmission was accounted for by the elevated pH of blackwater which shifted the ammonium equilibria toward volatile ammonia which is poorly separated by RO polymers, and is free to diffuse through the gas-filled micropores of the membrane distillation membrane. In contrast, greater ammonia and VOC separation was evidenced for the pervaporation membrane due to advanced polymer–solute interactions. In a preliminary assessment, the hydrophilicity exhibited by the membrane was also advantageous to withstanding fouling. If complemented with a polishing step to target the residual COD and VOCs (that may be of similar origin), pervaporation could deliver to category A standard for non-potable reuse. This is particularly advantageous for water scarce regions where solar or liquified fuels may be applied in favour of electricity for off-grid sanitation.
Water impactThermally driven membrane processes have demonstrated water reuse potential from concentrated blackwater in terms of water quality, odour management and flux robustness. Facilitated by off grid heat sources such as waste heat or cooking gas, this presents an economically accessible alternative to conventional pressure driven membrane processes which rely on electricity. |
1. Introduction
Two billion people lack access to adequate toilet facilities, resulting in over 673 million people having to defecate in the open, engendering transmission of diseases such as cholera, typhoid, polio, hepatitis A and trachoma.1 The United Nations' Sustainable Development Goal 6 (SDG 6) recognises this substantive challenge to human health, setting an agenda to provide access to clean water and sanitation for all by 2030, whilst mandating water reuse as a critical tool to alleviate water scarcity.2 However, implementing capital intensive centralised (sewered) wastewater infrastructure into low income countries (LICs) is economically challenging,3 instead deferring to decentralised sanitation, primarily using pit latrines, which pose health risks through contamination of local water sources via poor installation, operation or through the unregulated environmental discharge of faecal sludge.4 This challenge is not limited to LICs, where comparable inequity is identified in developed nations. For example, more than 2 million US citizens live without running water and basic indoor plumbing, whilst inadequate wastewater systems have been identified in up to 90% of households in discrete low-income regions of the US, resulting in high incidence of hookworm infection, transmitted via the faecal–oral route.1Barrier technology that can be practically implemented within a decentralised context presents an opportunity to break the source–receptor pathway, and offers protection to 29% of the global population that depend on water supplies of unknown provenance, which are commonly contaminated from poor sanitation practice.2 This is the key remit of the recently published ISO 30500 standard on non-sewered sanitation, which places emphasis on water reuse, to readdress water inequality introduced through water scarcity. This was exemplified by the approach of ‘day zero’ in Chennai and Cape Town, where communal water supply was no longer sustainable,5 which emphasised how important recovering even small water volumes could be, and may help to revalue barrier technology for implementation into the decentralised context. Membrane processes are an established barrier technology capable of direct and indirect potable water reuse schemes from municipal wastewater.6 The conventional two-stage ultrafiltration (UF) and reverse osmosis (RO) system used is commercially available for point-of-use potable applications (single household), demonstrating that economies of scale exist and may be amenable for decentralised sanitation. However, for point-of-use, power consumption is avoided through pre-pressurisation of the main supply, whereas a UF-RO sanitation system requires a feed pump to overcome osmotic pressures of ∼30 bar, due to the high salt content of urine (∼248 mEq L−1).7,8 This increases system cost and introduces a high peak power demand (but a low net energy demand). Membrane distillation (MD) and pervaporation (PV) employ a vapour pressure gradient to deliver permeate quality comparable to RO for desalination applications, due to the selectivity that is achievable for non-volatile contaminants.9–11 Whilst the source of heat restricts MD and PV, market penetration of liquefied fuels (propane and butane) has accelerated through government schemes (e.g. PMUY program) seeking to grow cleaner cooking fuels, and to ensure energy equity for women in below-poverty-line households.12 This has resulted in market penetration of liquified fuels into India of 95%, which exceeds grid produced electricity coverage, and as the unitary cost ($ kWh−1) is around 20% of power, thermally driven membrane processes may present a viable economic alternative for decentralised sanitation. This is complementary to other accessible heat sources including low grade waste heat and solar heat energy ranging from 4.5–7.5 kWh m−2 in water scarce areas.13
Non-sewered sanitation (NSS) systems typically accept blackwater, often with limited or no flush water, as transport within the sewerage network is no longer demanded.14 The resultant blackwater therefore comprises urine and faeces and is more concentrated than conventional blackwater, by over an order of magnitude.15 This feed chemistry is considerably more complex, comprising higher concentrations of pathogens, a broader MW (molecular weight) range of organics, and a transient solution chemistry (e.g. pH), all of which risk reducing permeate quality. The ISO 30500 standard assesses the safety of the outputs of an NSS technology treating blackwater. Pressure driven UF-RO and thermally driven MD have shown to comply to most of the ISO 30500 standards treating CB,10,16–18 while the robust MD operation achieving all ISO 30500 parameters has been demonstrated with upfront UF, which through size exclusion reduces the particulate and colloidal fraction contributing to membrane wetting, or utilising smaller pore sizes to increase the liquid entry pressure.18,19
The transport mechanisms in RO and MD are distinct and their role in fostering selectivity remains poorly understood for a wider variety of compound chemistries distinct to CB, particularly gaseous compounds or odour causing volatile organic compounds. While odour emissions are considered in the ISO 30500 standards, they are specific to the gas phase and not the liquid phase. However, taste and odour (T&O) is an aspect critical to water reuse as data from LIC surveys indicate that even if safe, malodourous water would likely be discarded, in preference for visually lower quality water that was odourless.20 Due to the volatile nature of these contaminants, the selectivity of the thermally driven processes is also challenged. Mercer et al.21 demonstrated the rejection and selective enrichment of faecal volatile T&O compounds by PV using hydrophilic and hydrophobic polymers respectively. This ultimately altered the compound concentration and mixture profile of VOCs, which are known to influence perception.21–24 Pervaporation is a thermally driven membrane process like MD. Although, unlike MD which is microporous like UF, it also possesses similarities to RO through the use of a dense membrane, where mass transfer is governed by interactions between the compound and polymer through a solution-diffusion model, with additional selectivity though steric hindrance size exclusion.25 Pervaporation may therefore provide an additional selective barrier for gas phase separation in thermal processes, with the polymer offering distinctive selectivity to the polymers employed in RO. While PV has been investigated for water recovery from urine26–32 and model faecal volatile organic compounds, it has not been examined with CB.
