Topography of the free energy landscape of Claisen–Schmidt condensation: solvent and temperature effects on the rate-controlling step†
Abstract
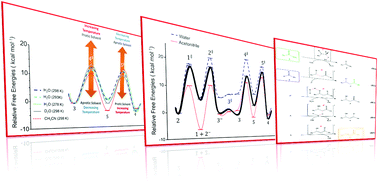
Recent studies have found that hydroxide elimination and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond formation step in base-promoted aldol condensation have a strong influence on the overall rate of the reaction, in contrast to the well-accepted first enolization or C–C bond formation step. Here, applying theoretical models to the prototypical reaction of chalcone formation, the complete free energy profile of Claisen–Schmidt condensation is assessed, revealing how a protic solvent and a slight increase in temperature can induce the second enolization as the rate-controlling step (RCS). It is also observed: i) the nonexistence of a step with a much higher energetic barrier than the others, making the concept of RCS debatable; and ii) that the overall inverse kinetic isotopic effect does not exclude second enolization as a RCS in protic continuum medium. We expect that these results can expand the understanding of the decisive role of physicochemical factors on the choose of the RCS in the aldol condensation.
C bond formation step in base-promoted aldol condensation have a strong influence on the overall rate of the reaction, in contrast to the well-accepted first enolization or C–C bond formation step. Here, applying theoretical models to the prototypical reaction of chalcone formation, the complete free energy profile of Claisen–Schmidt condensation is assessed, revealing how a protic solvent and a slight increase in temperature can induce the second enolization as the rate-controlling step (RCS). It is also observed: i) the nonexistence of a step with a much higher energetic barrier than the others, making the concept of RCS debatable; and ii) that the overall inverse kinetic isotopic effect does not exclude second enolization as a RCS in protic continuum medium. We expect that these results can expand the understanding of the decisive role of physicochemical factors on the choose of the RCS in the aldol condensation.


 Please wait while we load your content...
Please wait while we load your content...