Validated HPLC-DAD method for stability study of sulbutiamine HCl
Nada S. Abdelwahab* and
Nehal F. Farid
Pharmaceutical Analytical Chemistry Department, Faculty of Pharmacy, Benisuef University, Benisuef, Egypt. E-mail: nadasayed2003@yahoo.com; Fax: +20-02-0822317950; Tel: +20-01117236884/01110398881
First published on 11th June 2014
Abstract
Sulbutiamine (SUL) is a synthetic thiamine derivative widely used for treatment of memory disorders. In this study, a newly developed gradient HPLC-DAD method demonstrating no interference from the different degradation products of SUL has been optimized and validated. The drug was subjected to variable stress conditions including hydrolysis (at different pH values), oxidation, photolysis and dry heat. The drug was found to be labile to hydrolysis, oxidation and photolysis but stable in thermal and neutral hydrolytic degradation. Successful chromatographic separation of SUL from all degradation products with significantly different tR values was achieved on a ZORBAX Eclipse Plus C18 column using a mobile phase containing a gradient mixture of solvent A (50 mM KH2PO4 (pH 3.6 ± 0.2)) and solvent B (methanol). UV detection was performed at 254 nm using a photodiode array detector (DAD). The reliability of the method was assessed by evaluation of accuracy, precision, specificity, robustness and ruggedness according to USP guidelines. The linear regression analysis data for the calibration curve showed a good relationship in the range of 2–40 μg mL−1. System suitability tests were performed, and selectivity (α) and resolution (Rs) factors were found to be greater than 1.5 and 2, respectively. The assay method was successfully used to estimate SUL in Arcalion Forte® tablets and good percentage recoveries were obtained. The developed method compared favorably with the reported spectrophotometric method.
1. Introduction
Sulbutiamine HCl (SUL), is a nonpharmacopoeial drug, which is chemically known as N,N′-{dithiobis[2-(2-isobutyryloxyethyl)-1-methylvinylene]}bis[N-(4-amino-2-methyl pyrimidin-5-yl-methyl)formamide].1 It is a more efficient version of vitamin B1, which mimics the effect of thiamine at a more drastic level. It is more efficient than thiamine in crossing the blood–brain barrier and can be used alone or stacked with other nootropics. It is used to treat asthenia, improve memory and improve erectile dysfunction.2 In addition, many athletes use SUL as a legal performance-enhancing drug to enhance their performance in sports.3Only two methods have been found in the literature for determination of SUL in biological fluids and pharmaceutical dosage form. The first method was a kinetic spectrophotometric method, which depended on the catalytic effect on the reaction between sodium azide and iodine in aqueous solution, and then it measures the decrease in absorbance of iodine at 348 nm.4 The second reported method was HPLC with fluorescence detection for analysis of SUL along with other thiamine disulphides.5 It depended on gradient elution using methanol and 0.011 M phosphate buffer, pH 4.5 (from 10% to 62% methanol) with an analysis time of 50 minutes using λex = 365 nm and λem = 433 nm.
During manufacturing, storage and distribution, many drugs are susceptible to different environmental factors such as light, temperature and humidity; thus, forced degradation studies are necessary for providing information about chemical and physical factors that result in drug instability and hence help in selecting suitable packing and storage conditions. Moreover, results of stability studies are necessary for setting expiration dates for the API (active pharmaceutical ingredients) or drug products.6–10 According to ICH guidelines Q1AR2,10 the stability testing of an active ingredient should be performed under variable accelerated conditions (hydrolysis under different pH values, oxidation, photolysis and thermal degradation). A stability-indicating method is a method that can selectively analyze the parent constituent (API) from the pharmaceutical product, and it is developed to separate and determine the active drug in the presence of its impurities and degradation products.11
On reviewing the current literature, none of the known pharmacopoeias described any method of analysis for SUL. Moreover, the two published methods are time consuming and cannot be used to analyze SUL samples in the presence of its different degradation products.
Due to the importance of drug stability studies and the absence of information about SUL stability, the work in this manuscript aimed to perform a stability study for SUL according to ICH guidelines10 through a validated stability indicating gradient HPLC-DAD method. The newly developed method has the advantages of being the first stability-indicating method for SUL with high sensitivity, precision and accuracy. Moreover, minimum sample preparation is required and the analysis can be performed within 10 minutes.
