Nanochitin for sustainable and advanced manufacturing
Pei Lin
Chee
ab,
Thenapakiam
Sathasivam
b,
Ying Chuan
Tan
 b,
Wenya
Wu
b,
Wenya
Wu
 a,
Yihao
Leow
a,
Quentin Ray Tjieh
Lim
ac,
Pek Yin Michelle
Yew
a,
Yihao
Leow
a,
Quentin Ray Tjieh
Lim
ac,
Pek Yin Michelle
Yew
 ab,
Qiang
Zhu
ab,
Qiang
Zhu
 ad and
Dan
Kai
ad and
Dan
Kai
 *abd
*abd
aInstitute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), 2 Fusionopolis Way, Innovis #08-03, 138634, Singapore. E-mail: kaid@imre.a-star.edu.sg
bInstitute of Sustainability for Chemicals, Energy and Environment (ISCE2), Agency for Science, Technology and Research (A*STAR), 2 Fusionopolis Way, Innovis #08-03, 138634, Singapore
cDepartment of Materials Science and Engineering, National University of Singapore, 9 Engineering Drive 1, 117576, Singapore
dSchool of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, 62 Nanyang Dr, Singapore 637459
First published on 24th January 2024
Abstract
Presently, the rapid depletion of resources and drastic climate change highlight the importance of sustainable development. In this case, nanochitin derived from chitin, the second most abundant renewable polymer in the world, possesses numerous advantages, including toughness, easy processability and biodegradability. Furthermore, it exhibits better dispersibility in various solvents and higher reactivity than chitin owing to its increased surface area to volume ratio. Additionally, it is the only natural polysaccharide that contains nitrogen. Therefore, it is valuable to further develop this innovative technology. This review summarizes the recent developments in nanochitin and specifically identifies sustainable strategies for its preparation. Additionally, the different biomass sources that can be exploited for the extraction of nanochitin are highlighted. More importantly, the life cycle assessment of nanochitin preparation is discussed, followed by its applications in advanced manufacturing and perspectives on the valorization of chitin waste.
1 Introduction
The generation of 6–8 million tons of crustacean waste annually1 presents an issue of inefficient resource utilization where instead of being exploited to produce value-added products, these valuable resources are discarded, contributing to the problem of waste accumulation. This increasing accumulation of waste does not conform to the expected trend of sustainability. Thus, to achieve the goals of sustainability and circular economy and considering the advantages of chitin, research efforts have been devoted to its valorization.The discovery of chitin dates back to 1811. Subsequently, after more than a century of investigation and exploration, various approaches have been developed to extract/process chitin on different scales, and thus it can be employed in various applications (Fig. 1a). As a linear polysaccharide composed of β(1 → 4)-linked 2-acetoamido-2-deoxy-β-D-glucose units, chitin is the second most abundant renewable biopolymer in the world after cellulose. Moreover, chitin can be further classified as α, β and γ depending on the alignment of its molecular chains.2 In α-chitin, molecular chains are arranged in an antiparallel fashion, whereas β-chitin has parallel molecular chains.2 Alternatively, γ-chitin is made up of both parallel and antiparallel molecular chains. Among them, α-chitin is the most stable form and it exists as the backbone of the exoskeleton of crustaceans and insects and in the cell walls of microorganisms.2 In contrast, β-chitin can be found in squid pen, tubeworms, and cuttlefish bone,2 while γ-chitin is found in some cocoons and mushrooms.3 Thus, chitin has attracted interest from researchers not just because it is abundant and affordable, but also because it can impart toughness and resistance to materials.1 Furthermore, by adjusting its concentration, the toughness and resistance of the corresponding materials can be varied and closely regulated to achieve the intended purpose. Furthermore, chitin has many other attractive benefits such as biodegradability, low allergenicity, biocompatibility and easy processability.1
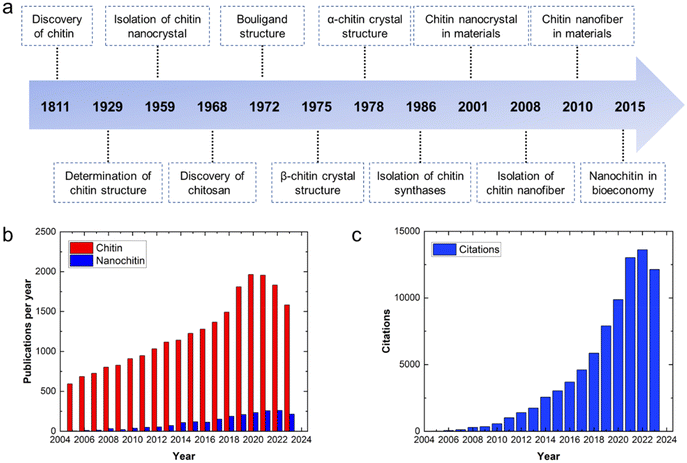 | ||
| Fig. 1 (a) Timeline of chitin and nanochitin from their discovery to analysis and applications. Adapted with permission.1 Copyright 2022, the American Chemical Society. (b) Annual publications on chitin and nanochitin from 2005 to 2023. (c) Annual citations on nanochitin from 2005 to 2023. The data was obtained from the Web of Science, 14th December 2023. | ||
Nanochitin is structurally made up of a bundle of semicrystalline chitin nanofibrils that are held together by van der Waals forces and hydrogen bonding.1 It usually exists together with protein and minerals. Hence, deproteinization and demineralization are necessary to isolate nanochitin. To date, researchers have explored various forms of nanochitin, such as nanocrystals and nanofibers.4 By breaking down chitin to nanoscale dimensions, the reactivity of the chitin can be preserved, while its solubility/dispersibility can be improved,1,5 thereby extending its applications. Nanochitin is an attractive polymer given that it is the only natural polysaccharide that contains nitrogen, which can serve as a natural nitrogen source to produce nitrogen-containing chemicals, e.g. compounds used in pharmaceuticals.6 Also, as an elemental building block of chitin, nanochitin possesses the advantages of chitin such as toughness, easy processability and biodegradable. Additionally, the presence of acetamide groups on the nanochitin endows it with antimicrobial activities, non-toxicity and wound-healing ability.3 However, although there are an increasing number of publications on nanochitin annually, it research still lags behind its parent chitin (Fig. 1b). The increasing attention on nanochitin is illustrated by its increasing number of citations, as shown in Fig. 1c.
