Open Access Article
Open Access ArticleSeparation of neodymium and dysprosium by solvent extraction using ionic liquids combined with neutral extractants: batch and mixer-settler experiments
Sofía Riaño†
 *,
Simona Sobekova Foltova†
*,
Simona Sobekova Foltova† and
Koen Binnemans
and
Koen Binnemans
KU Leuven, Department of Chemistry, Celestijnenlaan 200F, P. O. Box 2404, B-3001 Leuven, Belgium. E-mail: Sofia.Riano@kuleuven.be
First published on 2nd January 2020
Abstract
A solvent extraction method based on the combination of the ionic liquid trihexyl(tetradecyl)phosphonium thiocyanate or nitrate ([C101][SCN], [C101][NO3]) and the neutral extractants Cyanex 923 or tri-n-butyl phosphate (TBP) has been investigated for the separation of Nd(III) and Dy(III) from chloride media. High distribution ratios and separation factors were obtained when using Cyanex 923 diluted in [C101][SCN] 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 (wt%) and extracting from chloride media. The addition of Cyanex 923 to the ionic liquid has four advantages: (1) increase in the distribution ratios of the rare earths, (2) decrease of the viscosity of the organic phase, hence an improved mass transfer, (3) increase in the loading capacity of the ionic liquid and (4) improvement of the coalescence and phase disengagement, which is of importance when carrying out separations in continuous mode. Different extraction parameters were optimized: concentration of Cyanex 923, chloride concentration in the aqueous phase, equilibration time, pH of the aqueous phase, type of scrubbing and stripping agents. The ionic liquid combined with Cyanex 923 was recycled up to three times without losing its extraction efficiency. McCabe–Thiele diagrams were constructed to determine the number of stages needed for the separation of Nd(III) and Dy(III). Stripping of Dy(III) from the organic phase was easily achieved with water. The feasibility to run this process in continuous mode was tested in a battery of small mixer-settlers (0.12 L and 0.48 L effective volume in the mixer and the settler, respectively). As a result, this process constitutes a novel and scalable alternative for the separation of Nd(III) and Dy(III).
60 (wt%) and extracting from chloride media. The addition of Cyanex 923 to the ionic liquid has four advantages: (1) increase in the distribution ratios of the rare earths, (2) decrease of the viscosity of the organic phase, hence an improved mass transfer, (3) increase in the loading capacity of the ionic liquid and (4) improvement of the coalescence and phase disengagement, which is of importance when carrying out separations in continuous mode. Different extraction parameters were optimized: concentration of Cyanex 923, chloride concentration in the aqueous phase, equilibration time, pH of the aqueous phase, type of scrubbing and stripping agents. The ionic liquid combined with Cyanex 923 was recycled up to three times without losing its extraction efficiency. McCabe–Thiele diagrams were constructed to determine the number of stages needed for the separation of Nd(III) and Dy(III). Stripping of Dy(III) from the organic phase was easily achieved with water. The feasibility to run this process in continuous mode was tested in a battery of small mixer-settlers (0.12 L and 0.48 L effective volume in the mixer and the settler, respectively). As a result, this process constitutes a novel and scalable alternative for the separation of Nd(III) and Dy(III).
Introduction
The rare-earth elements (REEs) are a group of 17 metals containing scandium, yttrium and the 15 lanthanides that can be classified into two categories, namely light rare-earth elements (LREEs) and heavy rare-earth elements (HREEs).1 The production of permanent magnets based on REEs has gained considerable attention during the last decades because it has led to the consecution of powerful magnets with improved properties. Around 20% of the consumption of rare earths is employed in neodymium–iron–boron (NdFeB) permanent magnets that are used in wind turbines, electric motors, hard disc drives, loudspeakers, etc.2–5 Recycling of rare earths can help mitigate the balance problem and provide supply for scarce elements that are difficult to mine and avoid exhaustion of geological resources.1,2,6–10 Magnets can be recycled by manual dismantling or by fragmenting the device that contains the magnet and the magnet itself using shedders. Afterwards, the rare earths can be leached out from these fragments and purified through solvent extraction.2,3,11,12Since the middle of the 1950s, rare earths have been separated into groups or individual elements using different methods such as selective precipitation, solvent extraction and ionic exchange.13–15 At present, solvent extraction is the most commonly employed methodology for the separation of rare earths on industrial scale. In general, three different kinds of extractants are widely employed: cation exchangers (acidic extractants), anion exchangers (basic extractants) and solvating extractants (neutral extractants). Carboxylic acids and derivatives of phosphorous acids are acidic extractants usually employed for the separation of rare earths in hydrochloric media, the efficiency of the extraction is pH dependent and usually the stripping process has to be carried out by using concentrated acids.15–20 Another disadvantage is that at high metal loadings and low acidities, gels can be formed in the organic phase making difficult the separation process.15 Basic extractants such as primary amines and tertiary amines have been used to extract rare earths as anionic complexes from sulfate and nitrate media and stripping is easily carried out.19,21–23 Neutral extractants are preferred over acidic and basic extractants to avoid pH influence, as well as high consumption of acid during the stripping steps and gel formation in the organic phase when working at high metal loadings.2,15,24–26
Neutral extractants such as tri-n-butyl phosphate (TBP) have been widely used for the separation of metal ions.27 The extractability of the rare earth ions with TBP increases with increasing atomic number and the extraction is more efficient from nitrate solutions than from chloride solutions. In this kind of extraction, the rare earths in a neutral nitrato complex are coordinated by the phosphoryl group of TBP.28 Cyanex 923 is another neutral extractant that consists of a mixture of different trialkyl phosphine oxides and has been employed in the extraction of mineral acids and metal ions. Cyanex 923 has been used diluted in heptane to extract lanthanum from nitrate media.29 The extraction is independent of the pH of the aqueous solution and the mechanism of extraction is similar to that of TBP. Commercially, Cyanex 923 and TBP have been also employed for the separation of rare earths from impurities such as calcium in nitric acid.30 A study on the effect of the diluents in the separation of Pr(III) and Sm(III) with Cyanex 923 has shown that the extraction percent of the rare-earth ions from nitrate media decreases when increasing the dielectric constant of the diluent.31
