Open Access Article
Open Access ArticleAccess to tetracoordinate boron-doped polycyclic aromatic hydrocarbons with delayed fluorescence and aggregation-induced emission under mild conditions†
Long
Jiang
a,
Yu
Wang
b,
Dehui
Tan
a,
Xiaobin
Chen
a,
Tinghao
Ma
a,
Baoliang
Zhang
 *a and
Deng-Tao
Yang
*a and
Deng-Tao
Yang
 *a
*a
aSchool of Chemistry and Chemical Engineering, Northwestern Polytechnical University, Xi'an, Shanxi 710072, China. E-mail: blzhang@nwpu.edu.cn; dtyang@nwpu.edu.cn
bSchool of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing, 100081, China
First published on 29th April 2022
Abstract
Boron-doped polycyclic aromatic hydrocarbons (PAHs) have attracted ongoing attention in the field of optoelectronic materials due to their unique optical and redox properties. To investigate the effect of tetracoordinate boron in PAHs bearing N-heterocycles (indole and carbazole), a facile approach to four-coordinate boron-doped PAHs was developed, which does not require elevated temperature and pre-synthesized functionalized boron reactants. Five tetracoordinate boron-doped PAHs (NBNN-1–NBNN-5) were synthesized with different functional groups. Two of them (NBNN-1 and NBNN-2) could further undergo oxidative coupling reactions to form fused off-plane tetracoordinate boron-doped PAHs NBNN-1f and NBNN-2f. The investigation of photophysical properties showed that the UV/vis absorption and fluorescence emission are significantly red-shifted compared to those of the three-coordinate boron-doped counterparts. In addition, the emission of NBNN-1–NBNN-3 consisted of prompt fluorescence and delayed fluorescence. The compounds NBNN-1f and NBNN-2f showed aggregation-induced emission.
Introduction
Chemically doping heteroatoms into aromatic compounds has proven to be one of the most effective methods to tune their optoelectronic properties and intermolecular interactions.1–12 Due to the isoelectronic relationship between boron and carbon cations and between the boron–nitrogen (BN) unit and two carbon atom (CC) motif, boron becomes an important element that can induce novel and complementary properties.13–21 BN-doped aromatic compounds have a long history of evolution since the first example of BN-arene was reported by Alfred Stock in 1926.22 Much the same to many other topics of chemistry, not much attention was paid until the new millennium. Nowadays, the synthesis of BN-doped arenes has become one of the most popular topics in organic chemistry and materials chemistry, and numerous new BN-doped aromatic compounds have been synthesized and demonstrated to have very distinct properties in optoelectronic materials.23–27 Representative applications of B-doped polycyclic aromatic hydrocarbons (PAHs) have been demonstrated in organic field-effect transistors (OFETs)21,28–30 and organic light emitting diodes (OLEDs).21,29,31–37 Particularly, since PAHs decorated with para-positioned boron and nitrogen atoms have been reported to show thermally activated delayed fluorescence (TADF) with narrow full-width at half-maximum (FWHM) of emission based on efficient HOMO–LUMO separation by the multiple resonance effect in 2016,31 BN-doped PAHs have been extensively studied for the next-generation OLEDs.With the further development of BN-PAH chemistry by advances in the synthetic methodology and by the application potential of BN-doped π-conjugated systems, attention to the structure–property relationship has arisen.38,39 The location of the BN unit was found to be important for the optoelectronic properties because whether the BN unit could fully conjugate with PAHs is highly dependent on its locations.40–44 Although both the number of BN units45–50 and different orientations of BN units6,51–54 are believed to exhibit non-negligible impact on the optoelectronic properties and intermolecular interactions, only a few of examples have been documented due to the limited synthetic methodology.
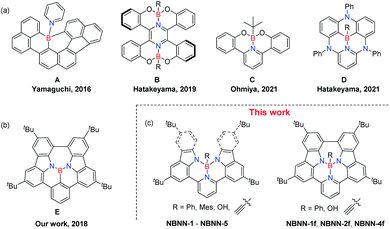
In addition, the majority of previous attention has been paid to the tricoordinate boron units in PAHs,55–60 tetracoordinate boron has been viewed more as a functional group instead of a dopant.61–66 In fact, the coordination number of the boron atom shows noteworthy impact on the photophysical properties. Recently, effort to replace the three-coordinate boron of the BN unit with four-coordinate boron has been made (Scheme 1a). The formation/dissociation of the four-coordinate boron unit through a dynamic B–N coordination bond in B-PAHs (A)67–69 has been exploited for the solution-processed fabrication of semiconducting films of poorly soluble B-PAHs. Hatakeyama et al. reported tetracoordinate boron-fused helicenes (B)70 as cathode active materials for lithium batteries. The photoexcitable borate (C),71 which is structurally similar to B, can form an alkyl radical via single electron transfer. Very recently, Hatakeyama et al. replaced tricoordinate boron by tetracoordinate boron to form compound D.72
 | ||
| Scheme 1 Representative examples of tetracoordinate B-doped PAHs (a), our previous work (b) and chemical structures of this work (c). | ||
With respect to the synthetic methodology of introducing boron atoms, electrophilic borylation as the symbolic reaction to synthesize borylated compounds has shown advantages in efficiency, scalability and avoidance of previous reagents. However, this type of electrophilic borylation requires high temperature with high boiling point solvents, which needs long reaction time and makes subsequent purification difficult.18,73–76
We have longstanding interest both in developing the synthetic methodology and applications of BN-doped PAHs.77–80 We have developed photo-driven elimination reactions to generate BN-PAHs77 and further expand this elimination using heat.78 We also proposed a new strategy named exciton-driven elimination (EDE) to enable the in situ solid-state conversion of non-emissive tetracoordinate boron-substituted heterocycles to highly emissive BN-PAHs in OLEDs.79 In addition, we recently demonstrated combination of electrophilic borylation and oxidative coupling to be a facile and viable approach to new NBN-doped molecular systems with tri-coordinate boron atoms (Scheme 1b).80 In this work, we further disclose the strategy of combining electrophilic borylation and oxidative coupling for producing tetra-coordinate boron-doped PAHs. Unlike the typical electrophilic borylation driven by high temperature, our system can quickly undergo borylation at room temperature followed by addition of different nucleophilic reagents to build tetra-coordinate BN-PAHs bearing different functional groups on boron atoms (Scheme 1c).
