Bi-functional Li2B12H12 for energy storage and conversion applications: solid-state electrolyte and luminescent down-conversion dye†
Abstract
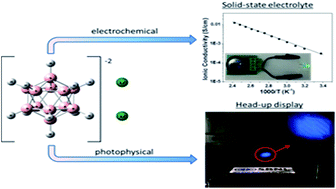
Our investigation of the chemical and physical properties of the alkali-metal dodecahydro-closo-dodecaborate, Li2B12H12, determined that it is a bi-functional material that can be used as a solid state electrolyte in lithium ion batteries and as a luminescent down conversion dye in scalable transparent displays. A series of electrochemical measurements of morphologically altered samples, via mechanical milling, was conducted. The measurements indicated that mechanical alternations of the Li2B12H12 morphology makes it an excellent lithium ion conductor in the solid state with exceptional ionic conductivity at room temperature (0.31 mS cm−1) and is compatible with a metallic lithium electrode up to 6.0 V. In addition, all solid state half and full electrochemical cells were assembled and successfully cycled using Li2B12H12 as a solid state electrolyte at temperatures as low as 30 °C with good capacity retention. The photophysical properties of Li2B12H12 were also investigated. Li2B12H12 has an emission maximum of ∼460 nm in a variety of solvents with Stokes' shifts up to 175 nm observed. Li2B12H12 was incorporated in a polyvinyl alcohol (PVA) thin film to demonstrate its application as a luminescent down-conversion dye in a transparent head-up display when excited by a UV projection source.

 Please wait while we load your content...
Please wait while we load your content...