This study therefore aims to evaluate the suitability of pressure driven (dense RO) and thermally driven membrane processes (microporous MD and dense PV-hydrophilic and hydrophobic) for delivering safe sanitation and water reuse within a decentralised context. Specific objectives are to: (i) benchmark permeate quality against water reuse standards proposed for non-sewered sanitation (ISO 30500); (ii) evaluate the transport of the volatile fraction to determine how the driving force and material characteristics influence reuse quality; and (iii) undertake preliminary assessment of membrane permeability in response to concentrated blackwater treatment.
2. Materials and methods
2.1 Experimental setup
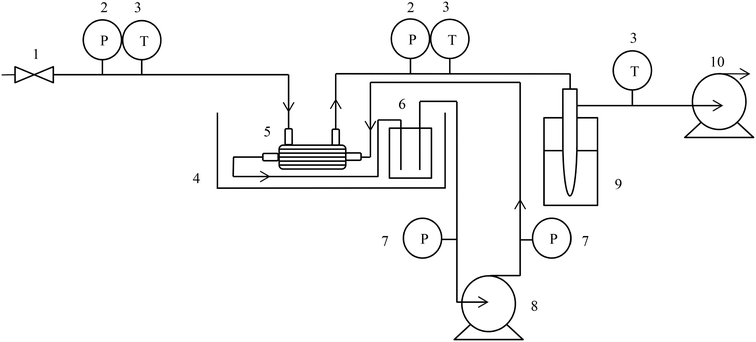
Pressure driven RO was operated in a HP4750 cell (Sterlitech, USA) at 12 bar (supplied by compressed air) in dead-end batch mode (Table 1). A magnetic stirrer (part of the HP4750 cell) was operated at 400 rpm on the feed side of the membrane to limit concentration polarisation. Flat sheet membranes from Sterlitech (TriSep X201, USA) were cut to an effective membrane area of 0.00146 m2. Permeate samples were collected within an airtight 50 mL glass conical flask. Flux was determined by measurement of the temporal permeate mass up to a standardised filtered volume of 10 L m−2 (PR410 Symmetry, Cole-Parmer Ltd., London, UK). For thermally driven membranes (PV and MD, Fig. 1 and Table 1), the feed was circulated at 0.2 L min−1 and vacuum was applied at 50 mbar on the permeate side using a diaphragm vacuum pump (MD 4C NT, Vacuubrand, Brackley). Flat sheet hydrophilic polyvinyl alcohol (PVA) membranes (DeltaMem, Pervap 4101, Switzerland) provided a membrane area of 0.0153 m2 (Model Products, UK). Hollow fibre (HF) polydimethylsiloxane (PDMS) (Permselect, PDMSXA-2500, USA) and hydrophobic microporous polypropylene (pore size 0.04 μm) (3M-Liqui-Cel, MM 1.7 × 5.5, USA) possessed membrane areas of 0.25 m2 and 0.54 m2 respectively. The permeate was collected through a condenser (2 °C) until a standardised filtered volume of 1 L m−2 was reached (Fig. 1). A liquid nitrogen cold trap (−196 °C) was used for permeate collection during the determination of VOCs. All membranes were operated at 50 °C within a thermostatic bath (Grant TC120, Cambridge, UK) to provide parity between each process. Pressure driven membranes were also operated at ambient temperature (20 °C). A virgin membrane was utilised for each experiment. Relative water flux was calculated by: | (1) |
| Reverse osmosis | Pervaporation | Membrane distillation | ||
|---|---|---|---|---|
| a Vacuum pressure; NAp: not applicable; NAv: not available. b Baker et al.25 c Liu et al.33 d Experimentally derived using the sessile drop method, Strobel et al.34 e Mark.35 f Bormashenko et al.36 | ||||
| Manufacturer (model) | TriSep | DeltaMem | Permselect | 3M™ Liqui-Cel™ |
| Model | (X201) | (Pervap™ 4101) | (PDMSXA-2500) | (MM1.7 × 5.5) |
| Material | Polyamide-urea | Polyvinyl alcohol | Polydimethylsiloxane | Polypropylene |
| Typical application | Desalination | Purification of organic mixtures | Removal of trace organic solvents from in industrial wastewaters | Desalination, process water treatment |
| Driving force | Pressure | Vapour pressure gradient | Vapour pressure gradient | Vapour pressure gradient |
| Membrane area (m2) | 0.00146 | 0.0153 | 0.25 | 0.54 |
| Membrane thickness (μm) | 100–150 | 0.5 | 55 | NAv |
| Pore size (μm) | <0.0005b | <0.0005b | <0.0005b | 0.04 |
| Contact angle | 28.5c | 43d | 116d | 104e |
| Hansen solubility δ (MPa m−1/2) | NAv | 25.78f | 15.59f | NAp |
| Geometry | Flat sheet | Flat sheet | Hollow fibre | Hollow fibre |
| Feed volume (mL) | 300 | 600 | 500 | 500 |
| Permeate volume (mL) | 20 | 20 | 5, 10, 20 | 5, 10, 20 |
| Recommended pressure (bar) | 7–21 | ≤1 | ≤1 | 0.04–0.2 |
| Operating pressure (bar) | 12 | 0.05a | 0.05a | 0.05a |
| Recommended operating temperature (°C) | 2–45 | ≤50 | ≤60 | 40 |
| Operating temperature (°C) | 20, 50 | 50 | 50 | 50 |
2.2 Feed preparation and analysis
Concentrated blackwater was prepared at a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 urine to faeces mass ratio, as this represents the typical proportion produced by an individual per day.37 Samples were vortexed for 30 seconds to homogenise, to simulate conditions similar to a dynamic system where agitation occurs. This ratio can be considered representative of the maximum faecal contamination of the solution phase, expected within NSS systems using a waterless flush (i.e. no flush water). The sample was pretreated by a passive filtration step through sand and cotton wool to limit the coarse material (e.g. unmasticated food) which could lead to blockage during pumping. All experiments were performed in accordance with guidelines set out on the anonymous collection of fresh urine and faeces from consenting volunteers and disposed of as biohazardous waste or through the normal sewerage system. This protocol was approved by the Cranfield University Research Ethics System (CURES, project ID 3022). Informed consents were obtained from human participants volunteering for this study.