Results and discussion
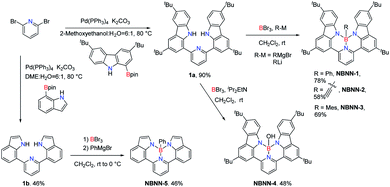
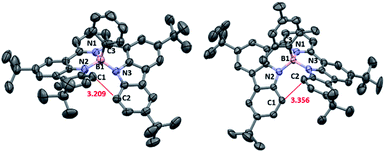
For the synthesis of tetracoordinate boron-doped PAHs NBNN-1–NBNN-5, we started from the commercially available 2,6-dibromopyridine, which could undergo the Suzuki coupling reaction with readily available 1-Bpin-carbazole and 7-Bpin-indole to produce the precursor compounds 1a and 1b in 90% and 46% yields, respectively (Scheme 2).Our first attempt to introduce the boron atom using BBr3 as the boron source with iPr2EtN in o-dichlorobenzene (o-DCB) at 180 °C was successful to produce phenyl group functionalized BN-PAH NBNN-1 when phenylmagnesium bromide was used as the nucleophilic reagent. The existence of o-DCB makes the purification difficult. We tried to use several other solvents with lower boiling points at lower reaction temperatures, all of which generated the target compound NBNN-1 with similar yields around 70%. Usually, the presence of the additive iPr2EtN is proven to be essential for the electrophilic borylation, but the absence of iPr2EtN did not show any influence on the borylation yield. To our delight, the reaction temperature was finally lowered down to room temperature without iPr2EtN in 78% yield (Scheme 2). In addition, the kind of nucleophile was also probed, and lithium reagents like phenyl lithium could produce compound NBNN-1 in slightly lower yield. While we were preparing the manuscript, the synthesis of NBNN-1 was reported at high temperature with PhBCl2 and the additive iPr2EtN.81 Our mild conditions are probably ascribed to the existence the pyridyl group which could act as a directing group coordinating with BBr3 and make the following borylation of N–H bonds less challenging. This process does not require to break the C–H bonds either. In addition, compared to the pre-functionalized boron reagents RBX2 (X = Cl, Br), the commercially available BBr3 has higher Lewis acidity and reactivity which also play a non-negligible role in the borylation reaction. The mild conditions without using high boiling point solvent make the purification of the product less disturbing. With the optimal conditions in hand, ethynylmagnesium bromide and mesitylmagnesium bromide were used as nucleophiles to generate compounds NBNN-2 and NBNN-3 in 58% and 69% yields, respectively. Compound NBNN-4 was obtained when the reaction was quenched by water without adding any nucleophiles in single-figure yield, however, the yield of NBNN-4 was significantly improved to 46% when iPr2EtN was used to quench the reaction. We also tried other hydroxy group containing reagents like phenol, which did not give the corresponding product at all. Compound NBNN-5 based on two indole fragments was also synthesized under the optimal conditions. Other nucleophiles like isopropylmagnesium bromide, vinylmagnesium bromide, and methyl lithium could also yield the corresponding tetracoordinate boron-doped PAHs, however, these compounds were slowly decomposing on the silica gel or alumina. Bulky nucleophiles like tbutylmagnesium bromide did not react, probably due to the steric hindrance. Compounds NBNN-1 to NBNN-5 were fully characterized by NMR and HRMS spectroscopy. The molecular structures of NBNN-1 and NBNN-3 were further determined by single-crystal X-ray diffraction analysis and are shown in Fig. 1.
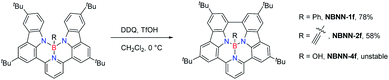
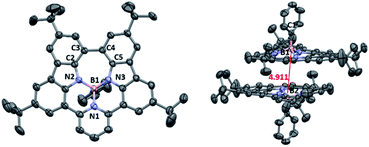
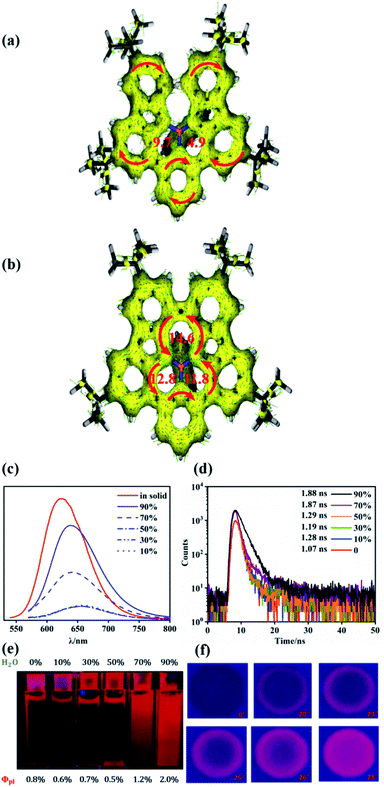
Similar to our previous work on synthesized tricoordinate boron-doped PAHs,80 the proximity and the steric repulsion between the hydrogen atoms of flanking units in NBNN-1 to NBNN-4 make them undergo the oxidative coupling reaction to form the fused forms. By combining 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) as the oxidant and TfOH as the acid, the fully fused compounds NBNN-1f, NBNN-2f and NBNN-4f were successfully observed. Compounds NBNN-1f and NBNN-2f were obtained in isolated yields of 78% and 58%, respectively (Scheme 3). Silica gel or alumina column chromatography was not compatible with NBNN-4f, which decomposed on the silica gel or alumina and was only confirmed by HRMS. NBNN-3 did not undergo the oxidative coupling reaction probably due to the longer distance (3.356 Å, Fig. 1) between two carbon atoms on the carbazole segments. Unlike the tricoordinate boron-doped PAH analogue (E in Scheme 1),80 the crystal structure of NBNN-1f showed that the four-coordinated boron atom adopts a tetrahedral geometry, which makes the molecular structure off-plane (Fig. 2). The B1–N1, B1–N2, and B1–N3 bond lengths are 1.640 Å, 1.523 Å, and 1.502 Å, respectively. Similar to tricoordinate boron-doped PAH E, the π-stacking interactions of NBNN-1f are limited to two neighbouring molecules with a longer separation distance than E.
