Catalyst design insights from modelling a titanium-catalyzed multicomponent reaction†
Abstract
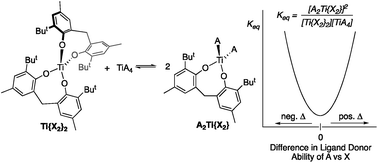
High oxidation state transition metal catalysis touches our daily lives through bulk chemical production, e.g. olefin polymerization, and through specialty chemical reactions common in organic synthesis, e.g. the Sharpless asymmetric epoxidation and olefin dihydroxylation. Our group has been expanding the reaction chemistry of titanium(IV) to produce a host of nitrogen-based heterocycles via multicomponent coupling reactions. One such multicomponent coupling reaction discovered in our laboratory is iminoamination, involving an amine, an alkyne, and an isonitrile. However, the experimental modeling of high oxidation state reactions lags far behind that of low oxidation state systems, where a great deal is known about ligands, their donor properties and how their structures affect catalysis. As a result, we have developed an experimental method for determining the donor abilities of anionic ligands on high oxidation state systems, which is based on the chromium(VI) nitride system NCr(NiPr2)2X, where X = the ligand being interrogated. The parameters obtained are simply called ligand donor parameters (LDP). In this contribution, a detailed optimization of the Ti(NMe2)2(dpm)-catalyzed iminoamination reaction was carried out, where dpm = 5,5-dimethyldipyrrolylmethane. During the course of these studies, dimeric {Ti(μ-N-tolyl)(dpm)}2 was isolated, which is proposed as the resting state of the catalyst. To destabilize this resting state, a more electron-rich bis(aryloxide) catalyst system was investigated. The more electron-rich system is somewhat more active for iminoamination under some conditions; however, the catalyst is prone to disproportionation. A study of heteroleptic titanium complexes revealed that the disproportionation equilibrium constant can be effectively modeled as a function of the square of the difference in LDP between the ligands, (ΔLDP)2. Using this methodology, one can estimate the stability of titanium complexes toward disproportionation.
- This article is part of the themed collection: Mechanistic processes in organometallic chemistry


 Please wait while we load your content...
Please wait while we load your content...