1 urine to faeces mass ratio, as this represents the typical proportion produced by an individual per day.37 Samples were vortexed for 30 seconds to homogenise, to simulate conditions similar to a dynamic system where agitation occurs. This ratio can be considered representative of the maximum faecal contamination of the solution phase, expected within NSS systems using a waterless flush (i.e. no flush water). The sample was pretreated by a passive filtration step through sand and cotton wool to limit the coarse material (e.g. unmasticated food) which could lead to blockage during pumping. All experiments were performed in accordance with guidelines set out on the anonymous collection of fresh urine and faeces from consenting volunteers and disposed of as biohazardous waste or through the normal sewerage system. This protocol was approved by the Cranfield University Research Ethics System (CURES, project ID 3022). Informed consents were obtained from human participants volunteering for this study.
The discharge and reuse standards contained within the recently published ISO 30500 standard on ‘Non Sewered Sanitation Systems’ sets out specified guideline values for ammonium (NH4+–N), total phosphorus (TP), chemical oxygen demand (COD), coliform forming units (CFU), total suspended solids (TSS) and pH as key parameters for evaluation.16 The purpose of this standard is for the certification of technologies explicitly developed to deliver safe sanitation at a single household scale, in order to reduce consumer risk at procurement. Consequently, membrane selectivity was characterised using these parameters together with conductivity. Data was triplicated however insufficient to conform to a 20% variance of at least five trials (as stated in the standard) and therefore the mean is only used as a quick reference to the guidelines, to benchmark the membrane technologies. For the determination of TP, NH4+–N and COD, proprietary wet chemistry methods were used coupled with quantitation by spectrophotometry (NOVA60 photometer, VWR, UK). The TSS, electrical conductivity and pH were measured using standard methods38 and a Jenway 4330 meter respectively (Cole Parmer, Staffordshire, UK). Total coliforms and E. coli coliform colony counts were based on methods 9215C, 9215D, 9922B and 9922D.38 Pathogen reduction was characterised using the log removal value (LRV):
 | (2) |
2.3 Volatile organic compound sampling and analysis
Membrane odour analysis was carried out using both a synthetic and real CB matrix. Odourant production within the feed occurred during the duration of the trial altering the feed concentration (Fig. S1†), as T&O causing compounds (VOCs) are bacterial by-products.39 Therefore, in order to accurately investigate polymer and process selectivity towards VOCs, the quantitative assessment of membrane separation was conducted using the synthetic matrix where the feed concentration was stable, and qualitative odour characterisation was carried out using the real matrix.Nine VOCs were identified that represent commonly occurring compounds with differing chemical structures found in urine and faeces:24 sulfides (dimethyl disulfide), aromatic heterocycles (indole, skatole), phenols (p-cresol), alcohols (1-butanol), aldehydes (benzaldehyde), ketones (2-butanone) and esters (ethyl propionate, ethyl butyrate) (Table S1†), to provide an understanding of membrane selectivity. For synthetic solutions, a 1000 mg L−1 stock solution containing pure VOCs was first prepared in propylene glycol to dissolve all compounds. An aliquot was subsequently added to a pH 6.5 (to mimic fresh urine)37 buffered solution according to Robinson and Stokes,40 for the preparation of a synthetic feed standardised at 10 mg L−1 for all VOCs. All the samples were stored within gas tight 10 ml glass centrifugal vials (Cole Parmer, UK) and analysed on the same day as the trial. Analysis of VOCs involved a solid phase pre-concentration step (Oasis HLB SPE cartridge, 1 g, Waters, USA) followed by quantification using gas chromatography mass spectrometry (GC-MS) (TQ 8040, Shimadzu, UK). Full details of quantification and method validation can be found in the ESI† and Mercer et al.21 Membrane processes were compared by determining the separation of odourous VOCs using the ratio between the water flux and VOC flux, defined as:
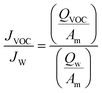 | (3) |
3. Results and discussion
3.1 Pervaporation offers competitive separation for ISO 30500 contaminants
The selective capabilities of each membrane were evaluated against key wastewater determinants set out in ISO 30500 (ref. 16) with the respective average loadings of 15 ± 3 gCOD L−1, 203 ± 142 mgTP L−1, 8.11 × 106 ± 9.43 × 106 CFU TC ml−1 and 998 ± 740 mgNH4–N L−1. Organic (as COD) removal efficiencies were >98.6% for RO (20 °C, 50 °C), MD and PV (Table 2). Liu et al.41 recorded a similar organic removal efficiency of 97% (as total organic carbon) for a RO membrane operated in forward osmosis (FO) mode at 25 °C, using a urine feed matrix, suggesting that RO can maintain high rejection capabilities with faecal organic loading (>3 times higher COD concentration). Kamranvand et al.10,42 demonstrated >95% COD rejection using MD for CB, which was improved to 98.9% with a reduced pore size of 0.1 μm. In this study, the MD pore size is 0.04 μm which could explain the enhanced organics separation efficiency of >99% due to the additional barrier of gas phase size exclusion. With respect to the ISO 30500 standard, COD category B (≤150 mg L−1) was achieved by the thermal processes hydrophilic PV and MD, which represents restricted urban reuse or safe discharge (Table 3). Non-compliance for RO could be attributed to the poor rejection of small and uncharged molecules such as urea (solute radius: 0.18 nm urea compared to 0.26 nm of water), where removal efficiencies have been reported to be as low as 22%,43 and the selective enrichment of the volatile organic compounds for hydrophobic PV.21| Conductivity | TP | COD | |
|---|---|---|---|
| Reduction (%) | Removal efficiency (%) | Removal efficiency (%) | |
| Feed water temperature 50 °C, unless stated otherwise. Average feed conductivity, TP and COD is 13.07 mS cm−1, 203.39 mg L−1 and 15.36 g L−1 respectively. RO (reverse osmosis); PA-UREA (polyamide-urea); PV (pervaporation); PVA (polyvinyl alcohol); PDMS (polydimethylsiloxane); MD (membrane distillation); PP (polypropylene). Error represented as standard deviation of a triplicated experiment. | |||