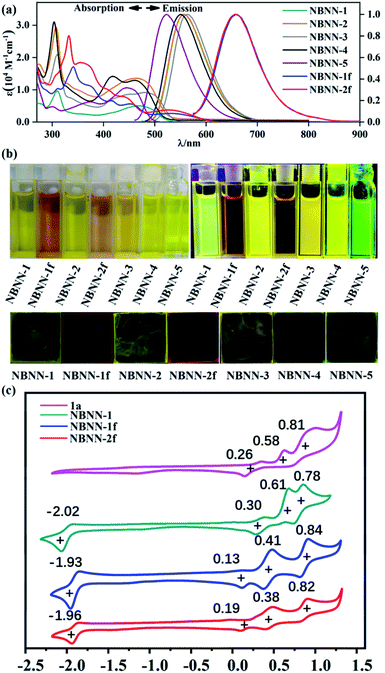
The absorption, emission and electrochemical data of these seven tetracoordinate boron-doped PAHs are shown in Fig. 3 and Table 1. The absorption peaks are in agreement with the colors of their THF solutions (Fig. 3b). The general trend of the experimental absorption bands agrees with the time-dependent density functional theory (TD-DFT) calculations (Fig. 4, and see the ESI† for details). The broad absorption bands of NBNN-1 to NBNN-5 between 400 nm and 520 nm are ascribable to S0 → S1 with transition configurations of HOMO → LUMO and S0 → S2 with the major transition configuration of HOMO−1 → LUMO. Due to the closer energy gap between the HOMO and HOMO−1, NBNN-1, NBNN-2 and NBNN-5 only exhibited one peak at 469 nm, 465 nm and 446 nm, respectively. The smaller HOMO–LUMO gap results in red-shifted absorption peaks of NBNN-3, while the larger HOMO–LUMO gap brings about the blue-shifted absorption peaks of NBNN-5. The strong absorption peaks at 310 nm for NBNN-1–NBNN-4 are likely attributed to the larger conjugation of the carbazole unit than the indole unit in NBNN-5. NBNN-1f and NBNN-2f showed five absorption bands with a maximum at λmax = 531 nm and 528 nm which could be attributed to S0 → S1 with the transition configuration of HOMO → LUMO, respectively. Different from NBNN-1–NBNN-5, the S0 → S2 transitions of NBNN-1f at 457 nm and NBNN-2f at 439 nm consisted of HOMO → LUMO+1 with charge transfer character. Except NBNN-5, molecules NBNN-1 to NBNN-4 showed near-identical fluorescence spectra with emission peaks between 551 nm and 564 nm, which suggests that the substituents on the boron atom of tetracoordinate boron-doped PAHs have a negligible contribution to fluorescence. The fluorescent peaks in the poly(methyl methacrylate) (PMMA) film of NBNN-1 to NBNN-4 are very similar to those in THF solution, while the λem of NBNN-5 in solid was red-shifted to 540 nm from bright green color to greenish yellow color as shown in Fig. 3b, which is probably because the less crowded structure of NBNN-5 results in the π–π packing in the solid. The quantum yields both in solution and in the PMMA film of the open form of compounds bearing carbazole units are ∼25%, except NBNN-1, which has much higher quantum yields in solution (Φpl = 62.3%) and PMMA film (Φpl = 55.7%) (Table 1). Compound NBNN-5 bearing indole units only has less than 10% quantum yield both in solution and in solid probably due to vibrational relaxation caused by the less crowded structure. Compared to NBNN-1 and NBNN-2, the emission peaks of the fused molecules NBNN-1f and NBNN-2f show remarkable red-shift up to 100 nm and exhibit indistinguishable fluorescent spectra (λem = 660 nm, Φ < 1%), while their absorption spectra are also similar with different absorption extinction coefficients. The fluorescence peak of NBNN-1f in the PMMA film was blue-shifted up to 39 nm compared to that in solution (see the ESI† for details), while NBNN-2f in the PMMA film exhibited two emission peaks at 624 nm and 659 nm. The blue-shifted emission peaks of NBNN-1f and NBNN-2f are probably ascribed to the structure constraint, which could also result in aggregation-induced emission (AIE). Both NBNN-1f and NBNN-2f showed a significant increase in quantum yields in solid up to 4%. The low quantum yields of NBNN-1f and NBNN-2f are likely due to the internal conversion between small S0–S1 gaps. The fluorescence peaks in solution and solid are consistent with the emission colors of their THF solutions and PMMA films (Fig. 3b). Ligand 1a has two absorption bands at 294 nm and 364 nm, while Ligand 1b has a broad band at 330 nm and a less intensive band at 364 nm (Fig. S5.16†). These two molecules 1a and 1b showed weak fluorescence with emission maxima at 636 nm and 656 nm, respectively. The quantum yields both in solution and in the PMMA film of the two molecules were less than 0.1%. Compared to the ligands, the boron-doped PAHs exhibited substantially red-shifted absorption spectra with enhanced emission quantum yields. As shown in Table 1 and Fig. S5.5–S5.11,† the phosphorescence decays at 77 K were recorded in the range of 200–400 ms for NBNN-1–NBNN-5, while the fused compounds NBNN-1f and NBNN-2f showed much shorter phosphorescence decays (0.012 ms and 5.62 ms, respectively).