Ionic liquids (ILs) are solvents which consist entirely of ions and have typically a melting points below 100 °C. They are characterized by a high chemical stability, negligible vapor pressure and low flammability, reasons why they are used as green solvents in solvent extraction.32–34 Additionally, they are electrically conductive, which means that there is no risk of accumulating static electricity.35 Information related to the environmental, health and safety impact of ionic liquids can be found in the literature.36,37 Ionic liquids have been mostly used to separate the rare earths from other metals ions, but recently they have also been applied to the separation of rare earths into individual elements.34,38–46 Ionic liquids can be used in split-anion extractions, a new approach in which the ionic liquid phase is used as the source of complex-forming anions.47 The main advantage of this approach is that ionic liquids containing anions such as thiocyanate or nitrate can be used to separate rare-earth mixtures from chloride aqueous media without the need of using acidic extractants.47 The latter is due to the fact that thiocyanate and nitrate anions have a strong affinity for the organic phase, while chloride anions will remain in the aqueous phase.48–50 Indeed, the ionic liquid phase needs to be rich in anions capable to form extractable complexes since the aqueous phase lacks of those anions. In this approach, the rare-earth ions are coordinated by thiocyanate or nitrate ions in the ionic liquid phase, while the chloride ions remain dissolved in the organic phase as counter anions for the ionic liquid cations that are not involved in the extraction of the rare-earth complex. The separation can be easily fine-tuned by the choice of the chloride concentration in the aqueous phase and the stripping can be carried out with water. Ionic liquids have been used in combination of di(2-ethylhexyl)2-ethylhexyl phosphonate (DEHEHP), TBP and Cyanex 925 to enhance the separation of rare-earth ions, increase the extraction capacity and decrease the viscosity of the organic phase.51–53
In this paper, the effect of molecular extractants on the extraction and separation of neodymium and dysprosium using split-anion was investigated. Since hydrophobic and low viscous ionic liquids with high availability are needed for the development of these processes, phosphonium and quaternary ammonium cations with long alkyl chains combined with nitrate and thiocyanate anions were employed. Special attention was paid to the effect on distribution ratios and separation factors between the rare earths that are most commonly found in NdFeB magnets (i.e. neodymium and dysprosium). Finally, proof of principle is given that ionic liquids combined with molecular extractants can be used in mixer-settlers without the need of high temperatures or molecular diluents.
Experimental
Chemicals
Tricaprylmethylammonium chloride (Aliquat 336, [A336][Cl], 88.2–90.6%), nitric acid (≥65%KSCN (99%) were purchased from Sigma-Aldrich (Diegem, Belgium). KNO3 (>99%) and tri, p.a.) and n-butyl phosphate (TBP) (97%) were purchased from Chem-Lab Analytical (Zedelgem, Belgium), trihexyl(tetradecyl)phosphonium chloride (Cyphos IL 101, [C101][Cl], 97.7%) and Cyanex® 923 (93%, mixture of trialkylphosphine oxides with hexyl and octyl groups) were obtained from Cytec Industries (Canada), Dy(NO3)3·6H2O (99%) and oxalic acid (99%) were purchased from Acros Oganics (Geel, Belgium), Toluene (99.8%, HPLC grade) and hydrochloric acid (37%, reagent grade) were obtained from Fischer Chemical (UK). Nd(NO3)3·6H2O (99%) was obtained from Alfa Aesar (Karlsruhe Germany), NdCl3·6H2O (99.9%) and DyCl3·6H2O (99.9%) from Strem Chemicals (Newburyport, USA). Standard solutions of individual elements (1000 mg L−1) for inductively coupled plasma optical emission spectrometer (ICP-OES) and CaCl2·2H2O (>99%) were obtained from Merck (Overijse, Belgium). The silicone solution in isopropanol was obtained from SERVA Electrophoresis GmbH (Heidelberg, Germany). Pure water (MilliQ, Millipore, >18 MΩ cm−1) was employed to make all the dilutions. All chemicals were used as received without further purification.Instrumentation
The extraction experiments were performed in 4 mL vials and using a temperature controllable Turbo Thermo Shaker (Model: TMS-200, Hangzhou Allsheng Instrument Co. Ltd., China). The separation of the aqueous and organic phases was speeded up by centrifugation using a Heraeus Megafuge 1.0 centrifuge. Viscosities and densities were measured using an automatic rolling-ball viscosity meter Lovis (Model 2000 M/ME, with a density measuring module MA 4500 ME, Anton Paar GmbH, Graz, Austria). A volumetric Karl Fischer titrator Mettler-Toledo with a Stromboli oven operating at 150 °C was used with HYDRANAL®-Composite 5 one-component reagent to determine the water content of the ionic liquids. Analysis of the aqueous phase was performed using a PerkinElmer Optima 8300 inductively coupled plasma optical emission spectrometer (ICP-OES) in dual view, with a GemTip CrossFlow II nebulizer, a Scott Spray Chamber Assembly, a sapphire injector and a Hybrid XLT ceramic torch. The calibration curve was constructed by fitting through the origin using standard solutions of Nd(III) and Dy(III) prepared in a 1 M HNO3 solution at four different concentrations: 2.5, 5, 10 and 20 mg L−1. Samples of the aqueous phase were prepared by taking an aliquot of 100 μL and diluting it to 10 mL with a 1 M HNO3 solution. A sample of 1 mL of this solution was further diluted to 10 mL with the 1 M HNO3 solution to measure Nd(III), which was in higher concentrations. All measurements were performed in triplicate. The chloride concentration in the organic phase was determined with a benchtop total reflection X-ray fluorescence (TXRF) spectrometer (S2 Picofox, Bruker). All the pH measurements were performed using an S220 Seven Compact pH/Ion meter (Mettler-Toledo) and a Slimtrode (Hamilton) electrode.Synthesis of ionic liquids
To prepare thiocyanate forms of the ionic liquids, the chloride ionic liquids were pre-equilibrated three times with a 3 M KSCN solution to exchange the chloride ions for thiocyanate ions. Afterwards, the thiocyanate ionic liquid was washed three times with pure water. This resulted in the water saturated forms of [C101][SCN] and [A336][SCN]. To obtain the nitrate forms of the ionic liquids, [C101][NO3] and [A336][NO3], the same procedure was followed by using a 3 M KNO3 solution. The chloride concentration in these ionic liquid phases after equilibration was checked by TXRF and was below the detection limit of the TXRF spectrometer (i.e. 20 ppm chlorine).54Solvent extraction