| RO (PA-UREA) 20 °C | 88.3 ± 3.3 | 99.4 ± 0.2 | 98.6 ± 0.5 |
| RO (PA-UREA) | 86.4 ± 1.4 | 97.4 ± 1.2 | 98.8 ± 0.1 |
| PV (PVA) | 93.8 ± 5.5 | 100 ± 0 | 99.1 ± 0.4 |
| PV (PDMS) | 76.5 ± 4.8 | 99.9 ± 0 | 98.8 ± 0.7 |
| MD (PP) | 60.6 ± 8.1 | 99.7 ± 0.2 | 99.1 ± 0.3 |
| RO (PA-UREA) | PV (PVA) | PV (PDMS) | MD (PP) | ||
|---|---|---|---|---|---|
| 20 ºC | 50 ºC | 50 ºC | 50 ºC | 50 ºC | |
| RO (reverse osmosis); PA-UREA (polyamide-urea); PV (pervaporation); PVA (polyvinyl alcohol); PDMS (polydimethylsiloxane); MD (membrane distillation); PP (polypropylene); COD (chemical oxygen demand); TP (total phosphorus); NH4+–N (ammoniacal nitrogen); TN (total nitrogen) CFU (colony forming units). Shaded rows represent urban wastewater discharge limits according to the European Commission (91/271/EEC) as a reference. | |||||
| COD Category A (≤50 mg L−1) | ✗ (202) | ✗ (159) | ✗ (134) | ✗ (231) | ✗ (149) |
| COD Category B (≤150 mg L−1) | ✗ (202) | ✗ (159) | ✓ (134) | ✗ (231) | ✓ (149) |
| TP (≤2 mg L−1) | ✓ (0.9) | ✗ (4) | ✓ (ND) | ✓ (0.05) | ✓ (1.19) |
| TP (≥80 % reduction) | ✓ (99) | ✓ (98) | ✓ (>99) | ✓ (>99) | ✓ (>99) |
| NH4+–N (TN ≤ 15 mg L−1) | ✗ (462) | ✗ (382) | ✗ (36) | ✗ (854) | ✗ (2375) |
| NH4+–N (TN ≥ 70 % reduction) | ✓ (74) | ✓ (78) | ✓ (87) | ✗ (-162) | ✗ (−736) |
| CFU mL−1 (≤0.1 CFU mL−1) | ✓ (ND) | ✓ (ND) | ✓ (ND) | ✓ (ND) | ✓ (ND) |
| pH (6–9) | ✗ (9.5) | ✗ (10.1) | ✗ (9.3) | ✗ (9.86) | ✗ (10.5) |
ISO 30500 compliance was met by all membranes for the non-volatile contaminants. Rejections of >99% for TP were encountered for all processes other than RO operated at 50 °C where rejection was reduced to 97% (Table 2). The high RO rejection in this study with CB was similarly observed by Davey et al.17 who also observed >99% rejection for TP after UF-RO, demonstrating the robustness of RO when directly fed with CB. This consistently high rejection could be attributed to the fact that the majority of the TP content is present as the phosphate ion which is preferentially rejected due to electrostatic interaction.44,45 Furthermore, TP is predominantly non-volatile and therefore its transport was restricted for all thermally driven processes. The TP limit of 2 mg L−1 set out in the EU directive for urban waste-water treatment (91/271/EEC)46 was met by RO at 20 °C and all thermal processes (Table 3). Decreased rejection of solutes by RO at elevated temperatures can be ascribed to changes in the polymer structure, combined with reduced solvent viscosity, and increased solute diffusivity.47 However, the less stringent ISO 30500 removal efficiency target could also be achieved by RO at 50 °C (Table 3). The permeate of all membranes also presented no coliforms above the detection limit when challenged with concentrated blackwater E. coli concentrations up to 107 CFU mL−1 (Fig. 3), which achieved the ISO 30500 specifications (≤0.1 CFU mL−1, Table 3). The LRVs obtained were influenced by feed concentration and available permeate volume which determined detection limits. Reverse osmosis is dense and provides an absolute barrier to pathogens, hence its wide scale adoption for potable reuse from wastewater.48 Similarly, PV and MD (despite being microporous) also act as absolute barriers since the separation constitutes both size exclusion and selectivity toward volatile constituents.
The volatile ISO 30500 contaminant, ammonia, challenged the selectivity of the membrane processes which demonstrated NH4+–N separations of 74 ± 0.1, 78 ± 3, 87 ± 3, −162 ± 91 and −736 ± 283% for RO (20 °C), RO (50 °C), PV (PVA), PV (PDMS) and MD respectively. This is because ammonium rejection was impacted by urea hydrolysis, a naturally occurring process during urine storage which converts urea into ammonium and increases pH:49,50
| CO(NH2)2 + 3H2O → 2NH4+ + OH− + HCO3− | (4) |
3.2 T&O is influenced by membrane selectivity, microbial activity and detection thresholds
The selectivity of taste and odour compounds was assessed with synthetic and real CB using a discrete range of VOCs including the key malodorous faecal compounds: indole, skatole, p-cresol and dimethyl disulfide; encompassing seven functional groups and a wide range of physico-chemical properties (Table S1†).The relative VOC to water flux for all membrane processes was first benchmarked using a synthetic VOC solution at 10 ppm (Fig. 4). Reverse osmosis demonstrated a VOC removal of 20–85% with selectivity (Fig. 5) strongly correlated to molecular weight (r = −0.85, n = 9, p = 0.003) and octanol water coefficient (r = −0.911, n = 9, p = 0.001) as reported by Altalyan et al.57 Rejection for RO was lower than PV(PVA) which removed VOCs by 65–80%. While transport phenomena are described by a solution-diffusion mechanism for both membranes, which comprise of glassy hydrophilic polymer characteristics, the difference can be ascribed to differences in the driving force applied, and distinctions in affinity of the polymeric substrate. For RO, pressure induces an osmotic gradient and the subsequent concentration polarisation causes solute flux to be higher than the water flux, thereby reducing selectivity. Concentration polarisation is similarly considered in pervaporation, but the role of the vapour pressure gradient is distinctive and thus selectivity is less sensitive to rejection. In contrast, the hydrophobic polydimethylsiloxane membrane concentrated all VOCs due to the material's selective preference to non-polar compounds.25 With the suite of compounds used in this study encompassing a large range of physico-chemical properties, selectivity in the PDMS was governed by vapour pressure, volatility and hydrophobicity as reported by Mercer et al.21 Membrane distillation illustrated a similar profile to PV(PDMS), however separation had a general dependency on boiling point (r = −0.862, n = 9, p = 0.003) or volatility (r = −0.701, n = 9, p = 0.036) due to the fact that the VOC transport is reliant on the vapour pressure gradient, rather than material interactions specifically.25
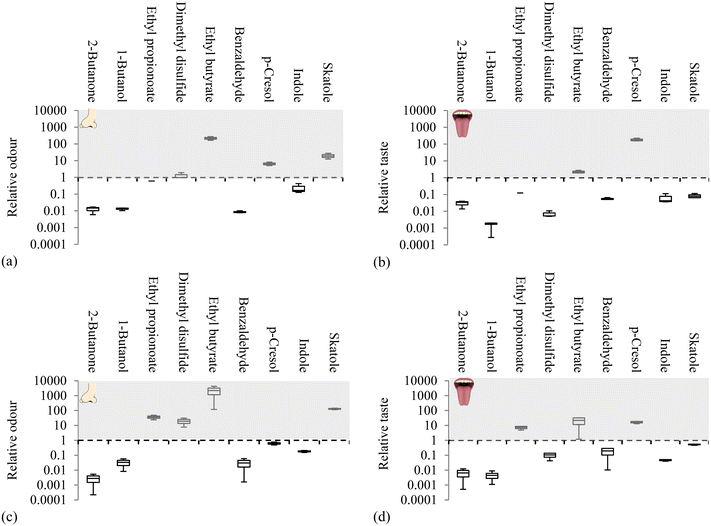 | ||