| UV/visa | Fluorescence | Phosphorescenceb | Electrochemical datac | |||||
|---|---|---|---|---|---|---|---|---|
| λ abs (nm) | λ em (nm)/λemb (nm) | Φ pl , (%)/Φplb,d (%) | τ pf (ns)/τdfa (μs) | λ em (nm) | τ ph (ms) | E red (V) | E ox (V) | |
| a 10−5 M in THF at 298 K. b PMMA film (10 wt%). c vs. Fc/Fc+ (ferrocene/ferrocenium) glassy carbon as the working electrode, a Pt wire as reference and counter electrodes, ferrocene (Fc/Fc+ = ferrocene/ferrocenium) was added to the solution as the internal reference, scan rate = 100 mV s−1. d Quantum yields were determined using a calibrated integrating sphere. e Not available. | ||||||||
| NBNN-1 | 310, 469 | 557/556 | 62.3/55.7 | 1.26/38.06 | 569 | 302 | −2.02 | 0.30 |
| NBNN-1f | 341, 376, 417, 457, 531 | 660/621 | 0.8/4.0 | 1.07/—e | 643 | 0.012 | −1.93 | 0.13 |
| NBNN-2 | 308, 465 | 558/562 | 23.4/22.6 | 1.89/8.15 | 567 | 270 | −2.16 | 0.26 |
| NBNN-2f | 332, 359, 404, 439, 528 | 659/624, 659 | 0.8/4.0 | 1.20/—e | 638 | 5.62 | −1.96 | 0.19 |
| NBNN-3 | 309, 445, 481 | 564/561 | 24.5/26.6 | 1.60/24.8 | 583 | 213 | −2.09 | 0.27 |
| NBNN-4 | 304, 416, 461 | 551/556 | 34.8/25.3 | 4.69/—e | 582 | 377 | −2.06 | 0.28 |
| NBNN-5 | 298, 370, 446 | 523/540 | 8.3/9.5 | 1.79/—e | 569, 621 | 355 | —e | —e |
The time-resolved photoluminescence spectra were recorded in THF to investigate the photoluminescence decay behaviors of these tetracoordinate boron-doped PAHs. As shown in Table 1 and Fig. 5, compounds NBNN-1, NBNN-2 and NBNN-3 consisted of prompt fluorescence with τpf = 1.26 ns, 1.89 ns and 1.60 ns and delayed emission with τdf = 38.06 μs, 8.15 μs and 24.8 μs, respectively. The fully fused compounds NBNN-1f and NBNN-2f, the carbazole-based compound with a hydroxy group on boron NBNN-4, and indole-based compound NBNN-5 only exhibited the prompt fluorescence component (τpf = 1.07 ns, 1.20 ns, 4.69 ns, 1.79 ns, respectively), likely due to the relatively large singlet-triplet band gap.
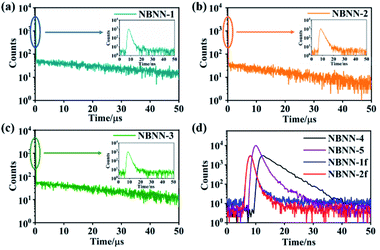 | ||
| Fig. 5 (a)–(c) PL decay curves in THF under N2 conditions at 298 K for NBNN-1 to NBNN-3. (d) Transient fluorescence in THF under N2 conditions at 298 K for NBNN-4, NBNN-5, NBNN-1f and NBNN-2f. | ||
To further investigate the delayed fluorescence behaviors of NBNN-1–NBNN-3, Φpl and the lifetimes of the prompt/delayed components (τpf/τdf) were exploited to estimate the photophysical rate constant of radiative decay (kr), intersystem crossing (kISC), and reverse intersystem crossing of (kRISC).81 As shown in Table 2, the kr value of NBNN-2 (8.62 × 107 s−1) is larger than those of NBNN-1 (2.38 × 107 s−1) and NBNN-3 (3.94 × 107 s−1). kISC (7.70 × 108 s−1) and kRISC (5.35 × 105 s−1) of tetracoordinate boron-doped PAH NBNN-1 with a phenyl group on the boron atom are the largest among those of NBNN-1 to NBNN-3. The kr value of NBNN-2 (8.62 × 107 s−1) is larger than those of NBNN-1 (2.38 × 107 s−1) and NBNN-3 (3.94 × 107 s−1). It is not clear why the different functional groups on the boron atom of the same skeleton could dramatically change kRISC.
| Cmpd | τ pf (ns)/τdfb (μs) | Φ pf/Φdfc (%) | k r (107 s−1) | k ISC (108 s−1) | k RISC (105 s−1) |
|---|---|---|---|---|---|
| a Measured to be 10−5 M in THF for NBNN-1 to NBNN-2 at 298 K. b Emission lifetime for prompt (τpf) and delayed (τdf) fluorescence. c Quantum yields for prompt (Φpf) and delayed (Φdf) fluorescence, Φpf + Φdf = Φpl. d Rate constant of fluorescence radiative decay (S1 → S0): kr = Φpf/τpf. e Rate constant of ISC (S1 → T1): kISC = (1 − Φpf)/τpf. f Rate constant of RISC (T1/S1): kRISC = Φdf/kISCτpfτdfΦpf. | |||||
| NBNN-1 | 1.26/38.06 | 3.0/59.3 | 2.38 | 7.70 | 5.35 |
| NBNN-2 | 1.89/8.15 | 16.3/7.1 | 8.62 | 4.43 | 0.64 |
| NBNN-3 | 1.60/24.8 | 6.3/18.2 | 3.94 | 5.86 | 1.24 |
Molecules NBNN-1 to NBNN-5 displayed pseudo-reversible reduction peaks at ∼−2.0 V (vs. Fc/Fc+) and multiple reversible oxidation peaks with the first oxidation peaks at ∼0.3 V (vs. Fc/Fc+) (Table 1 and ESI†). The fused forms of boron-doped PAHs NBNN-1f and NBNN-2f show almost identical cyclic voltammograms as shown in Fig. 3c. The first oxidation peaks of NBNN-1f and NBNN-2f are lower than those of NBNN-1 and NBNN-2, which can be attributed to the high-lying HOMO level (Fig. 4 bottom). The experimental data are in good agreement with the calculated HOMO and LUMO. Our previous study on tricoordinate boron-PAHs did not show such rich electrochemical properties.80 After we measured the CV curves of the ligand 1a and 1b (Fig. 3c, S2.8 and S2.9†), we found that the ligand 1a had similar multiple reversible oxidation peaks to the tetracoordinate boron-doped PAHs NBNN-1, NBNN-1f and NBNN-2f, as shown in Fig. 3c, which indicates that the ligand is likely ascribed to the multiple oxidation of NBNN-1, NBNN-1f and NBNN-2f rather than the introduction of the tetracoordinate boron atom. Compared to the reduction of ligands, the tetracoordinate boron-doped PAHs NBNN-1, NBNN-1f and NBNN-2f showed irreversible reduction at ∼−2.00 V (vs. Fc/Fc+), which indicates that the reduction peaks might originate from the boron atom.