For the solvent extraction experiments, a synthetic solution was prepared by dissolving NdCl3·6H2O and DyCl3·6H2O in water, HCl was added to help the dissolution, the final pH of the aqueous solution was 2.5 and the final concentrations were 64 g L−1 Nd(III) and 14 g L−1 Dy(III). These concentrations were chosen so that the ratio between the elements mimicked the ratio that is usually found in NdFeB magnets. The study of the behavior of Fe, B transition metals and impurities that can be found in the magnets was out of the scope of this paper. Batch experiments were carried out by contacting 1 mL of the aqueous phase with 1 mL of the organic phase (60% [C101][SCN], 40% Cyanex 923) in closed 4 mL vials. The vials were shaken at 25 °C and 2000 rpm during 60 min, unless otherwise is stated. Afterwards, the samples were centrifuged and the phases were separated. A sample of the aqueous phase was taken, diluted and measured with ICP-OES. For the scrubbing experiments, a loaded organic phase, 25 g L−1 Nd(III) and 9 g L−1 Dy(III), was put in contact with 1 mL of scrubbing agent (water, different concentrations of CaCl2) in 4 mL closed vials, and agitated during 60 min at 25 °C. After separation of the phases, a sample of the aqueous phase was taken, diluted and measured with ICP-OES. The maximum loading of Dy(III) and Nd(III) in the undiluted [C101][SCN] or the organic phase containing [C101][SCN] and Cyanex 923 (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 wt%) was determined by equilibrating the organic phase with an aqueous solution of 10 g L−1 Nd(III) and 4 g L−1 Dy(III) (total REE concentration 14 g L−1) in 3.0 M CaCl2 and then removing and measuring the aqueous phase after equilibrium. The organic phase was equilibrated again with fresh solution of the total rare earths and the aqueous phase was removed and measured again. This procedure was repeated six times. The extraction of Nd(III) from the aqueous phase at different concentrations of CaCl2 to [A33][NO3] was studied from a solution containing 41 g L−1 of Nd(III). The recycling was carried out by extracting from a solution containing 64 g L−1 Nd(III), 14 g L−1 Dy(III) and 3.0 M CaCl2 to the organic phase and afterwards stripping it completely with water (3 steps), then the organic phase containing [C101][SCN] and Cyanex 923 (60
40 wt%) was determined by equilibrating the organic phase with an aqueous solution of 10 g L−1 Nd(III) and 4 g L−1 Dy(III) (total REE concentration 14 g L−1) in 3.0 M CaCl2 and then removing and measuring the aqueous phase after equilibrium. The organic phase was equilibrated again with fresh solution of the total rare earths and the aqueous phase was removed and measured again. This procedure was repeated six times. The extraction of Nd(III) from the aqueous phase at different concentrations of CaCl2 to [A33][NO3] was studied from a solution containing 41 g L−1 of Nd(III). The recycling was carried out by extracting from a solution containing 64 g L−1 Nd(III), 14 g L−1 Dy(III) and 3.0 M CaCl2 to the organic phase and afterwards stripping it completely with water (3 steps), then the organic phase containing [C101][SCN] and Cyanex 923 (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 wt%) was reused in a new extraction.
40 wt%) was reused in a new extraction.
The distribution ratio D in case of equal volumes is defined as follows:
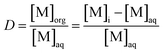 | (1) |
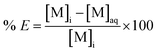 | (2) |
The efficiency of the separation of two metals can be described with the separation factor α, in which DM1 and DM2 correspond to the distribution ratios D of metals M1 and M2, respectively, DM1 > DM2:
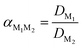 | (3) |
Mixer-settler experiments
A MEAB MSU-0.5 mixer-settler system comprised of two extraction and four stripping units in polytetrafluoroethylene (PTFE) was used to evaluate the feasibility to run the developed process in counter-current mode for the separation of neodymium and dysprosium. A mixer-settler unit consisted of: (a) the mixing chamber (0.12 L effective volume), where the aqueous and organic phases are mixed using a motor stirrer, (b) the settling chamber (0.480 L effective volume), where the phases were separated by density difference and (c) an aqueous phase outlet compartment, from which the aqueous phase exits the unit.54 Both phases were pumped into the mixer-settlers using solenoid metering pumps (ProMinent). The flow rates for the organic and aqueous phases during extraction and stripping were 4 mL min−1, the mixing speed in the mixer chambers was 500 rpm. The organic/aqueous phase ratio (O/A) was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for both the extraction and stripping of Dy(III). For the extraction experiments the organic phase consisted of a mixture of Cyanex 923 and [C101][SCN] wt% 40
1 for both the extraction and stripping of Dy(III). For the extraction experiments the organic phase consisted of a mixture of Cyanex 923 and [C101][SCN] wt% 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 and a solution of 65.7 g L−1 Nd(III) and 13.5 g L−1 Dy(III) in CaCl2 2.5 M, pH = 2.5 was used as aqueous phase. A solution of CaCl2 1.0 M was used as stripping agent for the stripping of Dy.
60 and a solution of 65.7 g L−1 Nd(III) and 13.5 g L−1 Dy(III) in CaCl2 2.5 M, pH = 2.5 was used as aqueous phase. A solution of CaCl2 1.0 M was used as stripping agent for the stripping of Dy.
Results and discussion
Two different molecular extractants, namely TBP and Cyanex 923 were tested to study their effect on the separation of Nd(III) and Dy(III) using the split-anion approach. One of the advantages of the split-anion extraction is that it offers the possibility to extract rare earths from chloride solutions using basic extractants. The latter is very useful for the design and development of separation processes for rare earths because no acidic extractants are needed and therefore, less chemicals are consumed. Furthermore, hydrochloric acid is easier to recycle and thus to be used in a closed-loop process than for example, nitric acid.47 The separation of Nd(III) and Dy(III) was first studied from chloride media to the undiluted ionic liquid [A336][NO3] or to this ionic liquid combined with Cyanex 923 or TBP. The results are presented in Table 1.| Organic phase | DNda ± SDb | DDya ± SDb | αNd/Dy ± SDb |
|---|---|---|---|
| a Equilibration time: 60 min, 2000 rpm, 25 °C, concentration of CaCl2 in the aqueous phase = 2.5 M.b SD = standard deviation. | |||
| [A336][NO3] | 0.38 ± 0.01 | 0.20 ± 0.01 | 1.90 ± 0.12 |
[A336][NO3]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TBP 90 TBP 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (wt%) 10 (wt%) |
0.41 ± 0.02 | 0.22 ± 0.01 | 1.86 ± 0.10 |
[A336][NO3]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cyanex 923 90 Cyanex 923 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (wt%) 10 (wt%) |
0.44 ± 0.01 | 0.29 ± 0.01 | 1.52 ± 0.09 |
From Table 1 it can be seen that the addition of Cyanex 923 to the ionic liquid slightly increases the distribution ratios of both metals. The addition of TBP does not have an impact on the distribution ratios or the separation factors. The distribution ratio of Nd(III) changes from 0.38 in the undiluted ionic liquid to 0.44 when 10 wt% Cyanex 923 is added. However, since the distribution ratios of Dy(III) are also increasing the separation factor decreases. The undiluted ionic liquid [A336][NO3] offers the best separation factor. This could be due to the fact that the extraction of rare earths from nitrate media follows a negative sequence (i.e. the LREES are extracted more than the HREES), in contrast to the fact that Cyanex 923 and TBP extract from nitrate media following a positive sequence (i.e. the HREES are extracted more than the LREES).16,49,55 Taking into account that the separation of rare earths from chloride media when employing [C101][SCN] or [A336][SCN] follows a positive sequence, which means that Dy(III) is preferentially extracted, the addition of TBP and Cyanex 923 was studied in thiocyanate-based ionic liquids and the results are presented in Table 2.