| Fig. 5 Concentrated blackwater relative odour and taste of reverse osmosis permeate (a and b) and hydrophilic pervaporation permeate (c and d) according to the lowest reported odour and taste thresholds in water.58 Note: dotted line represents the threshold in which the compound can be detected with grey above white below the threshold respectively. | ||
When assessing selectivity with a real CB matrix, it was evident that VOCs were being produced during the experiment due to feed side microbial activity.39 For example, in the PV(PVA) evaluation, after almost 5 hours, the malodorous faecal compound skatole increased by 11-fold within the feed (Fig. S1†). Coupled with the extremely low odour threshold of skatole in water (0.0002 mg kg−1),58 its odour would be detected in the permeates of RO and PV(PVA) (Fig. 5). However the taste threshold is higher (0.05 mg kg−1)58 and therefore skatole would not be tasted using RO and PV(PVA) (Fig. 5). The breaches in T&O are therefore dependent on the respective thresholds of the compounds as also observed for ethyl butyrate, ethyl propionate, p-cresol and dimethyl disulfide which can be smelt or tasted at concentrations at least three orders of magnitude lower than the other compounds examined.58
Using the method of wastewater odour wheel classification,59,60 odour was qualitatively characterised for the thermally driven processes which selectively enriched or rejected VOCs. Three different odour outcomes were encountered, with little resemblance of concentrated blackwater due to alterations in the respective odour profiles (Table 4). Hydrophobic pervaporation (PDMS) produced the most hedonistically pleasant odour which was characteristic of a cleaning product due to the selective enrichment of alcohols, aldehydes, esters and ketones over the key malodourous ‘faecal like’ odours of indole, skatole and p-cresol, from having a higher vapour pressure of over an order of magnitude (Table S1†).21 The reduction of indole and skatole within the odour profile changed their overpowering faecal notes to floral notes, and as such these compounds are key constituents of jasmine perfume.22 The permeate could be associated with ‘sanitised’ water and therefore widely accepted. Hydrophilic (PVA) pervaporation, which reduced the VOC concentration but did not change the T&O profile, represented a chemical odour with similarities to body odour, unpleasant however not repulsive. Membrane distillation permeate was overpowered by ammonia causing a repulsive odour, even with a VOC profile similar to PDMS (Fig. 4). Distinctive T&O profiles are therefore facilitated by thermal and pressure driven processes, and can be strongly modified with polymeric solution-diffusion interactions. Importantly, this study has demonstrated how membranes can mitigate T&O detection for water reuse by reduction in concentration, and how material characterisation alter perception as practiced in the perfume industry.22 Due to the concentration of CB, it may still be necessary for complementary technology for T&O polishing to increase willingness to adopt water reuse from this specific water source.
| Membrane process (material) | Permeate odour descriptor |
|---|---|
| PV (pervaporation); PVA (polyvinyl alcohol); PDMS (polydimethylsiloxane); MD (membrane distillation); PP (polypropylene). | |
| PV (PVA) | Sweaty, chemical, sweet, onion |
| PV (PDMS) | Sweet, chemical, earthy, floral |
| MD (PP) | Pungent, ammonia, fishy, citrus |
3.3 Water productivity may be more restricted from pressure driven membrane processes
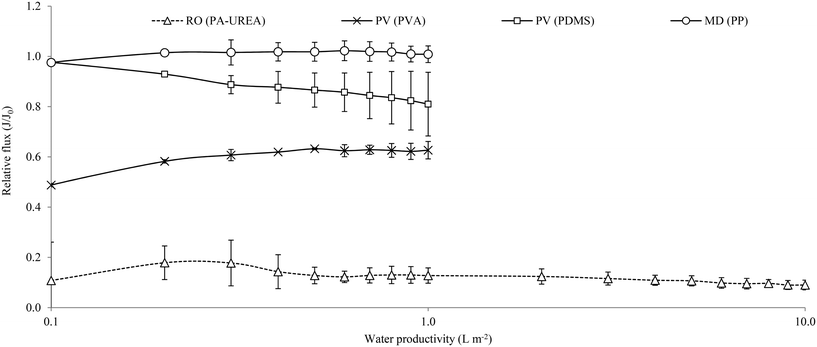
Preliminary analysis of water flux from each membrane was undertaken to provide an initial indication of how fouling mechanisms may differ based on driving force (thermal or pressure driven) and material characteristics when treating this challenging CB feed (Fig. 6). From an initial flux of 50 L m−2 h−1, a flux decline (80%) was demonstrated for the pressure driven RO, which we suggest is due to the convective mass transport of organics toward the membrane surface during filtration. Organic fouling can be mitigated through the use of a UF pretreatment which has shown to enhance the resilience of both RO and MD configurations,8,19 with sustainable UF operation evidenced for CB through hydrodynamic optimisation. Kamranvand et al.19 observed how UF retained the high molecular weight (MW) fraction of CB comprising colloidal organics greater than 500 kDa which represents 30% of organics in CB. The initial increase in flux for the PVA membrane can be attributed to polymer swelling which raises the internal polymer volume, subsequently increasing flux. A more stable flux (relative flux of 0.6) was observed for the hydrophilic PV(PVA) membrane. We propose this to be analogous to forward osmosis, where the limited applied convective force mitigates fouling.61 The hydrophobic microporous and dense thermally driven membranes facilitated similar recoveries to RO but only negligible permeability loss was identified. This can be ascribed to the material's resistance to mass transfer (as evidenced by the low water vapour flux of 0.06 and 0.08 L m−2 h−1 for PDMS and MD respectively, Table S4†), which was in excess of the resistance of heat and mass transfer provided by the fouling layer. Consequently, fouling did not inhibit water vapour mass transport as the rate of separation was determined by the material permeability. Fouling was visually evidenced as clogging within the interstitial spacing of the hollow fibre configuration (HF) by coarse faecal solids. This could be resolved using UF for pretreatment and further optimisation of thermal membrane module geometry for wastewater applications.4. Conclusions
The study assessed pressure and thermally driven membranes for their potential to realise water reuse as a household scale solution for sanitation:• Pressure driven and hydrophobic thermally driven membranes mostly achieved category B of the ISO 30500 standards within a single stage. The critical weakness in selectivity lies with volatile contaminants such as ammonia and organics (residual COD as VOCs). Thermally driven hydrophilic PV complied closely to the category A ISO 30500 standards and demonstrated the highest rejection of VOCs by providing the greatest selectivity for water over all the membrane processes, due to its affinity to water based on polarity.