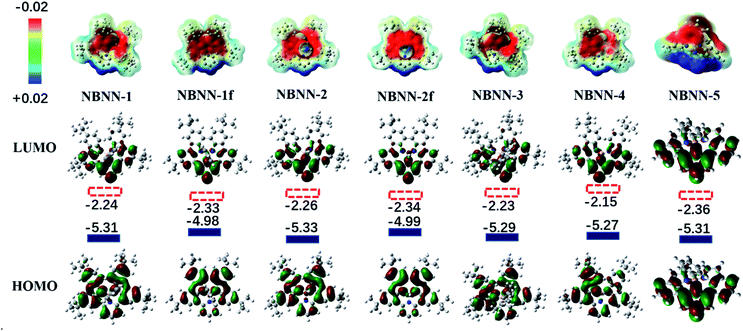
Unlike the tricoordinate boron-doped PAH analogues in which the boron atom has a significant contribution to the LUMO and the HOMO/LUMO spread over the entire molecule,80 the four-coordinate boron atom in NBNN-1 to NBNN-5, NBNN-1f and NBNN-2f has no contribution to the LUMO (Fig. 4), and their LUMO is mainly located on the pyridine and phenyl rings of carbazole and indole connected to pyridine. The calculated HOMO and LUMO prove that the substituents on the four-coordinate boron atom have trivial adjustment on the frontier molecular orbitals. Compared to NBNN-1 and NBNN-2, NBNN-1f and NBNN-2f have smaller HOMO–LUMO gaps due to the notably elevated HOMO and slightly lowered LUMO, which could indicate the reason why the fused product NBNN-4f of NBNN-4 was less stable and slowly decomposed on silica gel or alumina (Scheme 3). We note that the electrostatic potential maps of NBNN-1–NBNN-5, NBNN-1f and NBNN-2f display the development of negative characteristics on the tetracoordinate boron area and positive characteristics on the pyridine unit (Fig. 4 top).
To illustrate the aromaticity of these tetracoordinate boron-doped PAHs, the nucleus-independent chemical shift (NICS(1))82,83 of two C3NBN rings and the 7-memebered C4NBN ring (if exists) around boron atoms was calculated (Fig. 6a, b and ESI†). The two C3NBN rings in NBNN-1–NBNN-5 have positive NICS(1) values (see the ESI†), supporting their antiaromaticity. The two C3NBN rings in NBNN-1f and NBNN-2f have much more positive NICS(1) values than those of NBNN-1–NBNN-5, indicating that they are more antiaromatic. Similar to the tricoordinate boron-doped PAH,80 the 7-membered C4NBN has a larger NICS(1) value of 13.8 ppm (Fig. 6b). The anisotropy of the induced current density (ACID) calculations84,85 (Fig. 6a and b) that are based on the optimized structures further showed that there is no electron density around the boron atom and the central 13-membered C4NC3NC3N ring of NBNN-1f is antiaromatic with an anti-clockwise diatropic ring current circuit.
The fused molecules NBNN-1f and NBNN-2f exhibited AIE behavior,86,87 while compounds NBNN-1–NBNN-5 only have normal photoluminescence properties. AIE behaviors of NBNN-1f and NBNN-2f are probably due to their structure constraint. We used NBNN-1f as an example to investigate the AIE phenomenon in detail (Fig. 6c–f). The red emission became stronger after the water content reached 50% in volume in THF, and the emission intensity grew faster when water was further added, as shown in Fig. 6c and e. The THF solution with 90% water displayed bright red emission. The quantum yields gradually increased when the water fraction increased. In addition, we found that the AIE behavior of NBNN-1f was accompanied by the blue-shift of emission (Fig. 6c). The photoluminescence decays only slightly increased when the water fractions reached 70% and 90%, as shown in Fig. 6d. The AIE behavior of NBNN-1f was further demonstrated through change of emission intensity after adding one drop of NBNN-1f THF solution on a thin-layer chromatography plate (Fig. 6f). We observed a notable increase of the red emission under 365 nm UV lamp irradiation.
Conclusions
We developed an efficient strategy to construct a series of tetracoordinate boron-doped PAHs with various substituents on boron atoms at room temperature. Some of them were successfully converted to fully fused four-coordinate boron-doped NBN-PAHs via oxidative coupling. The tetracoordinate boron-doped PAHs exhibited delayed fluorescence. All the rings with tetracoordinate boron atoms are antiaromatic. The fused NBN-PAHs displayed blue-shifted AIE behavior.Data availability
The data sets supporting this article have been uploaded as part of the ESI.†Author contributions
L. J., B. Z. and D.-T. Yang conceptualized the work. L. J. developed the methodology and designed experiments. Y. W. recorded the time-resolved emission spectra. D. T. synthesized NBNN-3. X. C. and T. M. performed DFT calculations. D.-T. Y. wrote the original draft and created the original visuals. L. J., B. Z. and D.T. Y. edited the draft and visuals.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the National Natural Science Foundation of China (22001211) and Northwestern Polytechnical University for financial support. This project is also supported by special fund of Shaanxi Key Laboratory of Special Fuel Chemistry and Material (SPCF-SKL-2021-0014) and the Fundamental Research Funds for the Central Universities. The authors thank Prof. Lijie Liu, Dr Xiang Wang and Dr Guoyun Meng for insightful discussion.Notes and references
- X. Feng, J. Wu, M. Ai, W. Pisula, L. Zhi, J. P. Rabe and K. Müllen, Angew. Chem., Int. Ed., 2007, 46, 3033–3036 CrossRef CAS PubMed.