This review aims to provide readers with knowledge on the various methods to synthesize nanochitin. Generally, these methods are categorized as chemical and mechanical treatments. In contrast, we summarize the recent approaches in a new light by further grouping them into traditional and sustainable approaches to aid researchers to push the frontier of sustainable research. Furthermore, the different sources of biomass that can be exploited for the extraction of chitin are highlighted. Although the valorization of crustacean waste, specifically chitin, into value-added products is desirable to achieve a circular economy, its environmental impact from cradle to grave needs to be determined, which is attempted herein by life cycle assessments to present the true benefits of its valorization. Finally, this review is concluded with the potential range of applications of nanochitin in such as in the field of 3D printing, photonics, packaging and catalysis, followed by a perspective on the sustainable use of nanochitin.
2 Biomass sources for chitin
Chitin is traditionally sourced from the seafood industry, where the exoskeletons of crustaceans such as shrimps, crabs and crawfish are discarded as waste.7 Alternatively, to diversify the future sources of chitin, other forms of supplies are also studied. In general, chitin exists in three types of polymorphs (Fig. 2), where α-chitin is the main isomorph present in the exoskeletons of crustaceans and molluscs, β-chitin is found in squid pens, while γ-chitin exists in the cocoon fibers of Ptinus beetles.4 These polymorphs differ in the stacking arrangement of the polymeric chitin chains. Due to this difference, the degree of H-bonding interactions from the amide functional groups between the polymeric chains differ, and thus exhibit different properties. In particular, α-chitin and β-chitin contain polymeric chitin chains that are stacked in anti-parallel and parallel configurations, respectively. Alternatively, γ-chitin consists of both anti-parallel and parallel arrangements of chitin chains. Generally, chitin has been extracted and studied from three groups of sources, i.e., aquatic invertebrates, insects, and fungi.8 Generally, the extraction process involves demineralization (acid treatment), deproteinization (alkaline treatment) and decolorization steps. Furthermore, all these treatments need to be optimized according to the chitin source due to the differences in physicochemical properties.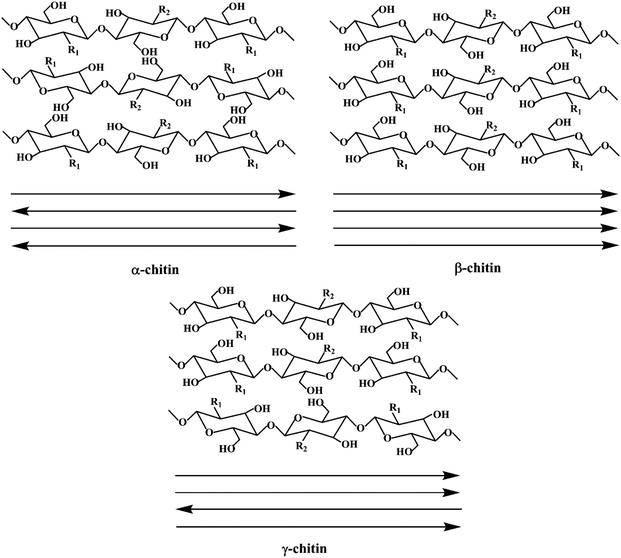 | ||
| Fig. 2 Schematic illustration of the three types of chitin polymorphs. Reproduced with permission.9 Copyright 2020, John Wiley and Sons. | ||
2.1 Aquatic invertebrates
Aquatic invertebrates, such as crustaceans, molluscs and cephalopods, represent an important food source globally. In particular, the exoskeletons of crustaceans, which are also major waste products in the seafood industry, have been extensively studied as a source of chitin.10 Presently, they are the primary sources of commercially produced chitin.7 More specifically, shrimp shells are more commonly used as the source of chitin due to their thinner exoskeleton, making the extraction of chitin easier than other forms of shells. Generally, crustacean shells are harder and more brittle than the other chitin sources due to the well-connected network among chitin, minerals and proteins. Therefore, the chitin extraction process is harsher, which requires crushing and concentrated acids for adequate demineralization. Alternatively, milder chemical or enzymatic treatments have also been proposed, although this approach is still not widely adopted in industry. Generally, crustacean shells are comprised of chitin (20–30%), proteins (30–40%), inorganic minerals (30–60%), and lipids (0–14%).11 Furthermore, these compositions vary significantly across species and seasons.Rajeevgandhi and team studied the extraction of chitin from various crustacean shells, i.e. crab, lobster, shrimp and squilla.12 Utilizing the traditional chemical treatments for chitin extraction, the chitin yields from the four types of crustaceans were 21.25%, 17.50%, 20.00% and 23.75%, respectively. As presented in Fig. 3a, the surface morphologies of the extracted chitin differed among the sources. The shrimp and crab chitins exhibited porous structures at low magnifications and nanofibrous structures at high magnifications. The squilla chitin showed the opposite characteristics, while lobster chitin mainly displayed nanofibrous structures. Considering that the surface structures can affect the potential functionalities of nanochitin, it is important to choose the appropriate chitin source. The authors also found that the chitin obtained from this work has a low molecular weight, which is classified as below 50 kDa. In general, chitin with low molecular weight is desirable for chemo-drug delivery applications.
