Evaluation of cyrhetrenyl and ferrocenyl precursors as 5-lipoxygenase inhibitors – biological and computational studies†
Abstract
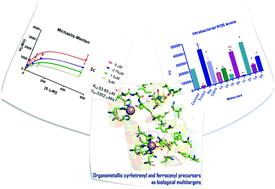
A series of ferrocenyl and cyrhetrenyl complexes [(η5-C5H4R)MLn] (MLn = [(η5-C5H5Fe) (1); MLn = Re(CO)3 (2)) with R = H (1a; 2a), R = aldehyde (1b; 2b), R = carboxylic acid (1c; 2c), and R = amino (1d; 2d) were synthesized. A new synthetic route was reported to obtain 2c, using the Tollens reagent. This route allowed us to obtain cyrhetrenyl carboxylic acid as a pale yellow crystalline solid. The anti-inflammatory activity of all complexes was evaluated through the inhibition of 5-hLOX. Complexes 1c and 2c showed a remarkable inhibitory activity against 5-hLOX compared to its organic analog (89%, 41%, and 20% inhibition, respectively); highlighting the ferrocenyl carboxylic acid (1c) with an IC50 of 2.5 ± 0.2 μM (mixed inhibitor, Ki = 1.0 ± 0.4 μM). Computational studies (docking and molecular dynamics) showed that the carboxylate group of the ferrocenyl complex (1c) could be oriented towards the catalytic site of the enzyme (Eb −10.93 kcal mol−1) or could also be located at the cavity entrance of the active site (Eb −3.18 kcal mol−1). This suggests that 1c competes with the arachidonic acid (AA) substrate, and the possible interaction at another site (a mixed mechanism), which agrees with the experimental results. Additionally, the antioxidant activity of these complexes was evaluated using DPPH-scavenging, FRAP, and ROS assays, where complexes 1b and 1c showed better antioxidant activity than the known antioxidant Trolox, whereas its cyrhetrenyl analog 2c was an excellent ROS generator.


 Please wait while we load your content...
Please wait while we load your content...