2. Experimental
2.1. Instruments
The HPLC (Agilent 1260 Infinity, Germany) instrument was equipped with an Agilent 1260 Infinity preparative pump (G1361A), Agilent 1260 Infinity Diode array detector VL (G131SD), Agilent 1260 Infinity Thermostated column compartment (G1316A) and Agilent 1260 Infinity preparative Autosampler (G2260A). Separation and quantitation was performed on a ZORBAX Eclipse Plus C18 column (250 × 4.6 mm i.d, 5 μm particle size) (USA).2.2. Samples
2.3. Chemicals and solvents
• Methanol [(Sigma-Aldrich, Chromasolv®, Germany), and (Fisher Scientific, UK)].• Deionized water (SEDICO Pharmaceuticals Co., Cairo, Egypt).
• Potassium dihydrogen phosphate, sodium hydroxide orthophosphoric acid, hydrochloric acid and hydrogen peroxide were of analytical grade and were purchased from El-NASR Pharmaceutical Chemicals Co., Abu-Zabaal, Cairo, Egypt.
2.4. Solutions
Stock standard solution of sulbutiamine (1 mg mL−1) was prepared by accurately weighing 0.1 g SUL in a 100 mL volumetric flask and dissolving in methanol.50 mM KH2PO4 buffer (pH = 3.6) was prepared by dissolving 6.8 g KH2PO4 in 1 L deionized water and then adjusting the pH to 3.6 ± 0.2 with aqueous phosphoric acid.
Working standard solution of sulbutiamine (0.1 mg mL−1) was prepared by transferring 10 mL from SUL stock standard solution (1 mg mL−1) into a 100 mL volumetric flask and then diluting with methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mM KH2PO4 buffer (pH = 3.6) (50
50 mM KH2PO4 buffer (pH = 3.6) (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v).
50, v/v).
3. Procedure
3.1. Chromatographic conditions
Chromatographic separation was performed on a ZORBAX Eclipse Plus C18 column using a gradient mixture of methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mM KH2PO4 buffer (pH = 3.6 ± 0.2) as a mobile phase. The gradient elution program is given in Table 1. The injection volume was 50 μL, and detection was carried out at 254 nm using DAD, maintaining the column temperature at 25 °C. The run time was 10 min, and the peak area ratio (using an area of 20 μg mL−1 SUL as an external standard) was used to quantify SUL.
50 mM KH2PO4 buffer (pH = 3.6 ± 0.2) as a mobile phase. The gradient elution program is given in Table 1. The injection volume was 50 μL, and detection was carried out at 254 nm using DAD, maintaining the column temperature at 25 °C. The run time was 10 min, and the peak area ratio (using an area of 20 μg mL−1 SUL as an external standard) was used to quantify SUL.
| Time (minutes) | % Methanol | Flow rate (minutes) |
|---|---|---|
| 0 | 55 | 1.5 |
| 2 | 55 | 1.5 |
| 4 | 85 | 2 |
| 8 | 85 | 2 |
| 9 | 55 | 1.5 |
| 10 | 55 | 1.5 |
3.2. Validation of the method
Validation of the method was performed with respect to USP guidelines.12Repeatability (intra-day variation) was checked by analyzing three concentrations of pure SUL (16, 20 and 38 μg mL−1) three times within the same day using the previously mentioned procedure under chromatographic conditions, and the %RSD values were then calculated.
Intermediate precision was verified on three different days using the previously chosen concentrations in the same laboratory using the specifications under chromatographic conditions, and the %RSD values were then calculated.