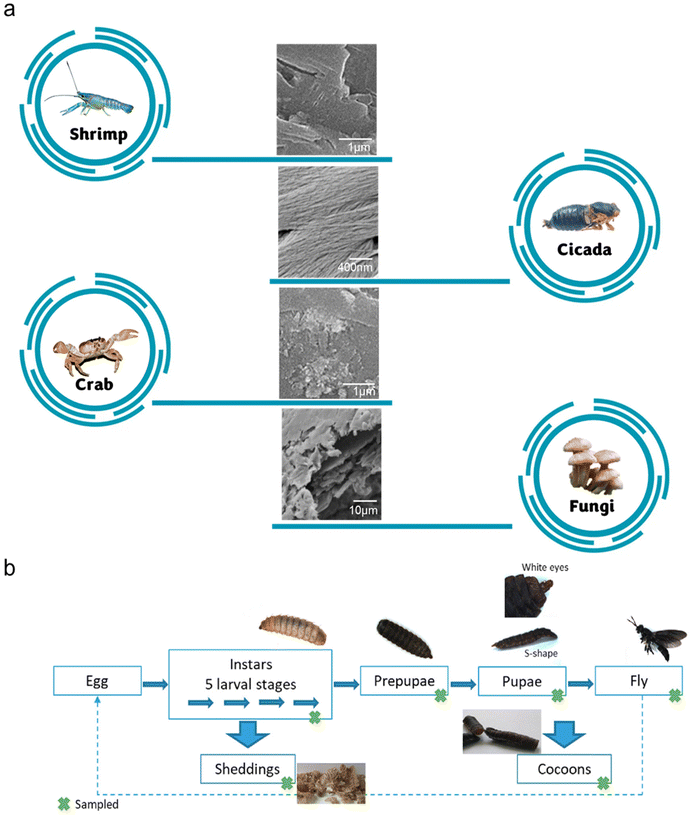 | ||
| Fig. 3 (a) SEM images of chitin from various sources. Adapted with permission.12 Copyright 2021, Elsevier Ltd.16 Copyright 2016, Elsevier Ltd.17 Copyright 2023, Elsevier B.V. (b) Investigation of chitin extraction from various life stages of BSF. Reproduced with permission.15 Copyright 2020, Elsevier Ltd. | ||
2.2 Insects
There has been an increasing number of industrial farms that produce insects as a new source of protein for feed and food.13 Thus, considering that insects contain chitin, insect farms can be a potential continuous source of chitin. Furthermore, the exoskeletons of insects contain less inorganic minerals than the shells of crustaceans, and thus the yield of chitin can be potentially higher, while the chitin extraction process milder.Huet and colleagues investigated the extraction of chitin from Bombyx eri larvae.14 The authors highlighted that the chitin contents in the cuticles of the larvae and the shells of shrimps were 45% and 19%, respectively. Furthermore, the mineral content in the studied insect chitin was significantly lower than that in shrimp chitin (1.9% vs. 21.7%). Therefore, it is theoretically possible to achieve a higher chitin yield and milder extraction process from this insect source. The authors demonstrated this by obtaining a chitin yield of 31.1% with a purity of 89.9% via a single-step extraction process without demineralization. With the same extraction process applied to shrimp shells, the chitin yield was 17.1% with a purity of less than 65.0%.
Black soldier flies (BSFs), Hermetia illucens, are one of the most studied insects as feed. This is largely due to the ability of this species to thrive on various organic streams, especially manure and food wastes. In addition, the byproducts formed at various lifecycle stages of the insects contain chitin. Soetemans et al. studied the extracted chitin at different stages of the lifecycle of BSFs, namely, the larvae, sheddings, prepupae, pupae, cocoons and flies (Fig. 3b).15 All these chitin samples were found to be mainly composed of α-chitin, similar to shrimp chitin. Among them, the sheddings were the most difficult to purify (75.7%) even though they exhibited one of the highest chitin contents. This can be attributed to their higher mineral content, which requires a harsher extraction process. Alternatively, the cocoons of BSFs were identified to possess a high chitin content of 24% and the extracted chitin exhibited a crystallinity index and purity of 94% and 97%, respectively. Nonetheless, the authors concluded that the various chitin samples generally had minor differences in their physicochemical properties, and thus it is still possible to perform a convenient homogeneous chitin extraction process with all chitin-containing byproducts simultaneously.