| Organic phase | DNd a ±SDb | DDy a ± SDb | αDy/Nd ± SDb |
|---|---|---|---|
| a Equilibration time: 60 min, 2000 rpm, 25 °C, concentration of CaCl2 in the aqueous phase = 2.5 M.b SD = standard deviation. | |||
| [A336][SCN] | 0.27 ± 0.01 | 0.54 ± 0.02 | 2.11 ± 0.12 |
[A336][SCN]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TBP wt% 90 TBP wt% 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
0.28 ± 0.01 | 0.59 ± 0.03 | 2.10 ± 0.18 |
[A336][SCN]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cyanex 923 wt% 90 Cyanex 923 wt% 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
0.32 ± 0.01 | 0.72 ± 0.03 | 2.25 ± 0.14 |
| [C101][SCN] | 0.16 ± 0.01 | 0.37 ± 0.02 | 2.31 ± 0.15 |
[C101][SCN]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TBP wt% 90 TBP wt% 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
0.19 ± 0.01 | 0.49 ± 0.01 | 2.57 ± 0.09 |
[C101][SCN]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cyanex 923 wt% 90 Cyanex 923 wt% 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
0.23 ± 0.01 | 0.79 ± 0.05 | 3.43 ± 0.24 |
Cyanex 923![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Toluene wt% 90 Toluene wt% 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
0.01 ± 0.001 | 0.070 ± 0.006 | 6.99 ± 0.21 |
Comparing the results from Tables 1 and 2, it can be seen that the separation is more efficient when using ionic liquids with thiocyanate anions than when using ionic liquids with nitrate anions. Thiocyanate-based ILs have larger separation factors than the nitrate-based ILs. When extracting to [A336][SCN], higher distribution factors for Dy(III) and Nd(III) are obtained than in the case of [C101][SCN], but the separation factors are smaller. This is because quaternary ammonium ILs trend to extract the rare earths more strongly than the quaternary phosphonium ILs, which is in part due to the fact that the ammonium ionic liquid has a higher loading capacity due to its lower molecular mass. Besides this, the aqueous media has a high content of Nd(III) (i.e. 64 g L−1) which is 4.5 times the amount of Dy(III) present (i.e. 14 g L−1). The addition of TBP to the thiocyanate-based ionic liquids does not significantly increase the distribution ratios. On the other hand, the addition of Cyanex 923 increased the distribution ratios and separation factors for [C101][SCN] due to the stronger capacity of extraction of Cyanex 923. When using Cyanex 923 diluted in toluene instead of in the ionic liquid, the distribution factors were extremely low, a high separation factor was obtained but the separation is not feasible, the percentages of extraction were just 6.4% for Dy and 0.5% for Nd. This can be explained by the fact that Cyanex 923 alone cannot extract the rare earths from chloride media under the studied experimental conditions. The thiocyanate anions are necessary for the extraction because they will form extractable complexes with the rare earths. The distribution ratios reported on Tables 1 and 2 were too low and an optimization of the system was necessary to increase them. Since larger separation factors were obtained when using Cyanex 923 combined with the ionic liquid [C101][SCN], this system was further optimized for the separation of Nd(III) and Dy(III).
The effect of the concentration of Cyanex 923 in [C101][SCN] on the extraction was studied only from 0 to 60 wt%, since percentages higher than 60 wt% resulted in the formation of a third phase. The results are presented in Fig. 1.
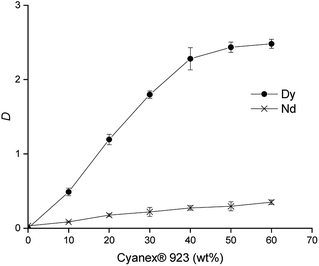 | ||
| Fig. 1 Effect of the concentration of Cyanex® 923 diluted in [C101][SCN] on the separation of Nd(III) and Dy(III) in 2.5 M CaCl2. Equilibration time: 60 min. Shaking speed: 2000 rpm at 25 °C. | ||
From Fig. 1, it can be seen how the distribution ratios of both metals increase while increasing the concentration of Cyanex 923 in [C101][SCN] and especially for Dy(III). The separation factors also increase up to a concentration of 40 wt% Cyanex 923 (α = 8.3), at higher concentrations of Cyanex 923 the distribution ratios of Dy(III) remain almost constant and the separation factors decrease. A concentration of 40 wt% was chosen as optimal. Concentrations of Cyanex 923 higher than 60 wt% led to third phase formation and it was not possible to determine distribution ratios. The chloride concentration has a major impact on the distribution ratios and can be used to fine-tune the extraction. Therefore the effect of the concentration of CaCl2 in the aqueous phase was studied (Fig. 2).