3.3. System suitability testing parameters
In all chromatographic systems, system suitability testing parameters should be checked before starting sample analysis. The peak symmetry, capacity, selectivity and resolution factors, number of theoretical plates and height equivalent to theoretical plates were calculated for the principle peak.3.4. Assay of pharmaceutical preparation
A SUL sample with a concentration of 16 μg mL−1 was prepared from Arcalion Forte® working solution (0.1 mg mL−1) and injected in triplicate following the procedure described for the chromatographic conditions. The concentration of SUL in the prepared solution was then calculated from the constructed regression equation. A standard addition technique was carried out to prove the accuracy of the suggested method, and it was performed by spiking the pre-analyzed SUL sample (16 μg mL−1) with an extra 80%, 100% and 120% of standard SUL.3.5. Forced degradation studies
Sulbutiamine stock standard solution (1 mg mL−1) was used during forced degradation studies, and each degraded sample with a concentration of 25 μg mL−1 was prepared in a mixture of methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mM KH2PO4 buffer (pH = 3.6) (50
50 mM KH2PO4 buffer (pH = 3.6) (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v). Then, the procedure described under chromatographic conditions was followed. From the relative peak area of SUL in each chromatographed sample, and the SUL % degradation was then calculated.
50, v/v). Then, the procedure described under chromatographic conditions was followed. From the relative peak area of SUL in each chromatographed sample, and the SUL % degradation was then calculated.
A separate 5 mL SUL stock standard solution (1 mg mL−1) was transferred to four separate 25 mL volumetric flasks, and then mixed with 5 mL of either 0.1 N HCl, 1 N HCl, 0.1 N NaOH or deionized water. The prepared solutions were kept away from light to prevent possible photodegradation and at 80 °C except for 0.1 N NaOH, which was maintained at room temperature. The samples were cooled and neutralized with an amount of acid or base equivalent to that of the previously added amount, and then the volume was completed to the mark with a mixture of methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mM KH2PO4 buffer (pH = 3.6) (50
50 mM KH2PO4 buffer (pH = 3.6) (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) to prepare sample working solutions of 200 μg mL−1 each.
50, v/v) to prepare sample working solutions of 200 μg mL−1 each.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mM KH2PO4 buffer (pH = 3.6) (50
50 mM KH2PO4 buffer (pH = 3.6) (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) to prepare sample working solutions of 200 μg mL−1 each.
50, v/v) to prepare sample working solutions of 200 μg mL−1 each.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mM KH2PO4 buffer (pH = 3.6) (50
50 mM KH2PO4 buffer (pH = 3.6) (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) to prepare sample working solutions of 200 μg mL−1 each.
50, v/v) to prepare sample working solutions of 200 μg mL−1 each.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mM KH2PO4 buffer (pH = 3.6) (50
50 mM KH2PO4 buffer (pH = 3.6) (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) to obtain sample solutions of 200 μg mL−1.
50, v/v) to obtain sample solutions of 200 μg mL−1.4. Results and discussion
Because of the complex nature of separating multiple components during analysis of stability samples, chromatographic methods have taken priority over the conventional methods of analysis.13–15 They possess greater accuracy and sensitivity for even small quantities of degradation products produced. The popularity of HPLC in stability studies is due to its high-resolution capacity, sensitivity and specificity.16 Stability-indicating methods are traditionally performed using gradient elution, in order to ensure that degradants of various chemical compositions are all detected.17To date, no stability-indicating method was found in the literature for determination of SUL. In this manuscript, we aimed to perform a stability study for SUL and to develop a novel stability-indicating HPLC-DAD method for its analysis. Complete separation of the analyte was accomplished in less than 10 minutes, and the method can be successfully applied to perform long-term and accelerate stability studies of SUL.
4.1. Method development and optimization
The initial method development was conducted on pure SUL samples, and the samples were obtained from different degradation conditions in order to select conditions that achieve good resolution between the drug and degradation products. The suitability of the mobile phase was determined on the basis of suitability for stability studies, time required for analysis and SUL peak broadening.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile (30
acetonitrile (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, v/v) at a flow rate of 1.0 mL min−1. Under these conditions, the SUL peak was very broad. Replacing acetonitrile with methanol slightly improved the peak broadening. Several trials were performed on the mobile phase consisting of water
70, v/v) at a flow rate of 1.0 mL min−1. Under these conditions, the SUL peak was very broad. Replacing acetonitrile with methanol slightly improved the peak broadening. Several trials were performed on the mobile phase consisting of water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (in the ratio of 30
methanol (in the ratio of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, v/v) in order to enhance the chromatographic resolution and decrease SUL peak broadening such as the addition of 0.25% triethyl amine (pH 5 adjusted with H3PO4 acid), 0.5% trifluoroacetic acid (pH 5 adjusted with NaOH) or the addition of 0.15 mM heptane sulfonic acid. Unfortunately, neither the chromatographic resolution nor the peak shape were improved.
