The effect of terminal N-donor aromatic ligands on the sensitization and emission of lanthanide ions in Zn2Ln (Ln = Eu, Tb) complexes with 4-biphenylcarboxylate anions†
Abstract
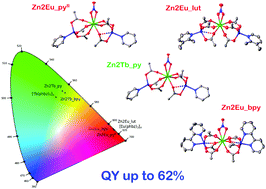
The systematic series of Zn–Ln trinuclear carboxylate molecular complexes of general formula [Zn2Ln(NO3)(phbz)6(L)2] (Ln = Eu, Gd, and Tb, where phbz is the anion of 4-biphenylcarboxylic acid, and L is pyridine, 2,3-lutidine or 2,2′-bipyridine) were synthesized. It was found that [Zn2Eu(NO3)(phbz)6(py)2] can be obtained in two phases, crystallized in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and monoclinic P21/n space groups, and solvated acetonitrile molecules are able to prevent the formation of intermolecular π–π stacking interactions between the aromatic fragments of [Zn2Eu(NO3)(phbz)6(py)2]. Heterometallic Zn2Eu and Zn2Tb compounds possess bright metal-centered luminescence with quantum yields up to 62%. The influence of an N-donor ligand type on the efficiency of luminescence sensitization was studied.
and monoclinic P21/n space groups, and solvated acetonitrile molecules are able to prevent the formation of intermolecular π–π stacking interactions between the aromatic fragments of [Zn2Eu(NO3)(phbz)6(py)2]. Heterometallic Zn2Eu and Zn2Tb compounds possess bright metal-centered luminescence with quantum yields up to 62%. The influence of an N-donor ligand type on the efficiency of luminescence sensitization was studied.


 Please wait while we load your content...
Please wait while we load your content...