2.3 Fungi
Chitin is also present as the major polymeric component in the cell wall of certain groups of fungi, e.g., Ascomycetes, Basidiomycetes, Deuteromycetes, and Zygomycetes.8 In fact, chitin was first isolated from a fungus source by Braconnot in the early 1800s. Although fungi generally contain a lower chitin content compared to crustaceans (10–26%), fungi chitin is attracting increasing academic and commercial attention. This is because fungal-based chitin is advantageous compared to animal-based chitin due to its absence of inorganic minerals, more uniform composition, abundant non-seasonal availability and vegan source.18 However, the chitin in fungi exists as a component of a network with other polysaccharides, e.g., cellulose, mannan, and glucan, thus complicating the chitin extraction process. Specifically, chitin and β-glucan are connected via covalent bonds. Therefore, a chitin-β-glucan complex is commonly obtained after performing the alkaline treatment on fungi sources (without demineralization step). Thus, to obtain pure chitin from this complex, acid treatments are required to selectively degrade the glucan components.19Due to the species-richness of fungi, it can be expected that the properties of the extracted chitin will vary considerably. Vetter investigated the chitin content of various cultivated edible mushrooms (Agaricus bisporus, Pleurotus ostreatus and Lentinula edodes).20 Among them, the saprotrophic A. bisporus contained the highest chitin composition of 6.7–8.8% (other two: 2.2–8.1%). Bamba et al. showed that α-chitin nanofibrils from the microalgae Phaeocystis globosa had comparable tensile strength as that extracted from squid pens and tubeworms.21 Considering the large amount of spent biomass produced during fermentation, such as the production of citric acid, studies have also been conducted to investigate the chitin content of Aspergillus niger.22 The yield of the extracted chitin-β-glucan complex was 44% with a good chitosan content of more than 32% (Table 1).
| Source | Chitin yield (%) | Properties | Ref. |
|---|---|---|---|
| Crab (shells) | 21.3 | Porous, nanofibrous | 12 |
| Lobster (shells) | 17.5 | Nanofibrous | 12 |
| Shrimp (shells) | 20.0 | Porous, nanofibrous | 12 |
| Squilla (shells) | 23.8 | Nanoporous, fibrous | 12 |
| Bombyx eri larvae (cuticles) | 31.1 | No demineralization required | 14 |
| Black soldier flies (cocoons) | 24.0 | High crystallinity and purity | 15 |
| Mushroom A. bisporus | 6.7–8.8 | Higher chitin level in the pileus (cap) | 20 |
| Microalgae Phaeocystis globosa | — | Nanofibrils with high tensile strength | 21 |
| Aspergillus niger | 44.0% | Exists as chitin-β-glucan complex | 22 |
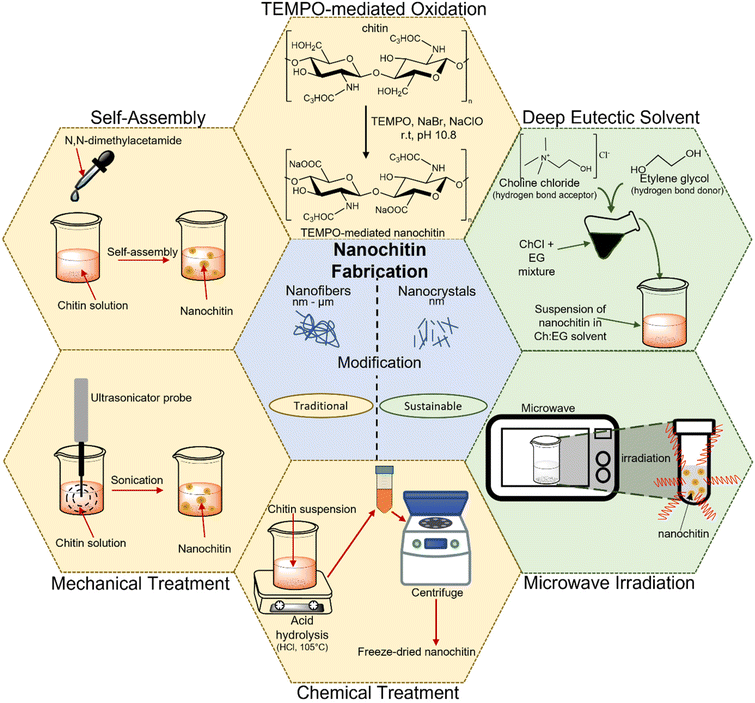
3 Nanochitin fabrication
The preparation of nanochitin usually involves two main steps. It starts with the purification step of chitin, which primarily involves (i) deproteinization using alkali or enzyme hydrolysis, (ii) demineralization achieved by using acid and (iii) removal of lipids and pigment via bleaching treatment. Then, the purified chitin can be attained in either the dry or wet state for further processing.23,24 The subsequent step to attain nanochitin (i.e., nanocrystals and nanofibers) is comprised of the microfabrication of the purified chitin and the various methods to achieve it can be categorized into either the traditional approach or sustainable approach. Fig. 4 illustrates the various methods for the preparation of nanochitin.3.1 Traditional approach
The use of chemicals, mechanical disintegration, electrospinning, wet spinning and self-assembly methods to obtain nanochitin are considered the traditional approaches. These are either the earliest methods developed for the extraction of nanochitin or the methods that emphasize efficiency and yield. These techniques can be further classified into top-down (i.e., chemical treatment, mechanical treatment, and TEMPO-mediated oxidation) and bottom-up approaches (i.e., electrospinning, wet spinning, and self-assembly method). In the former, the synthesis of nanoparticles is performed by breaking down the bulk material, whereas in the latter, they are created from small building blocks.For instance, Li and colleagues effectively isolated the crystalline region of nanochitin from shrimp chitin powder via HCl hydrolysis and obtained rod-like nanoparticles with a length of 50–150 nm and width of 30–50 nm.36 Similarly, Zhou et al. also extracted nanochitin from shrimp chitin powder and obtained a slender rod with sharp points with a broad distribution of 100–150 nm in length and 15–30 nm in width.37 Qin and team replaced HCl with H2SO4 to produce nanochitin and attained size in the range of 100 to 400 nm in length and 10 to 50 nm in width.38 This procedure enables sulphate half-ester functionalities to be present at the nanochitin surface. It is undeniable that acid hydrolysis is effective in generating nanochitin. However, the handling of corrosive chemicals and the generation of wasteful effluents are undesirable and detrimental with respect to sustainable development.