70, v/v) in order to enhance the chromatographic resolution and decrease SUL peak broadening such as the addition of 0.25% triethyl amine (pH 5 adjusted with H3PO4 acid), 0.5% trifluoroacetic acid (pH 5 adjusted with NaOH) or the addition of 0.15 mM heptane sulfonic acid. Unfortunately, neither the chromatographic resolution nor the peak shape were improved.The second step was to replace water with 50 mM KH2PO4 buffer at different pH values (3–8). It was noticed that changing the buffer pH greatly affected the tR of SUL, and hence the resolution between SUL and the closest eluted degradation product. Extremely acidic pH resulted in decreasing SUL tR, leading to bad chromatographic resolution, whereas an alkaline pH increased SUL tR, which gives rise to a broader SUL peak and increases the analysis time.
After extensive trials, gradient elution was tested by using a gradient solvent mixture consisting of methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 mM KH2PO4 (pH 3.6 ± 0.2). Different gradient elution programs were tried until optimum system suitability testing (SST) parameters were obtained with a symmetric SUL peak. The details of the gradient elution program used are given in Table 1.
50 mM KH2PO4 (pH 3.6 ± 0.2). Different gradient elution programs were tried until optimum system suitability testing (SST) parameters were obtained with a symmetric SUL peak. The details of the gradient elution program used are given in Table 1.
4.2. Application of the method
After optimization of all factors affecting method selectivity and sensitivity, the method was applied for determination of SUL in its pharmaceutical formulation. Firstly, a calibration curve relating the integrated relative peak area of SUL (using 20 μg mL−1 SUL as an external standard) to its corresponding concentration was constructed. Good linear calibration was achieved in the range of 2–40 μg mL−1, and the calibration equation was as follows:| A = 0.0520C + 0.0265, r = 0.9999 |
| Parameters | SUL |
|---|---|
| a The intra-day (n = 9) average of three different concentrations (16, 20 and 38 μg mL−1) repeated three times within 1 day.b The inter-day (n = 9) average of three different concentrations (16, 20 and 38 μg mL−1) repeated three times in 3 successive days. | |
| Linearity | |
| Range | 2–40 μg mL−1 |
| Slope | 0.0520 |
| Intercept | 0.0265 |
| Correlation coefficient (r) | 0.9999 |
| Accuracy (mean ± %RSD) | 100.76 ± 0.997 |
| Precision (%RSD) | |
| Repeatabilitya | 0.533 |
| Intermediate precisionb | 1.831 |
| LOD | 0.50 μg mL−1 |
| LOQ | 1.51 μg mL−1 |
Secondly, and in order to evaluate the suitability of the method, the method was applied to Arcalion Forte® tablets. A single peak at tR = 5.55 ± 0.03 minutes was observed in the chromatogram of the drug samples extracted from tablets, indicating no interference from the excipients that routinely occur in tablets. The mean % recovery of the drug content was found to be 102.75 ± 0.799 as shown in Table 3. The recovery studies were carried out at 80%, 100%, and 120% of the test concentration. The % recovery of SUL at all three levels was found to be acceptable (Table 3).
| Pharmaceutical formulation | Taken | Found | % Founda, ±%RSD | Standard addition technique | |
|---|---|---|---|---|---|
| Pure added (μg mL−1) | % Foundb | ||||
| a Average of 6 determinations.b Average of 3 determinations. | |||||
| Arcalion Forte® tablets, (B. N. 18255), claimed to contain 400 mg SUL per tablet | 16.00 | 16.44 | 102.75 ± 0.799 | 12.00 | 96.88 |
| 16.00 | 99.65 | ||||
| 20.00 | 99.25 | ||||
| Mean ± %RSD | 98.59 ± 1.497 | ||||
The suggested method compared favorably with the reported spectrophotometric method4 as shown from the values of the calculated Student's t and F-ratio, confirming that there was no significant difference within a probability of 95% between the two methods (Table 4).