• Improved ammonia separation could be practically implemented by limiting faecal bacterial enzymic activity to mitigate urea hydrolysis through reducing faecal contamination, shortening residence time and increasing operating temperature. Such modifications will also assist in the mitigation of feed side bacterial VOC production.
• As direct reuse from blackwater is considered high risk, ISO 30500 (ref. 16) advises that category A effluent should be reused for flushing or toilet cleaning purposes which saves up to 25% of household water.62 However, this risk can be reduced if a multibarrier approach is implemented and the effluent is frequently monitored (i.e. through sudden changes in electrical conductivity as an indicator), enabling a further saving of 51% of household water if extended to laundry and all other non-food related reuse applications.62
Thermally driven PV membranes may offer a more robust solution to water reclamation by offering greater mitigation of volatile components (NH3, VOCs) than RO, a more robust barrier to pathogens than MD, and less sensitivity to fouling due to the limited convection experienced in permeation. By complementing PV with UF pretreatment, as would be standard for the household application of RO, a lower organic load to PV could improve resilience and achieve category A water reuse compliance. Importantly, the use of thermal membrane separation can broaden the applicability and affordability of high-performance off-grid sanitation through exploiting widely accessible thermal energy. Increased crosslinking of PVA, or use of ceramic zeolites may further enhance robustness in the long-term by reducing swelling which can further enhance selectivity above that illustrated within the present study. Common to both technologies is the challenge of concentrate production, which must be managed locally either through collection or treatment, but also offers significant opportunities for localised resource recovery enabling synergistic valuation to maintenance business models.
Abbreviations
| CB | Concentrated blackwater |
| CFU | Coliform forming units |
| COD | Chemical oxygen demand |
| HF | Hollow fibre |
| ISO | International Standards Organisation |
| LD | Limit of detection |
| LIC | Low-income country |
| LRV | Log removal value |
| MD | Membrane distillation |
| MW | Molecular weight |
| NH3 | Ammonia |
| NH4+ | Ammonium |
| NH4–N | Ammoniacal nitrogen |
| NSS | Non-sewered sanitation |
| PA-UREA | Polyamide-urea |
| PDMS | Polydimethylsiloxane |
| PES | Polyethersulphone |
| PP | Polypropylene |
| PV | Pervaporation |
| PVA | Polyvinyl alcohol |
| RO | Reverse osmosis |
| SDG | Sustainable Development Goal |
| T&O | Taste and odour |
| TP | Total phosphorus |
| TSS | Total suspended solids |
| UF | Ultrafiltration |
| VOC | Volatile organic compound |
Data availability
All relevant data are available from an online repository or repositories. Data underlying this paper can be accessed from: https://dspace.lib.cranfield.ac.uk/handle/1826/20712.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication is based on research funded by the Bill & Melinda Gates Foundation. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the funders. Enquiries for access to the data referred to in this article should be directed to: E-mail: researchdata@cranfield.ac.uk.References
- J. Glausiusz, Toilets – what will it take to fix them?, Nature, 2021, 592, 345–346 CrossRef CAS.
- United Nations, Sustainable Development Goal 6, https://sustainabledevelopment.un.org/sdg6, (accessed 9 October 2018).
- G. Hutton and L. Haller, Evaluation of the Costs and Benefits of Water and Sanitation Improvements at the Global Level, World Heal. Organ., 2004, vol. 11, pp. 1–87 Search PubMed.
- L. Strande, M. Ronteltap and D. Brdjanovic, Faecal Sludge Management – Systems Approach for Implementation and Operation, IWA Publishing, 2014 Search PubMed.
- A. Trivedi and M. Chertock, Responding to Day Zero Equitably: Water Crisis Lessons from Cape Town and Chennai, https://www.wri.org/blog/2019/10/responding-day-zero-equitably-water-crisis-lessons-cape-town-and-chennai, (accessed 8 January 2021) Search PubMed.
- D. M. Warsinger, S. Chakraborty, E. W. Tow, M. H. Plumlee, C. Bellona, S. Loutatidou, L. Karimi, A. M. Mikelonis, A. Achilli, A. Ghassemi, L. P. Padhye, S. A. Snyder, S. Curcio, C. D. Vecitis, H. A. Arafat and J. H. Lienhard, A review of polymeric membranes and processes for potable water reuse, Prog. Polym. Sci., 2018, 81, 209–237 CrossRef CAS PubMed.
- D. F. Putnam, Composition and concentrative properties of human urine, Washington, United States, 1971 Search PubMed.
- C. J. Davey, N. Thomas and E. J. McAdam, Downscaling reverse osmosis for single-household wastewater reuse: towards low-cost decentralised sanitation through a batch open-loop configuration, J. Water Reuse Desalin., 2022, 12, 191–205 CrossRef CAS.