- M. Takase, V. Enkelmann, D. Sebastiani, M. Baumgarten and K. Müllen, Angew. Chem., Int. Ed., 2007, 46, 5524–5527 CrossRef CAS PubMed.
- J. Wei, B. Han, Q. Guo, X. Shi, W. Wang and N. Wei, Angew. Chem., Int. Ed., 2010, 49, 8209–8213 CrossRef CAS PubMed.
- T. Hatakeyama, S. Hashimoto, S. Seki and M. Nakamura, J. Am. Chem. Soc., 2011, 133, 18614–18617 CrossRef CAS PubMed.
- Z. Zhou, A. Wakamiya, T. Kushida and S. Yamaguchi, J. Am. Chem. Soc., 2012, 134, 4529–4532 CrossRef CAS PubMed.
- X. Wang, F. Zhang, J. Liu, R. Tang, Y. Fu, D. Wu, Q. Xu, X. Zhuang, G. He and X. Feng, Org. Lett., 2013, 15, 5714–5717 CrossRef CAS PubMed.
- L. Zou, X.-Y. Wang, K. Shi, J.-Y. Wang and J. Pei, Org. Lett., 2013, 15, 4378–4381 CrossRef CAS PubMed.
- E. Gońka, P. J. Chmielewski, T. Lis and M. Stępień, J. Am. Chem. Soc., 2014, 136, 16399–16410 CrossRef PubMed.
- V. M. Hertz, M. Bolte, H.-W. Lerner and M. Wagner, Angew. Chem., Int. Ed., 2015, 54, 8800–8804 CrossRef CAS PubMed.
- H. Oshima, A. Fukazawa, T. Sasamori and S. Yamaguchi, Angew. Chem., Int. Ed., 2015, 54, 7636–7639 CrossRef CAS PubMed.
- M. Hirai, N. Tanaka, M. Sakai and S. Yamaguchi, Chem. Rev., 2019, 119, 8291–8331 CrossRef CAS PubMed.
- N. Ando, T. Yamada, H. Narita, N. N. Oehlmann, M. Wagner and S. Yamaguchi, J. Am. Chem. Soc., 2021, 143, 9944–9951 CrossRef CAS PubMed.
- Z. Liu and T. B. Marder, Angew. Chem., Int. Ed., 2008, 47, 242–244 CrossRef CAS PubMed.
- J. F. Araneda, B. Neue and W. E. Piers, Angew. Chem., Int. Ed., 2012, 51, 9977–9979 CrossRef CAS PubMed.
- J. S. A. Ishibashi, J. L. Marshall, A. Mazière, G. J. Lovinger, B. Li, L. N. Zakharov, A. Dargelos, A. Graciaa, A. Chrostowska and S.-Y. Liu, J. Am. Chem. Soc., 2014, 136, 15414–15421 CrossRef CAS PubMed.
- M. Krieg, F. Reicherter, P. Haiss, M. Ströbele, K. Eichele, M.-J. Treanor, R. Schaub and H. F. Bettinger, Angew. Chem., Int. Ed., 2015, 54, 8284–8286 CrossRef CAS PubMed.
- J. Dosso, J. Tasseroul, F. Fasano, D. Marinelli, N. Biot, A. Fermi and D. Bonifazi, Angew. Chem., Int. Ed., 2017, 56, 4483–4487 CrossRef CAS PubMed.
- K. Matsui, S. Oda, K. Yoshiura, K. Nakajima, N. Yasuda and T. Hatakeyama, J. Am. Chem. Soc., 2018, 140, 1195–1198 CrossRef CAS PubMed.
- S. Nakatsuka, N. Yasuda and T. Hatakeyama, J. Am. Chem. Soc., 2018, 140, 13562–13565 CrossRef CAS PubMed.
- S. Limberti, L. Emmett, A. Trandafir, G. Kociok-Köhn and G. D. Pantoş, Chem. Sci., 2019, 10, 9565–9570 RSC.
- J. Hoffmann, B. Geffroy, E. Jaques, M. Hissler and A. Staubitz, J. Mater. Chem. C, 2021, 9, 14720–14729 RSC.
- A. Stock and E. Pohland, Chem. Ges., 1926, 59, 2215–2223 Search PubMed.
- M. Fingerle, C. Maichle-Mössmer, S. Schundelmeier, B. Speiser and H. F. Bettinger, Org. Lett., 2017, 19, 4428–4431 CrossRef CAS PubMed.
- D. Shimizu, K. Furukawa and A. Osuka, Angew. Chem., Int. Ed., 2017, 56, 7435–7439 CrossRef CAS PubMed.
- J. Huang and Y. Li, Front. Chem., 2018, 6, 341 CrossRef PubMed.
- J.-K. Li, X.-Y. Chen, Y.-L. Guo, X.-C. Wang, A. C. H. Sue, X.-Y. Cao and X.-Y. Wang, J. Am. Chem. Soc., 2021, 143, 17958–17963 CrossRef CAS PubMed.
- J. Wang, X. Fang, X. Guo, Q. Wu, Q. Gong, C. Yu, E. Hao and L. Jiao, Org. Lett., 2021, 23, 4796–4801 CrossRef CAS PubMed.
- X.-Y. Wang, F.-D. Zhuang, X. Zhou, D.-C. Yang, J.-Y. Wang and J. Pei, J. Mater. Chem. C, 2014, 2, 8152–8161 RSC.
- S. K. Mellerup and S. Wang, Trends Chem., 2019, 1, 77–89 CrossRef CAS.
