Reply to the ‘Comments on “Experimental and theoretical studies of the nanostructured {Fe3O4@SiO2@(CH2)3Im}C(CN)3 catalyst for 2-amino-3-cyanopyridine preparation via an anomeric based oxidation”, RSC Adv., 2016, 6, 50100–50111, and “The first computational study for the oxidative aromatization of pyrazolines and 1,4-dihydropyridines using 1,2,4-triazolinediones: an anomeric-based oxidation”, RSC Adv., 2016, 6, 102280–102291’ by S. Salehzadeh, RSC Adv., 2017, 7, 39704–39707, DOI: 10.1039/c6ra27033f†
Abstract
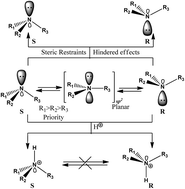
This response to Dr Salehzadeh’s comments on the papers mentioned in the title contains the comments where they have mentioned disagreement with basic chemistry concepts. The response to the comments include: (i) the experimental (X-ray) and theoretical reported results of the epimerism and the differences in the stereoisomer properties, as they are definitely not pair of enantiomers, and (ii) a discussion regarding internal molecular orbital (MO) electron transfer.


 Please wait while we load your content...
Please wait while we load your content...