Isolation of a C3-metalated indolizine complex and a phosphonium ring-fused bicyclic metallafuran from the osmium-induced transformation of pyridine-tethered alkynes†
Abstract
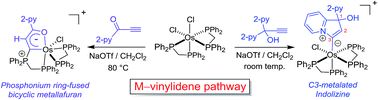
The first C3-metalated indolizine complex was prepared from the reaction between cis-[Os(dppm)2Cl2] (dppm = 1,1-bis(diphenylphosphino)methane) and propargylic pyridine, HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC(OH)(2-py)2. A phosphonium ring-fused bicyclic osmafuran complex was also prepared from the reaction between cis-[Os(dppm)2Cl2] and pyridyl ynone, HC
CC(OH)(2-py)2. A phosphonium ring-fused bicyclic osmafuran complex was also prepared from the reaction between cis-[Os(dppm)2Cl2] and pyridyl ynone, HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C(C
C(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)(2-py). The formation of these two products revealed the intermediacy of metal–vinylidene species regarding [Os(dppm)2Cl]+-mediated alkyne transformations. Comparison of the d6 transition-metal precursors employed to activate HC
O)(2-py). The formation of these two products revealed the intermediacy of metal–vinylidene species regarding [Os(dppm)2Cl]+-mediated alkyne transformations. Comparison of the d6 transition-metal precursors employed to activate HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC(OH)(2-py)2 suggests that precursors with higher π-basicity favor the vinylidene-involving pathway.
CC(OH)(2-py)2 suggests that precursors with higher π-basicity favor the vinylidene-involving pathway.
- This article is part of the themed collection: Mechanistic processes in organometallic chemistry


 Please wait while we load your content...
Please wait while we load your content...