- R. Zhao, Y. Min, C. Dou, B. Lin, W. Ma, J. Liu and L. Wang, ACS Appl. Polym. Mater., 2020, 2, 19–25 CrossRef CAS.
- T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Adv. Mater., 2016, 28, 2777–2781 CrossRef CAS PubMed.
- H. Nakanotani, T. Furukawa, T. Hosokai, T. Hatakeyama and C. Adachi, Adv. Opt. Mater., 2017, 5, 1700051 CrossRef.
- H. Fukagawa, T. Oono, Y. Iwasaki, T. Hatakeyama and T. Shimizu, Mater. Chem. Front., 2018, 2, 704–709 RSC.
- X. Liang, Z.-P. Yan, H.-B. Han, Z.-G. Wu, Y.-X. Zheng, H. Meng, J.-L. Zuo and W. Huang, Angew. Chem., Int. Ed., 2018, 57, 11316–11320 CrossRef CAS PubMed.
- G. Meng, X. Chen, X. Wang, N. Wang, T. Peng and S. Wang, Adv. Opt. Mater., 2019, 7, 1970043 CrossRef.
- J. U. Kim, I. S. Park, C.-Y. Chan, M. Tanaka, Y. Tsuchiya, H. Nakanotani and C. Adachi, Nat. Commun., 2020, 11, 1765 CrossRef CAS.
- Y. H. Lee, Y.-S. Shin, T. Lee, J. Jung, J.-H. Lee and M. H. Lee, Chem. Eng. J., 2021, 423, 130224 CrossRef CAS.
- M. Stępień, E. Gońka, M. Żyła and N. Sprutta, Chem. Rev., 2017, 117, 3479–3716 CrossRef PubMed.
- A. Borissov, Y. K. Maurya, L. Moshniaha, W.-S. Wong, M. Żyła-Karwowska and M. Stępień, Chem. Rev., 2022, 122, 565–788 CrossRef CAS PubMed.
- X.-Y. Wang, F.-D. Zhuang, X.-C. Wang, X.-Y. Cao, J.-Y. Wang and J. Pei, Chem. Commun., 2015, 51, 4368–4371 RSC.
- M. Numano, N. Nagami, S. Nakatsuka, T. Katayama, K. Nakajima, S. Tatsumi, N. Yasuda and T. Hatakeyama, Chem.–Eur. J., 2016, 22, 11574–11577 CrossRef CAS PubMed.
- S. K. Mellerup, C. Li, T. Peng and S. Wang, Angew. Chem., Int. Ed., 2017, 56, 6093–6097 CrossRef CAS PubMed.
- Y. Fu, X. Chang, H. Yang, E. Dmitrieva, Y. Gao, J. Ma, L. Huang, J. Liu, H. Lu, Z. Cheng, S. Du, H.-J. Gao and X. Feng, Angew. Chem., Int. Ed., 2021, 60, 26115–26121 CrossRef CAS.
- M. Zhao and Q. Miao, Angew. Chem., Int. Ed., 2021, 60, 21289–21294 CrossRef CAS PubMed.
- T. Agou, J. Kobayashi and T. Kawashima, Org. Lett., 2006, 8, 2241–2244 CrossRef CAS PubMed.
- F. Qiu, F. Zhang, R. Tang, Y. Fu, X. Wang, S. Han, X. Zhuang and X. Feng, Org. Lett., 2016, 18, 1398–1401 CrossRef CAS PubMed.
- Z.-C. He, S. K. Mellerup, L. Liu, X. Wang, C. Dao and S. Wang, Angew. Chem., Int. Ed., 2019, 58, 6683–6687 CrossRef CAS PubMed.
- Y. Chen, W. Chen, Y. Qiao, X. Lu and G. Zhou, Angew. Chem., Int. Ed., 2020, 59, 7122–7130 CrossRef CAS PubMed.
- S. M. Suresh, E. Duda, D. Hall, Z. Yao, S. Bagnich, A. M. Z. Slawin, H. Bässler, D. Beljonne, M. Buck, Y. Olivier, A. Köhler and E. Zysman-Colman, J. Am. Chem. Soc., 2020, 142, 6588–6599 CrossRef CAS PubMed.
- D. Shimoyama, N. Baser-Kirazli, R. A. Lalancette and F. Jäkle, Angew. Chem., Int. Ed., 2021, 60, 17942–17946 CrossRef CAS PubMed.
- X. Wang, F. Zhang, J. Gao, Y. Fu, W. Zhao, R. Tang, W. Zhang, X. Zhuang and X. Feng, J. Org. Chem., 2015, 80, 10127–10133 CrossRef CAS PubMed.
- X.-Y. Wang, A. Narita, X. Feng and K. Müllen, J. Am. Chem. Soc., 2015, 137, 7668–7671 CrossRef CAS PubMed.
- Q. Zhang, Z. Sun, L. Zhang, M. Li, L. Zi, Z. Liu, B. Zhen, W. Sun and X. Liu, J. Org. Chem., 2020, 85, 7877–7883 CrossRef CAS PubMed.
- P.-F. Zhang, J.-C. Zeng, F.-D. Zhuang, K.-X. Zhao, Z.-H. Sun, Z.-F. Yao, Y. Lu, X.-Y. Wang, J.-Y. Wang and J. Pei, Angew. Chem., Int. Ed., 2021, 60, 23313–23319 CrossRef CAS PubMed.
- T. Hatakeyama, S. Hashimoto, T. Oba and M. Nakamura, J. Am. Chem. Soc., 2012, 134, 19600–19603 CrossRef CAS PubMed.
- N. Ando, H. Soutome and S. Yamaguchi, Chem. Sci., 2019, 10, 7816–7821 RSC.
- J. Wang, N. Wang, G. Wu, S. Wang and X. Li, Angew. Chem., Int. Ed., 2019, 58, 3082–3086 CrossRef CAS PubMed.
- N. Baser-Kirazli, R. A. Lalancette and F. Jäkle, Angew. Chem., Int. Ed., 2020, 59, 8689–8697 CrossRef CAS PubMed.
