Comment on “Topography of the free energy landscape of Claisen–Schmidt condensation: solvent and temperature effects on the rate-controlling step” by N. D. Coutinho, H. G. Machado, V. H. Carvalho-Silva and W. A. da Silva, Phys. Chem. Chem. Phys., 2021, 23, 6738
Abstract
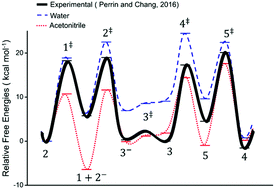
The referenced article in PCCP presents calculations of solvent kinetic isotope effects that indicate that the rate-limiting step in base-catalyzed chalcone formation in aqueous solution becomes the second enolization. This disputes our previous conclusion, based on experimental isotope effects in aqueous acetonitrile, that the rate-limiting step is the final loss of hydroxide and formation of the C–C double bond. That conclusion is here affirmed as general for any protic solvent, and it is further concluded that those calculations are flawed.


 Please wait while we load your content...
Please wait while we load your content...