- H. C. Duong, A. J. Ansari, R. H. Hailemariam, Y. C. Woo, T. M. Pham, L. T. Ngo, D. T. Dao and L. D. Nghiem, Membrane Distillation for Strategic Water Treatment Applications: Opportunities, Challenges, and Current Status, Curr. Pollut. Rep., 2020, 6, 173–187 CrossRef CAS.
- F. Kamranvand, C. J. Davey, L. Williams, A. Parker, Y. Jiang, S. Tyrrel and E. J. McAdam, Membrane distillation of concentrated blackwater: Effect of temperature, solids concentration and membrane pore size, Water Environ. Res., 2021, 93(6), 875–886 CrossRef CAS PubMed.
- Q. Wang, N. Li, B. Bolto, M. Hoang and Z. Xie, Desalination by pervaporation: A review, Desalination, 2016, 387, 46–60 CrossRef CAS.
- Y. He, Is India the marginal demand market for global butane?, https://ihsmarkit.com/research-analysis/is-india-the-marginal-demand-market-for-global-butane.html, (accessed 8 January 2021) Search PubMed.
- SolarGIS, Solar GIS world map, https://solargis.com/maps-and-gis-data/download/world, (accessed 30 October 2023) Search PubMed.
- G. Libralato, A. Volpi Ghirardini and F. Avezzù, To centralise or to decentralise: An overview of the most recent trends in wastewater treatment management, J. Environ. Manage., 2012, 94, 61–68 CrossRef CAS PubMed.
- E. van Voorthuizen, A. Zwijnenburg, W. van der Meer and H. Temmink, Biological black water treatment combined with membrane separation, Water Res., 2008, 42, 4334–4340 CrossRef CAS PubMed.
- International Organisation for Standardisation, ISO 30500, 2018 Search PubMed.
- C. J. Davey, N. Thomas and E. J. McAdam, Downscaling reverse osmosis for single-household wastewater reuse: towards low-cost decentralised sanitation through a batch open-loop configuration, J. Water Reuse Desalin., 2022, jwrd2022084 Search PubMed.
- C. J. Davey, P. Liu, F. Kamranvand, L. Williams, Y. Jiang, A. Parker, S. Tyrrel and E. J. McAdam, Membrane distillation for concentrated blackwater: Influence of configuration (air gap, direct contact, vacuum) on selectivity and water productivity, Sep. Purif. Technol., 2021, 263, 118390 CrossRef CAS PubMed.
- F. Kamranvand, C. J. Davey, L. Williams, A. Parker, Y. Jiang, S. Tyrrel and E. J. McAdam, Ultrafiltration pretreatment enhances membrane distillation flux, resilience and permeate quality during water recovery from concentrated blackwater (urine/faeces), Sep. Purif. Technol., 2020, 253, 117547 CrossRef CAS PubMed.
- P. H. Cruddas, A. Parker and A. Gormley, in 38th WEDC International Conference, Loughborough, UK, 2015 Search PubMed.
- E. Mercer, C. J. Davey, P. Campo, D. Fowler, L. Williams, A. Kolios, A. Parker, S. Tyrrel, C. Walton, E. Cartmell, M. Pidou and E. J. McAdam, Quantification of liquid phase faecal odourants to evaluate membrane technology for wastewater reuse from decentralised sanitation facilities, Environ. Sci.: Water Res. Technol., 2019, 5(1), 161–171 RSC.
- C. Starkenmann, in Springer Handbook of Odor, ed. A. Buettner, Springer, Berlin, 2017, pp. 921–936 Search PubMed.
- C. J.-F. Chappuis, Y. Niclass, C. Vuilleumier and C. Starkenmann, Quantitative Headspace Analysis of Selected Odorants from Latrines in Africa and India, Environ. Sci. Technol., 2015, 49, 6134–6140 CrossRef CAS PubMed.
- J. Lin, J. Aoll, Y. Niclass, M. I. Velazco, L. Wunsche, J. Pika and C. Starkenmann, Qualitative and Quantitative Analysis of Volatile Constituents from Latrines, Environ. Sci. Technol., 2013, 47, 7876–7882 CrossRef CAS PubMed.
- R. Baker, Membrane Technology and Applications, John Wiley and Sons, Chichester, 3rd edn, 2012 Search PubMed.
- Y. Hirabayashi, Pervaporation Membrane System for the Removal of Ammonia from Water, Mater. Trans., 2002, 43, 1074–1077 CrossRef CAS.
- K. Nitta, A. Ashida, K. Mitani, K. Ebara and A. Yamada, in 15th International Symposium on Space Technology and Science, Tokyo, 1986, pp. 1355–1359 Search PubMed.
- F. Volpin, U. Badeti, C. Wang, J. Jiang, J. Vogel, S. Freguia, D. Fam, J. Cho, S. Phuntsho and H. K. Shon, Urine treatment on the international space station: Current practice and novel approaches, Membranes, 2020, 10, 1–18 CrossRef PubMed.
- H. Ray, F. Perreault and T. H. Boyer, Urea recovery from fresh human urine by forward osmosis and membrane distillation (FO–MD), Environ. Sci.: Water Res. Technol., 2019, 5, 1993–2003 RSC.
- H. Ray, F. Perreault and T. H. Boyer, Rejection of nitrogen species in real fresh and hydrolyzed human urine by reverse osmosis and nanofiltration, J. Environ. Chem. Eng., 2020, 8, 103993 CrossRef CAS.
- M. M. Damtie, F. Volpin, M. Yao, L. D. Tijing, R. H. Hailemariam, T. Bao, K.-D. Park, H. K. Shon and J.-S. Choi, Ammonia recovery from human urine as liquid fertilizers in hollow fiber membrane contactor: Effects of permeate chemistry, Environ. Eng. Res., 2021, 26, 190520–190523 Search PubMed.
- F. Volpin, L. Chekli, S. Phuntsho, N. Ghaffour, J. S. Vrouwenvelder and H. K. Shon, Optimisation of a forward osmosis and membrane distillation hybrid system for the treatment of source-separated urine, Sep. Purif. Technol., 2019, 212, 368–375 CrossRef CAS.
- L.-F. Liu, S.-C. Yu, L.-G. Wu and C.-J. Gao, Study on a novel polyamide-urea reverse osmosis composite membrane (ICIC-MPD): II. Analysis of membrane antifouling performance, J. Membr. Sci., 2006, 283, 133–146 CrossRef CAS.