- J. Guo, Y. Yang, C. Dou and Y. Wang, J. Am. Chem. Soc., 2021, 143, 18272–18279 CrossRef CAS PubMed.
- F. D. Zhuang, J. H. Yang, Z. H. Sun, P. F. Zhang, Q. R. Chen, J. Y. Wang and J. Pei, Chin. J. Chem., 2021, 39, 909–912 CrossRef CAS.
- J. F. Araneda, W. E. Piers, B. Heyne, M. Parvez and R. McDonald, Angew. Chem., Int. Ed. Engl., 2011, 50, 12214–12217 CrossRef CAS PubMed.
- C. G. Claessens, D. González-Rodríguez, M. S. Rodríguez-Morgade, A. Medina and T. Torres, Chem. Rev., 2014, 114, 2192–2277 CrossRef CAS PubMed.
- S. Shimizu, Chem. Rev., 2017, 117, 2730–2784 CrossRef CAS PubMed.
- Y. Min, C. Dou, H. Tian, Y. Geng, J. Liu and L. Wang, Angew. Chem., Int. Ed., 2018, 57, 2000–2004 CrossRef CAS PubMed.
- S. Mula, N. Leclerc, P. Leveque, P. Retailleau and G. Ulrich, J. Org. Chem., 2018, 83, 14406–14418 CrossRef CAS PubMed.
- D. Cappello, R. R. Maar, V. N. Staroverov and J. B. Gilroy, Chem.–Eur. J., 2020, 26, 5522–5529 CrossRef CAS PubMed.
- K. Matsuo, S. Saito and S. Yamaguchi, J. Am. Chem. Soc., 2014, 136, 12580–12583 CrossRef CAS PubMed.
- K. Matsuo, S. Saito and S. Yamaguchi, Angew. Chem., Int. Ed., 2016, 55, 11984–11988 CrossRef CAS PubMed.
- S. Osumi, S. Saito, C. Dou, K. Matsuo, K. Kume, H. Yoshikawa, K. Awaga and S. Yamaguchi, Chem. Sci., 2016, 7, 219–227 RSC.
- S. Oda, T. Shimizu, T. Katayama, H. Yoshikawa and T. Hatakeyama, Org. Lett., 2019, 21, 1770–1773 CrossRef CAS PubMed.
- Y. Sato, Y. Goto, K. Nakamura, Y. Miyamoto, Y. Sumida and H. Ohmiya, ACS Catal., 2021, 11, 12886–12892 CrossRef CAS.
- H. Gotoh, S. Nakatsuka, H. Tanaka, N. Yasuda, Y. Haketa, H. Maeda and T. Hatakeyama, Angew. Chem., Int. Ed., 2021, 60, 12835–12840 CrossRef CAS PubMed.
- S. Nakatsuka, H. Gotoh, K. Kinoshita, N. Yasuda and T. Hatakeyama, Angew. Chem., Int. Ed., 2017, 56, 5087–5090 CrossRef CAS PubMed.
- S. A. Iqbal, J. Pahl, K. Yuan and M. J. Ingleson, Chem. Soc. Rev., 2020, 49, 4564–4591 RSC.
- P. B. Pati, E. Jin, Y. Kim, Y. Kim, J. Mun, S. J. Kim, S. J. Kang, W. Choe, G. Lee, H.-J. Shin and Y. S. Park, Angew. Chem., Int. Ed., 2020, 59, 14891–14895 CrossRef CAS PubMed.
- S. Oda and T. Hatakeyama, Bull. Chem. Soc. Jpn., 2021, 94, 950–960 CrossRef CAS.
- J.-S. Lu, S.-B. Ko, N. R. Walters, Y. Kang, F. Sauriol and S. Wang, Angew. Chem., Int. Ed., 2013, 52, 4544–4548 CrossRef CAS PubMed.
- D.-T. Yang, S. K. Mellerup, X. Wang, J.-S. Lu and S. Wang, Angew. Chem., Int. Ed., 2015, 54, 5498–5501 CrossRef CAS PubMed.
- S. Wang, D.-T. Yang, J. Lu, H. Shimogawa, S. Gong, X. Wang, S. K. Mellerup, A. Wakamiya, Y.-L. Chang, C. Yang and Z.-H. Lu, Angew. Chem., Int. Ed., 2015, 54, 15074–15078 CrossRef CAS PubMed.
- D. T. Yang, T. Nakamura, Z. He, X. Wang, A. Wakamiya, T. Peng and S. Wang, Org. Lett., 2018, 20, 6741–6745 CrossRef CAS PubMed.
- G. Meng, L. Liu, Z. He, D. Hall, X. Wang, T. Peng, X. Yin, P. Chen, D. Beljonne, Y. Olivier, E. Zysman-Colman, N. Wang and S. Wang, Chem. Sci., 2022, 13, 1665–1674 RSC.
- P. v. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao and N. J. R. van Eikema Hommes, J. Am. Chem. Soc., 1996, 118, 6317–6318 CrossRef CAS PubMed.
- P. v. R. Schleyer, M. Manoharan, Z.-X. Wang, B. Kiran, H. Jiao, R. Puchta and N. J. R. van Eikema Hommes, Org. Lett., 2001, 3, 2465–2468 CrossRef CAS PubMed.
- D. G. R. Herges, J. Phys. Chem. A, 2001, 3214–3220 CrossRef.
- K. H. D. Geuenich, F. Kohler and R. Herges, Chem. Rev., 2005, 105, 3758–3772 CrossRef PubMed.
- J. Luo, Z. Xie, J. W. Y. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu, D. Zhu and B. Z. Tang, Chem. Commun., 2001, 1740–1741 RSC.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2128933 (NBNN-1), 2128995 (NBNN-1f), 2129008 (NBNN-3). For ESI and crystallographic data in CIF or other electronic format see https://doi.org/10.1039/d2sc01722a |
| This journal is © The Royal Society of Chemistry 2022 |