The grinding process involves breaking down the hierarchical structure by using shearing forces produced by two countersense rotating grinding stones.42 For the chitin to be effectively fibrillated during the grinding process, an acidic environment is required. This is because a small percentage of amino groups in chitin becomes cationized when acid is added, which aids the fibrillation of chitin through electrostatic repulsion.43 A study was conducted to compare the chitin nanofibers obtained from a 1% slurry of crab shell chitin that was passed through a grinder with and without acetic acid.44 It was found that due to the strong hydrogen bonding between the chitin networks, it is challenging to fibrillate chitin without the presence of acid, leading to the formation of large, dense bundles of chitin nanofibers. Although an acidic environment is necessary for the grinding process to create chitin nanofibers, this condition may not be ideal for the production of certain nanocomposites, electrical devices, and biological materials. Hence, Ifuku and team attempted to produce chitin nanofibers via grinding under neutral condition, and they succeeded in extracting chitin nanofibers from prawn shell chitin with a consistent diameter of around 10–20 nm.43
Ultrasonication is another popular technique used for producing nanostructured materials, where the high energy generated induces the formation, growth, and rapid collapse of cavities in water.45 Typically, cavitation produces energy in the range of 10 to 100 kJ mol−1, which is equivalent to the energy level of hydrogen bonds.46 Therefore, the formation of chitin nanoparticles via the ultrasonication process is due to the rupturing of the strong hydrogen bonds between the network of chitin. Lu and colleagues extracted chitin nanofibers from dried prawn shell chitin via the high-intensity ultrasonication method at an optimal parameter of 60 kHz, 300 W and pH 7.47 The extent of the breakage of the weak van der Waals forces and hydrogen bonding in the fibers was found to vary with the ultrasonication duration, which allowed the diameter of the chitin nanofibers to be controlled in the range of 20 to 200 nm. The results showed that after 30 min of sonication, high-aspect-ratio nanofibers were obtained with a consistent width of 19.4 nm.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups in the presence of the TEMPO/co-oxidation system, producing chitin nanofibers. Fan and colleagues developed a method utilizing TEMPO-mediated oxidation to selectively oxidize the amorphous region of chitin through the radical oxidation pathway.48 Basically, this technique involves several steps, as follows: (i) chitin is suspended in water containing TEMPO and sodium bromide (NaBr) before sodium hypochlorite (NaClO) solution is added to initiate the TEMPO-mediated oxidation of chitin. (ii) Sodium hydroxide (NaOH) is continuously added to maintain the pH at 10 at room temperature. (iii) To stop the oxidation process, ethanol is added to the solution without using any alkali.49 In a study, β-chitin isolated from tubeworms was subjected to TEMPO/NaClO/NaBr oxidation at pH 10 to produce chitin nanofibers with a width in the range of 20–50 nm and length of over few microns.50 It was found that increasing the amount of NaClO caused the length of the chitin nanofibrils to become shorter. Most of the β-chitin was oxidized to carboxyl groups and transformed into water-soluble sodium chitin salt when an adequate amount of NaClO was added. A similar observation was noted in another independent study, in which higher NaClO concentrations resulted in shorter chitin nanofibrils, which was potentially caused by the intensification of the depolymerization reaction at the ends of the chitin nanofibrils.
O groups in the presence of the TEMPO/co-oxidation system, producing chitin nanofibers. Fan and colleagues developed a method utilizing TEMPO-mediated oxidation to selectively oxidize the amorphous region of chitin through the radical oxidation pathway.48 Basically, this technique involves several steps, as follows: (i) chitin is suspended in water containing TEMPO and sodium bromide (NaBr) before sodium hypochlorite (NaClO) solution is added to initiate the TEMPO-mediated oxidation of chitin. (ii) Sodium hydroxide (NaOH) is continuously added to maintain the pH at 10 at room temperature. (iii) To stop the oxidation process, ethanol is added to the solution without using any alkali.49 In a study, β-chitin isolated from tubeworms was subjected to TEMPO/NaClO/NaBr oxidation at pH 10 to produce chitin nanofibers with a width in the range of 20–50 nm and length of over few microns.50 It was found that increasing the amount of NaClO caused the length of the chitin nanofibrils to become shorter. Most of the β-chitin was oxidized to carboxyl groups and transformed into water-soluble sodium chitin salt when an adequate amount of NaClO was added. A similar observation was noted in another independent study, in which higher NaClO concentrations resulted in shorter chitin nanofibrils, which was potentially caused by the intensification of the depolymerization reaction at the ends of the chitin nanofibrils.