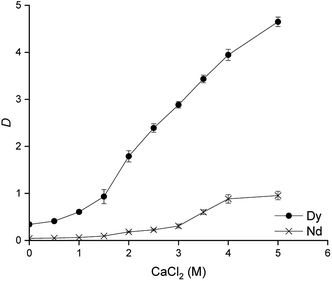 | ||
| Fig. 2 Effect of the CaCl2 concentration on the separation of Nd(III) and Dy(III). Equilibration time: 60 min. Shaking speed: 2000 rpm at 25 °C, 40 wt% Cyanex 923 in [C101][SCN]. | ||
In a split anion extraction process, the thiocyanate ions in the ionic liquid phase coordinate the rare earths because under the conditions of the present extraction, the rare-earth ions form more easily extracted complexes with thiocyanate ions than with chloride ions. The chloride ions act as counter anions for the ionic liquid cations that are not involved as counter cations for the anionic rare-earth complexes.47 Therefore, the addition of CaCl2 to the aqueous phase has a major impact on the distribution ratios of dysprosium. The best separation factors were obtained when using a concentration of 2.5 M CaCl2 (α = 9.7). When working at CaCl2 concentrations higher than 3 M, the selectivity is lost due to the co-extraction of neodymium. The marked effect that the CaCl2 concentration has over the distribution factors is also an indication that it could be used for the stripping and the scrubbing steps. The next parameter evaluated was the equilibration time and the results are presented in Fig. 3.
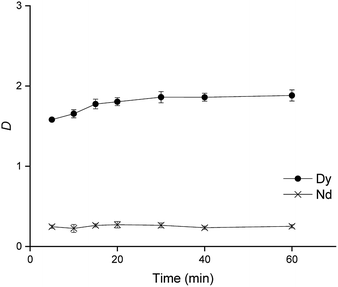 | ||
| Fig. 3 Influence of time on the separation of Nd(III) and Dy(III) from a 2.5 M CaCl2 matrix to an organic phase composed of 40 wt% Cyanex 923, 60 wt% [C101][SCN] shaking speed: 2000 rpm at 25 °C. | ||
From Fig. 3 it can be seen that equilibrium is reached at around 20 min, which still allows to carry out the process in large counter-current continuous scale. For the study of the pH effect on the distribution ratios of Nd(III) and Dy(III), different pH values between 0.25 and 4 were evaluated. The pH did not have a significant effect on the percentage extraction, the distribution ratios remained constant between pH 2 and 5 and therefore a pH of 2 was chosen as optimal. pH values higher than 5 are not recommended since at these values the rare earths can undergo hydrolysis.
When studying the influence of the concentration of Cyanex 923 it was observed how its addition decreased the viscosity of the organic phase and also accelerated the phase disengagement (except at concentrations higher than 60 wt% in which a third phase was formed). For this reason the viscosity of the loaded undiluted ionic liquid [C101][SCN] and the viscosity of the loaded organic phase composed of Cyanex 923 40 wt% in [C101][SCN] were compared. The loading capacity of the organic phases was calculated by contacting them with a solution containing 14 g L−1 total concentration of rare earths, after equilibrium, the aqueous phase was removed and the previously loaded organic phase was contacted again with fresh solution. This process was repeated 6 times to reach a maximum loading capacity. The results obtained regarding the loading capacity are presented in Table 3 and the viscosity values of the organic phases before and after loading are reported in Table 4.
| Organic phase | Viscosity (cP) |
|---|---|
| [C101][SCN] | 391 |
| 60 wt% [C101][SCN] + 40 wt% Cyanex 923 | 180 |
| [C101][SCN] loaded with 29.8 g L−1 REE | 958 |
| 60 wt% [C101][SCN] + 40 wt% Cyanex 923 loaded with 29.8 g L−1 REE | 370 |
The loading capacity of the organic phase is an important parameter in split-anion extraction. This is because the HREES have a crowing effect on the extraction of LREES (i.e. the rare earths with higher D values displaces the ones with lower D values at high loading values). The total loading increased from 19.2 g L−1 to 29.6 g L−1 when adding 40 wt% Cyanex 923 (Table 3). This effect is most likely due to the greater solubility of the extracted rare-earth complexes in the ionic liquid phase due to the presence of Cyanex 923. At the same time, the addition of Cyanex 923 decreased the viscosity of the loaded organic phase and improved the phase disengagement. The high loading, the low viscosity and the easy phase disengagement are parameters that are crucial in solvent extraction. In industrial processes in which concentrated feeds are treated, higher loading capacities are beneficial because allow to increase the process capacity and selectivity.
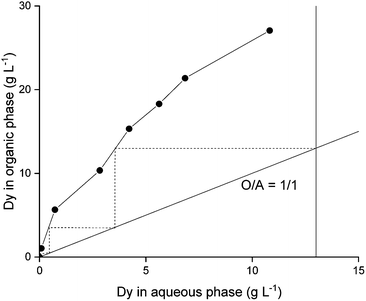
A McCabe–Thiele diagram was constructed to determine the number of stages required to separate Dy(III) from Nd(III) with the optimized system. As a result, it was estimated that two stages would be needed for the removal of Dy(III) using an O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratio of 1
A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 4).
1 (Fig. 4).
Scrubbing and stripping
The next parameters investigated were the stripping and the scrubbing of Nd(III). Scrubbing is important since due to the large content of Nd(III) in the aqueous phase, it can get coextracted along Dy(III). Taking into account that large concentrations of chloride ions increase the distribution of Dy(III) in the organic phase, the effect of the concentration of CaCl2 on the percentage scrubbing was studied.From Table 5, it can be seen how different concentrations of CaCl2 can offer selectivity in the scrubbing step. For instance, high concentrations of CaCl2 allow the scrubbing of Nd(III) while keeping most of the Dy(III) in the loaded organic phase. Concerning the stripping, it can be seen in Table 5 that this can be achieved with water or with a diluted solution of CaCl2 since the presence of the salt helps additionally the phase disengagement.
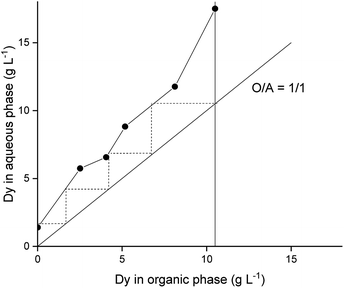
A McCabe–Thiele diagram was constructed to determine the number of stages required to strip Dy(III) from the loaded organic phase. As a result, it was estimated that four stages would be needed for the removal of Dy(III) using an O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratio of 1
A ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 5).
1 (Fig. 5).
Up to this point it has been shown how Dy(III) can be extracted, refined and stripped from the initial rare earth mixture. However, a part of Nd(III) will still remain in the aqueous phase together with Ca(II). Another advantage of the split-anion approach is that it allows the possibility to change the organic phases depending on the needs of the extraction, for example the distribution ratios of Nd(III) are larger when extracting to nitrate-based ionic liquids than when extracting to thiocyanate-based ionic liquids (Tables 1 and 2). Therefore, the aqueous phase containing Nd(III) can be contacted with [C101][NO3] or [A336][NO3] to separate it from Ca(II) (Table 6).