| Items | HPLC method | Reported methodb,4 |
|---|---|---|
| a Figures between parenthesis represent the corresponding tabulated values of t and F at P = 0.05.b Spectrophotometric method that depended on measuring the decrease in absorbance of I2 at 348 nm. | ||
| Mean | 100.76 | 99.90 |
| %RSD | 0.997 | 1.404 |
| Variance | 3.683 | 1.968 |
| n | 9 | 7 |
| Student's t-test | 1.411, (2.145)a | |
| F-value | 1.953, (3.581)a | |
4.3. Method validation
 | ||
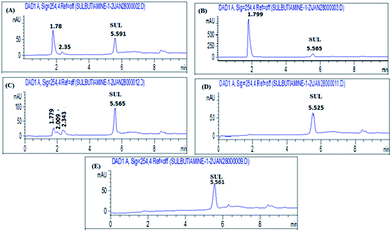
| Fig. 1 HPLC chromatograms of (A) sulbutiamine, and its hydrolytic degradation products by (B) 1 N HCl, (C) 0.1 N NaOH, (D) 0.1 N HCl and (E) water. | ||
| Factor | |
|---|---|
| Robustness (%RSD) | |
| 0.467 | 1-Mobile phase composition (±2% methanol) |
| 1.458 | 2-pH of phosphate buffer (±0.2 pH) |
| Ruggedness (%RSD) | |
| 0.914 | 1-Different methanol manufacturer |
| 1.975 | 2-Two analysts |
4.4. System suitability testing parameters
System suitability testing was carried out during method development and optimization as well as through the validation procedure.18 The resolution (Rs) and selectivity (α) factors were calculated between SUL and the nearest eluted peak and were found to be >1.5 and 2, respectively, in all degradation conditions. Moreover, the symmetry factor was calculated for the basic component and was equal to 1. Other parameters such as capacity factor, number of theoretical plates and height equivalent to theoretical plates were calculated, and their values were within the acceptable limits (Table 6).| Parameters | SUL | Reference value13 | |
|---|---|---|---|
| tR | 5.55 ± 0.03 min | ||
| Peak a symmetry | 1 | <1.5–2 or <2 | |
| K′ (capacity factor) | 2.36 | 1–10 acceptable | |
| N (number of theoretical plates) | 9417.44 | Increases with increasing efficiency of separation | |
| H (in cm) (Height equivalent to theoretical plates) | 2.65 × 10−3 | The smaller the value the higher the column efficiency | |
| Resolution (Rs) | 0.1 N NaOH | 14.25 | R < 2 |
| 0.1 N HCl | 8.67 | ||
| 1 N HCl | 14.25 | ||
| 3% H2O2 | 11.27 | ||
| 30% H2O2 | 14.22 | ||
| Light | 10.77 | ||
| Selectivity (α) | 0.1 N NaOH | 3.04 | α < 1.5 |
| 0.1 N HCl | 4.16 | ||
| 1 N HCl | 3.04 | ||
| 3% H2O2 | 5.70 | ||
| 30% H2O2 | 27.93 | ||
| Light | 5.75 | ||
4.5. Results of forced degradation studies
The results of SUL stability studies are given in Table 7.| Stress conditions | Number of degradates (tR) | Time of degradation (hours) | Degradation% | |
|---|---|---|---|---|
| 0.1 N NaOH at room temperature | 1-(1.83) | 1/2 | 100% | |
| 0.1 HCl at 80 °C | 2-(1.81, 2.55) | 3 | 93.6% | |
| 1HCl at 80 °C | 1-(1.71) | 3 | 100% | |
| H2O at 80 °C | No degradation | 5 | 0% | |
| 3% H2O2 at 80 °C | 2-(1.78, 2.35) | 5 | 38.14% | |
| 30%H2O2 a 80 °C | 1-(1.78) | 5 | 93.13% | |
| Photolysis | On liquid sample | 3-(1.78, 2.01, 2.34) | 3 | 26.8% |
| On solid sample | No degradation | 5 | 0% | |
| Dry heat at 80 °C | No degradation | 3 | 0% | |
The chromatograms (Fig. 1 and 2) verified the stability indicating properties of the proposed method and assessed its ability to resolve the peak of the studied drug from all degradation products.