Chitin can only be oxidized by the TEMPO/NaClO/NaBr system in an alkaline environment. Alternatively, for the first time, Pang and team successfully produced chitin nanofibers with a width of 5–10 nm and length of 200–400 nm by employing the TEMPO/NaClO2/NaClO system in a mildly acidic environment.51 They suspended chitin in a pH 6.86 sodium phosphate buffer solution containing TEMPO, sodium chlorite (NaClO2) and NaClO to produce chitin nanofibers via oxidation reaction. A similar TEMPO technique with acidic conditions was used by Jiang et al. to create chitin nanocrystals with dimensions of 200–600 nm in length and 6–15 nm in width.52
3.2 Sustainable approach
The increasing occurrence of extreme weather, global warming and rising water levels has attracted significant attention, and consequently it is necessary to find sustainable means consider the consequences of unsustainable development. In this case, nanotechnology is employed by researchers to improve and achieve cleaner and more environmentally friendly processes. To date, different approaches for the synthesis of nanochitin have been attempted, ranging from employing more environmentally friendly processes and using greener solvents to accelerating the reaction to save energy, which will be discussed in more detail in this section.Sharma and team were the first to report that by using conventional heating, microwave heating, and ultrasound-assisted heating, α-chitin could be dissolved in DESs in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mole ratio for a mixture of choline chloride
2 mole ratio for a mixture of choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thiourea, choline chloride
thiourea, choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea, chlorocholine chloride
urea, chlorocholine chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea, choline bromide
urea, choline bromide![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea and a mole ratio of 1
urea and a mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 for betaine hydrochloride
4 for betaine hydrochloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea.79 One year later, Mukesh and colleagues used the same DESs mole ratio mixture as Sharma et al.79 for choline chloride
urea.79 One year later, Mukesh and colleagues used the same DESs mole ratio mixture as Sharma et al.79 for choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thiourea and produced chitin nanofibers with a width of 25–45 nm and a length of 162–450 nm.80 Similarly, chitin nanocrystals were created with a diameter in the range of 42 to 49 nm and a length of 257 to 670 nm by utilizing choline chloride and various acids such as lactic acid, oxalic acid, citric acid, malonic acid, and DL-malic acid in a ultrasound-assisted procedure (Fig. 5).81 In a different investigation, a green, non-volatile solvent of DES containing choline chloride
thiourea and produced chitin nanofibers with a width of 25–45 nm and a length of 162–450 nm.80 Similarly, chitin nanocrystals were created with a diameter in the range of 42 to 49 nm and a length of 257 to 670 nm by utilizing choline chloride and various acids such as lactic acid, oxalic acid, citric acid, malonic acid, and DL-malic acid in a ultrasound-assisted procedure (Fig. 5).81 In a different investigation, a green, non-volatile solvent of DES containing choline chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zinc chloride with a mole ratio of 1
zinc chloride with a mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was employed to produce acetylated and esterified chitin nanocrystals in a one-step synthesis.82 Chitin nanocrystals with high yield (≈88.5%) were produced from a betaine chloride
2 was employed to produce acetylated and esterified chitin nanocrystals in a one-step synthesis.82 Chitin nanocrystals with high yield (≈88.5%) were produced from a betaine chloride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ferric chloride hexahydrate mixture with a molar ratio of 1
ferric chloride hexahydrate mixture with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.83
1.83
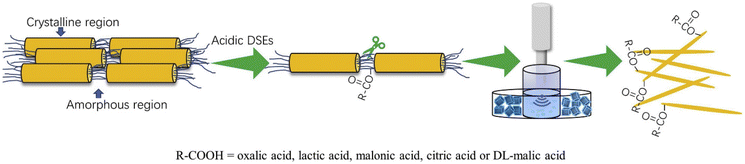 | ||
| Fig. 5 Illustration of nanochitin synthesis using organic acid DESs. Reproduced with permission.81 Copyright 2020, Elsevier Ltd. | ||
| Type of nano chitin | Typical source | Formation process | Characteristics | Application | Ref. | ||
|---|---|---|---|---|---|---|---|
| Length/nm | Width/nm | Diameter/nm | |||||
| Nanocrystals | Shrimp | Acid hydrolysis, ultrasound | 300 | 60 | — | Films for food coating and packaging | 86 |
| Nanofibrils | Crab | Acid hydrolysis, homogenization, ultrasound | 150–200 | 5–10 | — | Antimicrobial film for packaging | 87 |
| Nanocrystals | Cuttlefish bone | Acid hydrolysis, ultrasound | 22 | 14 | — | — | 88 |
| Nano whiskers | Rifia tubes | Acid hydrolysis, ultrasound | 500–104 | 18 | — | Nanocomposite films | 31 |
| Nanofibrils | Tubeworm, pens of squid | TEMPO-mediated oxidation | Several microns | 20–50 | — | Translucent gel | 50 |
| Nano whiskers | Crab shells | TEMPO-mediated oxidation, ultrasound | 150–500 | 20–55 | For fuel cell applications | 89 | |
| Nanofibers | Chitin powder | Electrospinning method | — | — | 163 | For wound healing and regeneration of oral mucosa and skin. | 90 |
| Nanofibers | Chitin powder | Electrospinning, 60Co gamma ray, deacetylation | — | — | 40–640 | Nanofibrous matrices | 60 |
| Nanofibers | Crab shell | Ultrasound | 650 | 9–120 | — | Textile industry for antibacterial finishing application | 91 |
| Nanofibers | Crab shell | Grinding | — | 10–20 | — | Nanomaterials | 44 |
| Nanofibers | Chitin powder | Grinding, HPWJ treatment | — | — | — | Nanofibrous matrices | 40 |
| Nanocrystals | Crab shell | TEMPO-mediated oxidation, partial deacetylation, starburst | 250 | 15 | — | Zwitterionic nanocrystals for biomedical field | 49 |