From Table 6, it can be seen how the addition of CaCl2 to the aqueous feed can be beneficial for the extraction of Nd(III). The percentage extraction of Ca(II) was very low and without significant deviations between the percentage extraction using the quaternary phosphonium or the quaternary ammonium ionic liquid. After extraction with [C101][NO3], Nd(III) can be stripped from the loaded ionic liquid using water as well (i.e. with a stripping efficiency up to 99.6%).
Mechanism of extraction
In general, the mechanism of extraction of a split-anion extraction of a rare-earth ion Ln3+ can be written as follows:47
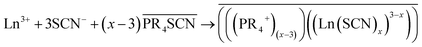 | (4) |
The bars in the equation mean that the species is in the organic phase, and x ≥ 3. Due to an anion exchange between the aqueous and the ionic liquid phase there is small amount of SCN− anions in the aqueous phase. The extracted complex is probably formed in the aqueous phase rather than in the ionic liquid, therefore coordinated water molecules could be present in the extracted species. On the other hand, Cyanex 923 can form complexes of the form Ln(SCN)3 n(Cya) with n = 4 and 3 for the light and heavy rare earths, respectively when extracting to Cyanex 923 diluted in xylene.55 It has been demonstrated that lanthanide thiocyanate complexes are soluble in hydrophobic ionic liquids and non-polar solvents.56 The stability constant for these complexes increases as the atomic number of the rare earths increases whereas in the case of nitrate complexes, the stability constant decreases.57 The interaction between thiocyanate anions and the lanthanide cation is stronger than between nitrates and the lanthanides, therefore it has been proposed that thiocyanate anions form inner sphere ion pairs.58 With the use of extended X-ray absorption fine structure (EXAFS) it has been demonstrated that when extracting to nitrate ionic liquids combined with molecular extractants, different species are formed, depending on the composition of the organic phase.51,59 A complete study of the species formed during the extraction in the extraction system proposed here is out of the scope of this work. A combination of complementary speciation techniques and modelling should be applied to obtain an accurate and reliable description of the mechanism of extraction. Different models to study and understand the complexation and aggregation in both the aqueous and the organic phases have been proposed for the extraction of rare earths using different types of extractants and diluents.60–62
Recycling of the organic phase
The organic phase composed of 60 wt% [C101][SCN] and 40 wt% Cyanex 923 was recovered after the stripping of dysprosium and recycled. With this aim, the ionic liquid was re-equilibrated with acidulated water to a pH = 2 and then contacted again with fresh feed. This procedure was repeated three times. The distribution ratios remained almost the same which indicates the feasibility of recycling the organic phase. It has to be highlighted that the prolonged exposition of thiocyanate ionic liquids to sunlight and high temperatures leads to some degradation of the thiocyanate ions.49 No degradation was observed (constant distribution ratios) in the experiments carried out at 25 °C. A conceptual flow sheet of the complete process is given in Fig. 6.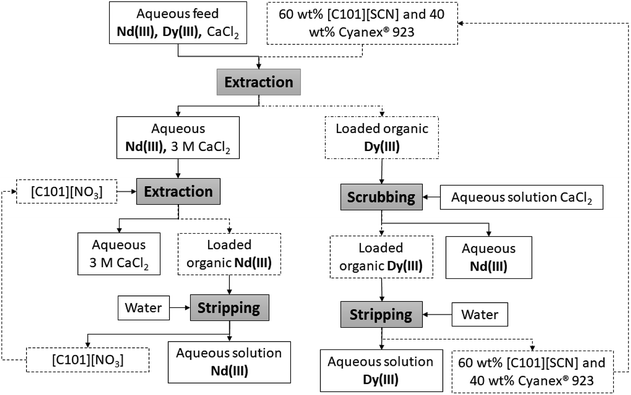 | ||
| Fig. 6 Conceptual flow sheet of the developed and optimized process for the extraction and purification of Nd(III) and Dy(III) mixtures in concentrated chloride media. | ||
Mixer-settler experiments
The feasibility to run the developed process in continuous mode at room temperature was tested in a small battery of mixer-settlers. The main goal was to demonstrate that the process has suitable physicochemical properties for its use in larger scales rather than only in batch experiments. A part of the solvent extraction community has prejudices against the use of ionic liquids in solvent extraction mainly because it has been commonly accepted that it is very difficult to use them in continuous equipment. While it is true that not all ionic liquids are suitable for liquid–liquid extraction and upscaling, some of them can still offer advantages over conventional systems and have been used in mixer-settlers.54 In this paper we show that it is completely possible to run a continuous extraction at room temperature using ionic liquids combined with neutral extractants without the need to add molecular solvents and without facing problems during the separation.Two parts of the flow sheet (Fig. 6) were selected to carry out the test in the mixer-settlers. The whole flowsheet was not fully tested due to the large consumption of time and chemicals that this would require. The removal of Dy(III) was studied because it is the crucial step of the process and the stripping of Dy(III) was chosen over the scrubbing because the scrubbing can be performed with aqueous solutions with high salt concentrations that facilitate the phase disengagement in the settling chamber in the mixer-settler. Stripping can be done with water but it is more challenging due to the stability of the emulsion that can be formed when mixing both phases.
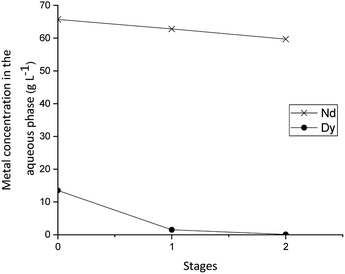
According to the McCabe–Thiele diagrams, it was estimated that two stages were needed for the extraction and four for the stripping (Fig. 4 and 5) when using O/A ratios of 1/1. The extraction and the stripping were equilibrated during 10 h and 16 h respectively and samples were taken from the aqueous chamber of each stage and analysed afterwards with ICP-OES. The extraction behaviour of Dy(III) is presented in Fig. 7. During the whole time of operation, no precipitate or third phase formation was observed and the phases disengaged quickly and easily in the settler without the need of coalescence plates. The results from the mixer-settlers showed that indeed two stages are needed to completely remove the Dy(III) from the mixture. There was some co-extraction of Nd(III) which was expected and is the reason why a scrubbing step would be required to further purity the Dy(III) extracted to the organic phase. A synthetic loaded organic phase containing only Dy(III) was prepared to carry out the stripping experiments in the mixer-settlers.
