Magnetostructural correlation in isolated trinuclear iron(iii) oxo acetate complexes†
Abstract
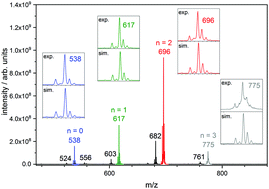
We elucidate the correlation between geometric structures and magnetic couplings in trinuclear iron(III) oxo acetate complexes [Fe3O(OAc)6(Py)n]+ (n = 0, 1, 2, 3) when isolated and trapped as gaseous ions. Structural information arises from Infra Red-Multiple Photon Dissociation (IR-MPD) and Collision Induced Dissociation (CID) experiments in conjuction with Density Functional Theory (DFT) based calculations. We simulate the antiferromagnetic couplings between the FeIII (d5) centers by employing a Broken Symmetry approach within our DFT calculations, and we extract the associated antiferromagnetic coupling constants. Coordination of one, two or three axial pyridine ligands to the [Fe3O(OAc)6]+ subunit distorts the geometry of the triangular Fe3O core. The Fe–Ocentral bond lengths are enlarged or shortened depending on number of coordinated pyridine ligands. This significantly affects the antiferromagnetic coupling constants between the FeIII centers ranging from −62 cm−1 to −28 cm−1 in [Fe3O(OAc)6(Py)n]+ (n = 0, 1, 2, 3). A detailed analysis of the associated exchange couplings indicates a switching of magnetic ground states by pyridine coordination. The total spin ST in the ground states of [Fe3O(OAc)6(Py)n]+ raises from ST = 1/2 (n = 0) to 3/2 (n = 1) and 5/2 (n = 2). Coordination of the third pyridine ligand (n = 3) re-establishes a spin ground state of ST = 1/2. We thus identify a coordination controlled switching of magnetic ground states.


 Please wait while we load your content...
Please wait while we load your content...