Galvanically replaced Au–Pd nanostructures: study of their enhanced elemental mercury sorption capacity over gold†
Abstract
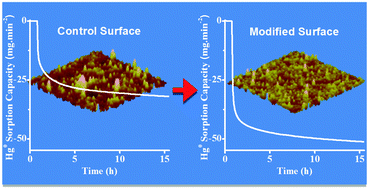
In this study, the efficiency of bimetallic (Au–Pd) nanostructures over Au and Pd substrates for elemental mercury (Hg0) vapor sensing and capturing was investigated. The quartz crystal microbalance (QCM) technique was utilized to determine the sorption kinetics and quantity of Hg0 captured by the developed Au–Pd surfaces. The Au–Pd nanostructures were synthesized directly on the QCM's Pd electrodes using galvanic replacement (GR) reactions for periods of 0.5 to 48 hours, which enabled the ratio of Au to Pd on the surface to be controlled. It was observed that the mercury affinity of the surface does not increase with increased Au loading, rather the Au : Pd ratio obtained after a GR reaction time of 1 hour was found to have the highest affinity towards Hg0 vapor under the GR reaction conditions used in this study. Any further increase in Au : Pd ratio at the surface resulted in reduced affinity for Hg0 with the Au-rich Au–Pd nanostructures behaving similar to an Au-control substrate. However, short reaction periods (i.e. 1 h) produced small Au nanoparticles increasing the surface to volume ratio for better sensitivity and response times. Remarkably, the QCM data showed that GR based Au–Pd nanostructures removed 2.5 μg cm−2 of Hg0 from a gas stream containing 9.1 mg m−3 of Hg0 vapor within the first 3 minutes of exposure. The control surfaces (Pd and Au based thin-films) on the other hand took a total of 106 and 159 minutes, respectively to reach the same Hg0 sorption capacity from the same gas stream.

 Please wait while we load your content...
Please wait while we load your content...