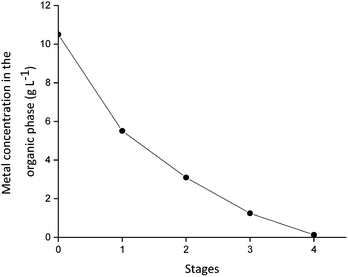
The stripping of Dy(III) was tested using an aqueous solution of 1.0 M CaCl2 as stripping agent. The stripping behavior of Dy(III) is presented in Fig. 8. Four stages were needed for the complete stripping of Dy(III). In a first approach, the stripping in the mixer-settler was carried out using water as stripping agent, but this resulted in emulsion formation and slow phase disengagement in the settling chamber. The presence of a small amount of salt, such as CaCl2 is crucial for the good performance of the process in continuous mode. No third phase formation was observed and the emulsion disengaged without problems in the settler. The organic phase was easily pumped due to the low viscosity of the ionic liquid when Cyanex 923 is added. These experiments demonstrate that it is possible to run a continuous solvent extraction process based on mixtures of ionic liquids and molecular extractants at room temperature and without the need of a diluent.
After stripping, Nd(III) and Dy(III) could be recovered from the aqueous stripping solutions by adition of oxalic acid, followed by calcination to the corresponding oxide as it has been previously reported.40,46
Conclusions
This paper proposes an alternative process based on the ionic liquid trihexyl(tetradecyl)phosphonium thiocyanate combined with Cyanex 923 for the separation of Nd(III) and Dy(III) from highly concentrated chloride aqueous solutions. By introducing Cyanex 923 into the system, the distribution ratios of Dy(III) increased, a higher metal loading was allowed while decreasing the viscosity and enhancing the phase disengagement process. As a result, the presented process is of relevance for the separation of Nd(III) and Dy(III) from used NdFeB magnets. Dy(III) was separated from Nd(III) by using [C101][SCN] combined with Cyanex 923 and easily stripped with water. Nd(III) was recovered from the aqueous phase using the ionic liquid [C101][NO3]. The latter demonstrated one of the biggest advantages of split-anion extractions: since the organic phase is the source of the complexing anion and not the aqueous phase, different extractions can be carried out from the same type of feed by changing the source of complexing anions in the organic phase. The proposed strategy was tested in continuous mode and it is compatible with current industrial processes for the separation of rare-earth elements from chloride media allowing the separation of two critical metals in relatively few steps.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research leading to these results has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement no. 720838 (NEOHIRE) and from the European Community's Horizon 2020 Programme (H2020/2014-2019) under grant agreement no. 674973 (MSCA-ETN DEMETER). This publication reflects only the author's view, exempting the Community from any liability.References
- A. Golev, M. Scott, P. D. Erskine, S. H. Ali and G. R. Ballantyne, Resour. Policy, 2014, 41, 52–59 CrossRef.
- K. Binnemans, P. T. Jones, B. Blanpain, T. Van Gerven, Y. Yang, A. Walton and M. Buchert, J. Cleaner Prod., 2013, 51, 1–22 CrossRef CAS.
- Y. Yang, A. Walton, R. Sheridan, K. Güth, R. Gauß, O. Gutfleisch, M. Buchert, B.-M. Steenari, T. Van Gerven, P. T. Jones and K. Binnemans, J. Sustain. Metall., 2017, 3, 122–149 CrossRef.
- D. Brown, B. M. Ma and Z. Chen, J. Magn. Magn. Mater., 2002, 248, 432–440 CrossRef CAS.
- V. Zepf, Rare earth elements a new approach to the nexus of supply, demand and use: exemplified along the use of neodymium in permanent, Augsburg University, 2013 Search PubMed.
- E. Machacek, J. L. Richter, K. Habib and P. Klossek, Resour. Conserv. Recycl., 2015, 104, 76–93 CrossRef.
- K. Binnemans, P. T. Jones, K. Acker, B. Blanpain, B. Mishra and D. Apelian, JOM, 2013, 65, 846–848 CrossRef.
- A. Rollat, D. Guyonnet, M. Planchon and J. Tuduri, Waste Manag., 2016, 49, 427–436 CrossRef CAS PubMed.
- G. G. Zaimes, B. J. Hubler, S. Wang and V. Khanna, ACS Sustain. Chem. Eng., 2015, 3, 237–244 CrossRef CAS.
- J. H. Rademaker, R. Kleijn and Y. Yang, Environ. Sci. Technol., 2013, 47, 10129–10136 CrossRef CAS PubMed.
- B. Sprecher, Y. Xiao, A. Walton, J. Speight, R. Harris, R. Kleijn, G. Visser and G. J. Kramer, Environ. Sci. Technol., 2014, 48, 3951–3958 CrossRef CAS PubMed.
- J. Kooroshy, G. Tiess, A. Tukker and A. Walton, Strengthening of the European Rare Earths Supply-chain, The European Rare Earths Competency Network (ERECON), 2015 Search PubMed.
- K. A. Gschneidner, J.-C. G. Bunzli and V. K. Pecharsky, Handbook on the physics and chemistry of rare earths, North-Holland, Amsterdam, 2003, vol. 33 Search PubMed.
- N. Krishnamurthy and C. Gupta, Extractive Metallurgy of Rare Earths, CRC Press, Boca Raton (Florida), 2nd edn, 2016 Search PubMed.
- F. Xie, T. A. Zhang, D. Dreisinger and F. Doyle, Miner. Eng., 2014, 56, 10–28 CrossRef CAS.
- Y. Wang, F. Li, Z. Zhao, Y. Dong and X. Sun, Sep. Purif. Technol., 2015, 151, 303–308 CrossRef CAS.
- X. Wang, W. Li and D. Li, J. Rare Earths, 2011, 29, 413–415 CrossRef.
- S. Radhika, B. N. Kumar, M. L. Kantam and B. R. Reddy, Sep. Purif. Technol., 2010, 75, 295–302 CrossRef CAS.
- R. Safarbali, M. R. Yaftian and A. Zamani, J. Rare Earths, 2016, 34, 91–98 CrossRef CAS.
- M. Anitha, M. K. Kotekar, D. K. Singh, R. Vijayalakshmi and H. Singh, Hydrometallurgy, 2014, 146, 128–132 CrossRef CAS.
- M. Černá, E. Volaufová and V. Rod, Hydrometallurgy, 1992, 28, 339–352 CrossRef.
