Preparation of Mo nanopowders through electroreduction of solid MoS2 in molten KCl–NaCl
Abstract
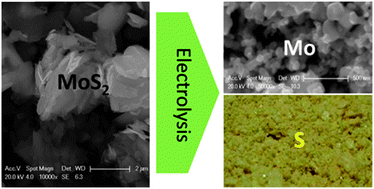
Electrolysis of MoS2 to produce Mo nanopowders and elemental sulfur has been studied in an equimolar mixture of NaCl and KCl at 700 °C. The reduction mechanism was investigated by cyclic voltammetry (CV), potentiostatic and constant voltage electrolysis together with spectroscopic and scanning electron microscopic analyses. The reduction pathway was identified to be MoS2 → LxMoS2 (x ≤ 1, L = Na or K) → L3Mo6S8 and LMo3S3 → Mo, and the last step to format metallic Mo was found to be relatively slow in kinetics. Electrolysis at a cell voltage of 2.7 V has led to a rapid reduction of MoS2 to nodular Mo nanoparticles (50–100 nm), with the current efficiency and energy consumption being about 92% and 2.07 kW h kg−1-Mo, respectively.


 Please wait while we load your content...
Please wait while we load your content...