- M. Strobel, S. Corn, C. S. Lyons and G. A. Korba, Surface modification of polypropylene with CF4, CF3H, CF3Cl, and CF3Br plasmas, J. Polym. Sci., Polym. Chem. Ed., 1985, 23, 1125–1135 CrossRef CAS.
- J. E. Mark, Polymer data handbook, Oxford University Press, 2009 Search PubMed.
- E. Bormashenko, R. Pogreb, G. Whyman, Y. Bormashenko, R. Jager, T. Stein, A. Schechter and D. Aurbach, The Reversible Giant Change in the Contact Angle on the Polysulfone and Polyethersulfone Films Exposed to UV Irradiation, Langmuir, 2008, 24, 5977–5980 CrossRef CAS PubMed.
- C. Rose, A. Parker, B. Jefferson and E. Cartmell, The Characterization of Feces and Urine: A Review of the Literature to Inform Advanced Treatment Technology, Crit. Rev. Environ. Sci. Technol., 2015, 45, 1827–1879 CrossRef CAS PubMed.
- APHA, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, Washington, D.C, 21st edn, 2012 Search PubMed.
- L. D. J. Bos, P. J. Sterk and M. J. Schultz, Volatile Metabolites of Pathogens: A Systematic Review, PLoS Pathog., 2013, 9, e1003311 CrossRef CAS PubMed.
- R. Robinson and R. Stokes, Electrolyte solutions, Dover Publications, Mineola, 2nd edn, 2002 Search PubMed.
- Q. Liu, C. Liu, L. Zhao, W. Ma, H. Liu and J. Ma, Integrated forward osmosis-membrane distillation process for human urine treatment, Water Res., 2016, 91, 45–54 CrossRef CAS PubMed.
- F. Kamranvand, C. J. Davey, H. Sakar, O. Autin, E. Mercer, M. Collins, L. Williams, A. Kolios, A. Parker, S. Tyrrel, E. Cartmell and E. J. McAdam, Impact of fouling, cleaning and faecal contamination on the separation of water from urine using thermally driven membrane separation, Sep. Sci. Technol., 2018, 53, 1372–1382 CrossRef CAS PubMed.
- S. Lee and R. M. Lueptow, Membrane Rejection of Nitrogen Compounds, Environ. Sci. Technol., 2001, 35, 3008–3018 CrossRef CAS PubMed.
- S. Lee and R. M. Lueptow, Rotating reverse osmosis for water recovery in space: influence of operational parameters on RO performance, Desalination, 2004, 169, 109–120 CrossRef CAS.
- D. Ek, Concentration of nutrients from urine and reject water from anaerobically digested sludge, Water Sci. Technol., 2006, 54, 437–444 CrossRef PubMed.
- European Commission, Urban wastewater treatment directive (91/271/EEC), 1991 Search PubMed.
- X. Jin, A. Jawor, S. Kim and E. M. V. Hoek, Effects of feed water temperature on separation performance and organic fouling of brackish water RO membranes, Desalination, 2009, 239, 346–359 CrossRef CAS.
- M.-L. Pype, M. G. Lawrence, J. Keller and W. Gernjak, Reverse osmosis integrity monitoring in water reuse: The challenge to verify virus removal – A review, Water Res., 2016, 98, 384–395 CrossRef CAS PubMed.
- Z. P. Zhao, L. Xu, X. Shang and K. Chen, Sep. Purif. Technol., 2013, 118, 369–376 CrossRef CAS.
- K. M. Udert, T. A. Larsen, M. Biebow and W. Gujer, Urea hydrolysis and precipitation dynamics in a urine-collecting system, Water Res., 2003, 37, 2571–2582 CrossRef CAS PubMed.
- A. Bódalo, J.-L. Gómez, E. Gómez, G. León and M. Tejera, Ammonium removal from aqueous solutions by reverse osmosis using cellulose acetate membranes, Desalination, 2005, 184, 149–155 CrossRef.
- PubChem, PubChem, https://pubchem.ncbi.nlm.nih.gov/, (accessed 10 February 2018).
- L. L. Tun, D. Jeong, S. Jeong, K. Cho, S. Lee and H. Bae, Dewatering of source-separated human urine for nitrogen recovery by membrane distillation, J. Membr. Sci., 2016, 512, 13–20 CrossRef CAS.
- M. S. EL-Bourawi, M. Khayet, R. Ma, Z. Ding, Z. Li and X. Zhang, Application of vacuum membrane distillation for ammonia removal, J. Membr. Sci., 2007, 301, 200–209 CrossRef CAS.
- R. Ö. Sürmeli, A. Bayrakdar and B. Çalli, Ammonia recovery from chicken manure digestate using polydimethylsiloxane membrane contactor, J. Cleaner Prod., 2018, 191, 99–104 CrossRef.
- X. Yang, T. Fraser, D. Myat, S. Smart, J. Zhang, J. C. Diniz da Costa, A. Liubinas and M. Duke, A pervaporation study of ammonia solutions using molecular sieve silica membranes, Membranes, 2014, 4, 40–54 CrossRef PubMed.
- H. N. Altalyan, B. Jones, J. Bradd, L. D. Nghiem and Y. M. Alyazichi, Removal of volatile organic compounds (VOCs) from groundwater by reverse osmosis and nanofiltration, J. Water Process Eng., 2016, 9, 9–21 CrossRef.
- L. van Gemert, Odour thresholds. Compilations in air water and other media, Oliemans Punter & Partners BV, Utrecht, 2nd edn, 2011 Search PubMed.
- R. M. Fisher, R. J. Barczak, I. H. “Mel” Suffet, J. E. Hayes and R. M. Stuetz, Framework for the use of odour wheels to manage odours throughout wastewater biosolids processing, Sci. Total Environ., 2018, 634, 214–223 CrossRef CAS PubMed.
- G. A. Burlingame, I. H. Suffet, D. Khiari and A. L. Bruchet, Development of an odor wheel classification scheme for wastewater, Water Sci. Technol., 2004, 49, 201–209 CrossRef CAS PubMed.
- R. W. Field, Q. She, F. A. Siddiqui and A. G. Fane, Reverse osmosis and forward osmosis fouling: a comparison, Discover Chem. Eng., 2021, 1, 6 CrossRef.
- S. Grego, The humble toilet is an opportunity for sustainable water reuse, Nat. Water, 2023, 1, 900–901 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ew00200h |
| This journal is © The Royal Society of Chemistry 2024 |