| Nanocrystals | Shrimp shells, squid pens, yellow lobster | Acid hydrolysis, microwave irradiation | 314–900 | 41–42 | — | — | 68 |
| Nanowhiskers | Crab shell | Partial deacetylation | 250 | 6.2 | — | Nano-composite materials for reinforcement | 53 |
| Nanofibers | Chitin powder | Self-assembly | — | — | 2.8–10.2 | Nanofibrous matrices | 64 |
| Nanocrystals | Shrimp shells | Deep eutectic solvent | 100–700 | 20–80 | — | — | 82 |
| Nanofibers | Shrimp shells, practical grade chitin powder, pure chitin powder | Ionic liquid | — | — | — | Film for medical application | 92 |
| Nanofibers | Chitin powder | Dissolution–regeneration | 3 × 104–9 × 104 | Nonwoven mat for wound dressing | 61 | ||
| Nanofibers | Crab shell | TEMPO-mediated oxidation, aqueous counter collision | 492–828 | — | — | Hydrogel with enhanced mechanical properties for various application | 71 |
| Nanofibers | Shrimp fragment powder | Partial deacetylation, high-pressure homogenization | 400–600 | 10–20 | — | Waterproof packaging, waterproof transparent membrane | 55 |
| Nanocrystals | Chitin powder | Enzymatic hydrolysis | 458 | — | 32.3 | For biomedicine such as tissue engineering and bioprinting | 84 |
| Nanofibers | Crayfish shell | Enzymatic hydrolysis, ultrasound | 130–195 | — | — | For emulsions | 85 |
3.3 Sustainable analysis of nanochitin extraction
Globally, energy and environmental concerns have reignited scientific interest in the development of materials derived from biomass, which are more sustainable than materials derived from fossil fuels. However, besides demonstrating desirable functionalities, it is necessary to perform a life cycle assessment (LCA) for nanochitin to quantify the sustainability of this nanobiopolymer.93 LCA is an established methodology to evaluate the environmental impact of the technology of interest from cradle to grave. The process to perform LCA involves three general steps (Fig. 6). Firstly, the scope of the study needs to be defined, which includes the boundaries and limitations. Secondly, the quantitative inputs (energy, resources, chemicals, etc.) and outputs (emissions/waste to the environment) during the life cycle of the processes involved will be modelled. Lastly, the impact analysis is conducted by converting the model data to interpretable environmental impacts, which are categorized into midpoint and endpoint indicators. Midpoint indicators focus on the short-term impacts, such as climate change, terrestrial acidification, and water depletion. Alternatively, endpoint indicators focus on the long-term eventual impacts, such as damage to human health, the ecosystem and resource availability.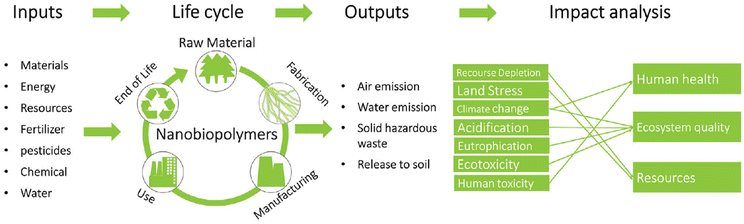 | ||
| Fig. 6 General process of LCA for nanobiopolymers such as nanochitin. Reproduced with permission.93 Copyright 2018, John Wiley and Sons. | ||
Generally, there are various factors that can affect the environmental impact score of the technology.93 Firstly, the raw material determines the chitin content, and thus the extent of chemical treatment required. Simultaneously, the raw material determines the resource availability and accessibility. Secondly, the isolation techniques of nanochitin can have different levels of impact, for example, replacing the use of concentrated hydrochloric acid (HCl) and sodium hydroxide (NaOH) will reduce the environmental impact. Thirdly, the manufacturing of nanochitin-based products has various extents of impact, which differ greatly according to the applications (biomedical, agricultural, catalysis, etc.). LCA is necessary to quantify the relative impacts of each factor. Presently, most of the reported nanochitin production processes are still at low technology readiness levels (TRL), and hence no comprehensive LCA has been conducted on this nanomaterial. Nonetheless, there are some reported LCAs that are relevant to provide a useful reference point for future studies.
Muñoz and co-workers presented a cradle-to-gate LCA of the production of bulk chitosan, which was derived from chitin via an additional deacetylation process.94 This study assessed the primary data from two producers located in India and Europe that produce chitosan for general purposes and medical use, respectively. Given that these two chitosan productions involve different supply chains, production locations and different uses, it was expected that they would have distinct environmental profiles. In the case of the Indian-produced chitosan (Fig. 7a and b), the major impact of chitin production (from shrimp shells) on climate change and acidification was related to the use of HCl and the ammonia emission from the use of protein-rich sludge as fertilizer. The HCl and NaOH treatments also have a significant impact on ecotoxicity and water quality. Regarding the European-produced chitosan (Fig. 7c and d), chitin (from crab shells) was sourced and produced from China. Given that coal is the main fuel used for heating and electricity in China, energy generation was identified as the main contributor to the impact on climate change, acidification and ecotoxicity. Notably, the acquisition of crab shells for chitin production resulted in a credit in the acidification impact indicator as the crab shells were diverted from being composted, thus avoiding ammonia and NOx emissions. This avoidance in acidifying emissions was actually more significant than the emissions associated with the production of chitin, i.e., a net reduction of acidification can be achieved through the production of chitin. This work highlights the important factors that can contribute to the global environmental impacts for chitosan (and thus, chitin) production, hence providing a useful benchmark for future investigations, although there will be some differences in chitin production on the nanoscale.
