Comments on “Experimental and theoretical studies of the nanostructured {Fe3O4@SiO2@(CH2)3Im}C(CN)3 catalyst for 2-amino-3-cyanopyridine preparation via an anomeric based oxidation”, RSC Adv., 2016, 6, 50100–50111, and “The first computational study for the oxidative aromatization of pyrazolines and 1,4-dihydropyridines using 1,2,4-triazolinediones: an anomeric-based oxidation”, RSC Adv., 2016, 6, 102280–102291
Abstract
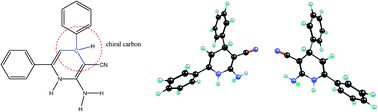
The papers mentioned in the title of the present comment article contain some calculations/results that are in disagreement with some basic concepts of chemistry. The calculations include (i) the calculation of an energy difference, more than 1000 J mol−1, between one pair of enantiomers, (ii) the calculation of an energy difference between the Lewis structures (resonance forms), and (iii) the calculation of bond orders in different pathways of resonance process.


 Please wait while we load your content...
Please wait while we load your content...