5. Conclusion
An accurate and reproducible HPLC-DAD method has been developed and validated for determination of SUL in pure form and marketed tablets. It is the first developed stability indicating method, in which all SUL degradation products were completely resolved from the parent drug. The short chromatographic run time of only 10 minutes makes this method suitable for processing many samples in a limited time, which is very important in quality control analysis of any drug.Acknowledgements
The authors would like to express their appreciation and thanks to SIGMA Pharmaceutical Industries S.A.E, Quesna City, Egypt for the provision of the necessary materials to carry out this work.References
- Through website, http://en.wikipedia.org/wiki/Sulbutiamine, accessed 22 February 2014.
- O. Van Reeth, Pharmacologic and Therapeutic Features of Sulbutiamine, Drugs Today, 1999, 35, 187–192 CAS.
- Through website, http://peaknootropics.com/shop/sulbutiamine, accessed 20 February 2014.
- M. E. S. Metwally and Y. El Shabrawy, Kinetic spectrophotometric determination of sulbutiamine in pharmaceutical preparations and in human serum, Anal. Sci., 2000, 16, 633–636 CrossRef CAS.
- P. Gele, C. Boursier-Neyret, M. Lesourd and C. Sauveur, Determination of sulbutiamine and its disulphide derivatives in human plasma by HPLC using online post column reactors and fluorimetric detection, Chromatographia, 1993, 36, 67 Search PubMed.
- I. A. Darwish1, H. F. Askal, A. S. Khedr and R. M. Mahmoud, Stability indicating thin-layer chromatographic method for quantitative determination of ribavirin, J. Chromatogr. Sci., 2008, 46, 3–9 Search PubMed.
- W. Grimm, in Drug Stability, Principles and Practices, ed. J. T. Carstensen and C. T. Rhodes, Marcel Dekker, Inc., New York, 2000 Search PubMed.
- H. Khan, M. Ali, A. Ahuja and J. Ali, Stability Testing of Pharmaceutical Products Comparison of Stability Testing Guidelines, Curr. Pharm. Anal., 2010, 6, 142–150 CrossRef CAS.
- ICH Guideline, Q1F: Stability Data Package for Registration Applications in Climatic Zones III and IV, London, 2003 Search PubMed.
- ICH Q1A (R2) Stability testing of new drug substances and products. proceedings of the International Conference on Harmonization, 2003, http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q1A_R2/Step4/Q1A_R2__Guideline.pdf, accessed 22 February 2014.
- N. V. V. S. S. Raman, K. A. Harikrishna, A. V. S. S. Prasad, K. Ratnakar Reddy and K. Ramakrishna, Development and validation of a stability-indicating RP-LC method for famciclovir, J. Pharm. Biomed. Anal., 2009, 50, 797–802 CrossRef CAS PubMed.
- The United States Pharmacopeia, National Formulary 35, United States Pharmacopeia Convention Inc., 30th edn, 2012 Search PubMed.
- T. K. Ravi, M. Gandhimathi, A. Suganthi and S. Sarovar, Forced-degradation study of valdecoxib as bulk drug and in tablet formulation by HPTLC, J. Sep. Sci., 2006, 29, 1647–1652 CrossRef CAS.
- A. F. Beebee Fauzee and R. B. Walker, Forced degradation studies of clobetasol 17-propionate in methanol, propylene glycol, as bulk drug and cream formulations by RP-HPLC, J. Sep. Sci., 2013, 36, 849–856 CrossRef PubMed.
- A. S. Fayed, M. A. Shehata, N. Y. Hassan and S. A. El-Weshahy, Validated HPLC and HPTLC stability-indicating methods for determination of alfuzosin hydrochloride in bulk powder and pharmaceutical formulations, J. Sep. Sci., 2006, 29, 2716–2724 CrossRef CAS.
- M. Bakshi and S. Singh, Development of validated stability-indicating assay methods—critical review, J. Pharm. Biomed. Anal., 2002, 28, 1011–1040 CrossRef CAS.
- Through website, http://www.velescopharma.com/stability-indicating.asp, accessed 22 February 2014.
- I. Hewalaa, H. El-Fatatreb, E. Emamc and M. Mubroukb, Development and application of a validated stability-indicating HPLC method for simultaneous determination of granisetron hydrochloride, benzyl alcohol and their main degradation products in parenteral dosage forms, Talanta, 2010, 82, 184–195 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2014 |