- J. Liu, W. Wang and D. Li, Colloid. Surf. Physicochem. Eng. Asp., 2007, 311, 124–130 CrossRef CAS.
- Y. Zuo, J. Chen and D. Li, Sep. Purif. Technol., 2008, 63, 684–690 CrossRef CAS.
- K. Larsson, C. Ekberg and A. Ødegaard-Jensen, Hydrometallurgy, 2013, 133, 168–175 CrossRef CAS.
- D. F. Haghshenas, D. Darvishi, H. Rafieipour, E. K. Alamdari and A. A. Salardini, Hydrometallurgy, 2009, 97, 173–179 CrossRef CAS.
- W. Liao, Q. Shang, G. Yu and D. Li, Talanta, 2002, 57, 1085–1092 CrossRef CAS PubMed.
- K. R. Barnard, N. J. Kelly and D. W. Shiers, Hydrometallurgy, 2014, 146, 1–7 CrossRef CAS.
- D. F. Peppard, J. P. Faris, P. R. Gray and G. W. Mason, J. Phys. Chem., 1953, 57, 294–301 CrossRef CAS.
- H. Tong, D. Li, Y. Wang and J. Lei, Wuhan Univ. J. Nat. Sci., 2003, 8, 871–874 CrossRef CAS.
- M. S. E. E. A. W. Al-Shawi, O. B. Jenssen and T. R. Jorgensen, Proc. ISEC 2002, 2002, 2, pp. 1064–1069 Search PubMed.
- Y. A. El-Nadi, J. Rare Earths, 2010, 28, 215–220 CrossRef CAS.
- M. J. Earle and K. R. Seddon, ACS Symp. Ser., 2002, 819, 10–25 CrossRef CAS.
- G.-T. Wei, Z. Yang and C.-J. Chen, Anal. Chim. Acta, 2003, 488, 183–192 CrossRef CAS.
- T. Vander Hoogerstraete, S. Wellens, K. Verachtert and K. Binnemans, Green Chem., 2013, 15, 919–927 RSC.
- J. N. Chubb, P. Lagos and J. Lienlaf, J. Electrost., 2005, 63, 119–127 CrossRef CAS.
- T. P. Thuy Pham, C. W. Cho and Y. S. Yun, Water Res., 2010, 44, 352–372 CrossRef PubMed.
- D. Zhao, Y. Liao and Z. Zhang, Clean: Soil, Air, Water, 2007, 35, 42–48 CAS.
- Y. Baba, F. Kubota, N. Kamiya and M. Goto, J. Chem. Eng. Jpn., 2011, 44, 679–685 CrossRef CAS.
- D. Dupont and K. Binnemans, Green Chem., 2015, 17, 856–868 RSC.
- T. Vander Hoogerstraete, B. Blanpain, T. Van Gerven and K. Binnemans, RSC Adv., 2014, 4, 64099–64111 RSC.
- X. Sun, C.-L. Do-Thanh, H. Luo and S. Dai, Chem. Eng. J., 2014, 239, 392–398 CrossRef CAS.
- J. Wang, G. Chen, S. Xu, Z. Yin and Q. Zhang, J. Rare Earths, 2016, 34, 724–730 CrossRef CAS.
- Y. Liu, J. Chen and D. Li, Sep. Sci. Technol., 2012, 47, 223–232 CrossRef CAS.
- L. Guo, J. Chen, L. Shen, J. Zhang, D. Zhang and Y. Deng, ACS Sustain. Chem. Eng., 2014, 2, 1968–1975 CrossRef CAS.
- A. Rout and K. Binnemans, Phys. Chem. Chem. Phys., 2016, 18, 16039–16045 RSC.
- S. Riano and K. Binnemans, Green Chem., 2015, 17, 2931–2942 RSC.
- K. Larsson and K. Binnemans, Hydrometallurgy, 2015, 156, 206–214 CrossRef CAS.
- D. Dupont, D. Depuydt and K. Binnemans, J. Phys. Chem. B, 2015, 119, 6747–6757 CrossRef CAS PubMed.
- R. Banda, F. Forte, B. Onghena and K. Binnemans, RSC Adv., 2019, 9, 4876–4883 RSC.
- S. Sobekova Foltova, T. Vander Hoogerstraete, D. Banerjee and K. Binnemans, Sep. Purif. Technol., 2019, 210, 209–218 CrossRef CAS.
- M. Regadío, T. Vander Hoogerstraete, D. Banerjee and K. Binnemans, RSC Adv., 2018, 8, 34754–34763 RSC.
- Y. Xiong, W. Kuang, J. Zhao and H. Liu, Sep. Purif. Technol., 2017, 179, 349–356 CrossRef CAS.
- X. Sun, D. Wu, J. Chen and D. Li, J. Chem. Technol. Biotechnol., 2007, 82, 267–272 CrossRef CAS.
- J. O. Liljenzin, G. Persson, I. Svantesson and S. Wingefors, in Transplutonium Elements—Production and Recovery, American Chemical Society, Washington DC, 1981, pp. 203–221 Search PubMed.
- M. L. P. Reddy, R. L. Varma, T. R. Ramamohan, S. K. Sahu and V. Chakravortty, Solvent Extr. Ion Exch., 1998, 16, 795–812 CrossRef CAS.
- P. Nockemann, B. Thijs, N. Postelmans, K. Van Hecke, L. Van Meervelt and K. Binnemans, J. Am. Chem. Soc., 2006, 128, 13658–13659 CrossRef CAS PubMed.
- P. K. Khopkar and J. N. Mathur, J. Inorg. Nucl. Chem., 1974, 36, 3819–3825 CrossRef CAS.
- G. R. Choppin and J. Ketels, J. Inorg. Nucl. Chem., 1965, 27, 1335–1339 CrossRef CAS.
- C. H. C. Janssen, N. A. Macías-Ruvalcaba, M. Aguilar-Martínez and M. N. Kobrak, Int. Rev. Phys. Chem., 2015, 34, 591–622 Search PubMed.
- Y. Chen, M. Duvail, P. Guilbaud and J. F. Dufrêche, Phys. Chem. Chem. Phys., 2017, 19, 7094–7100 RSC.
- M. Špadina, K. Bohinc, T. Zemb and J. F. Dufrêche, Langmuir, 2018, 34, 10434–10447 CrossRef PubMed.
- K. L. Lyon, V. P. Utgikar and M. R. Greenhalgh, Ind. Eng. Chem. Res., 2017, 56, 1048–1056 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2020 |