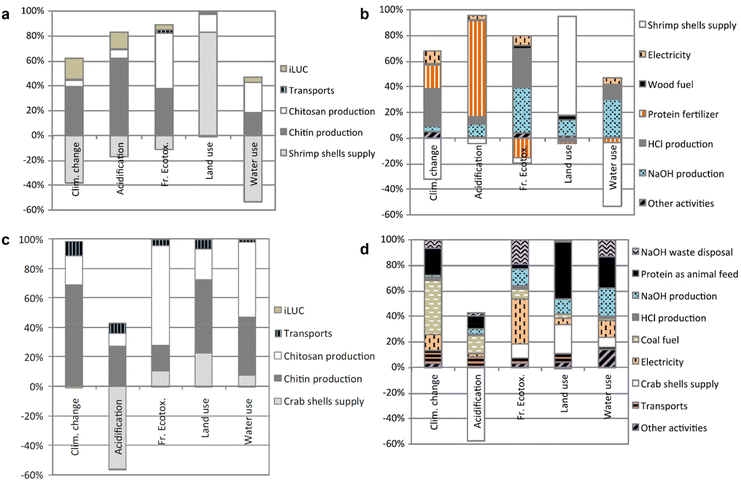 | ||
| Fig. 7 Impact assessment disaggregated into individual activities for the production of chitosan in (a and b) India and (c and d) Europe. Reproduced with permission.94 Copyright 2018, Springer Link. | ||
Cinelli et al. conducted an LCA study on a bio-plastic-based on polylactic acid and chitin nanofibrils (CNF).95 In the analysis of CNF production, the energy associated with the concentrating process of the CNF suspension, i.e., drying of water, was the main factor affecting the overall evaluation. Thus, the authors suggested the direct production of a concentrated CNF suspension of 20 wt% (vs. the original 2 wt%) to avoid the energy-intensive purification step. However, additives will be further required to aid the dispersion of CNF in the concentrated suspension, which may cause a change in bio-plastic properties or require additional steps to remove the additive present at a later process stage. Alternatively, the use of less energy-intensive separation methods, such as membrane filtration, can also be explored to concentrate the CNF suspension. It is important to note that this LCA is specifically for the production of CNF-containing bio-plastics, and therefore the requirement for the preparation of concentrated CNF suspension may not be applicable for other nanochitin materials with different applications.
As the production of nanochitin advances to a higher TRL, it will be increasingly crucial to evaluate the ecological footprint of these emerging materials through comprehensive LCAs. Additionally, LCA will be an important tool to provide a quantifiable justification for the sustainable production of chitin from alternative sources (crustaceans vs. insects vs. fungi).
4 Nanochitin applications towards advanced manufacturing
Advanced manufacturing can be defined as the adoption of innovative or cutting-edge technology in production, either to improve the process or product. The reduction of chitin to the nanoscale has seen the emergence of ingenious technology. In the nanoscale form, nanochitin gains new properties such as higher reactivity that arises with higher aspect ratio and new interaction with light. Besides the recently gained advantages, nanochitin still maintains the attributes as its predecessor, which makes it attractive for use in various sectors ranging from 3D printing and photonics to packaging and catalysis. This section summarizes the recent works on nanochitin in each field.4.1 Nanochitin for 3D printing
Recently, nanochitin has gained popularity in three-dimensional (3D) bioprinting applications. 3D bioprinting is a method of additive manufacturing that utilizes biocompatible filaments that may or may not be cell-laden to produce complex, tissue-like constructs by layer-by-layer deposition.96,97 Nanochitin provides biocompatibility and biodegradability to 3D bio-printed constructs used in regenerative medicine and tissue engineering. The high aspect ratio of nanochitin allows it to enhance the mechanical strength of objects constructed using bioinks, making it an attractive candidate as a filler in bioinks.98,99Karimipour-Fard et al. synthesized a nanocomposite filament of polycaprolactone (PCL) matrix with nano-hydroxyapatite (n-Hap) and ChNW fillers using a 1-butyl-3-methylimdazolium chloride (BMIMCl) ionic solvent. A preosteoblast mouse bone cell line was used to investigate the applicability of the nanocomposite filament for tissue engineering. It was found that ChNW increased the mechanical properties and the biodegradation rate of the PCL/n-Hap/ChNW filaments and enhanced the cell attachment and proliferation.100
Sadhasivam et al. fabricated a nanocomposite filament of poly(butylene adipate-co-terephthalate) (PBAT) and nanochitin filler for injection moulding. Similarly, nanochitin improved the thermal and mechanical properties of the PBAT/nanochitin filament, with the optimum composition being 30% nanochitin. 3D constructs made from the PBAT/nanochitin filament were stable and expected to be biodegradable. The PBAT/nanochitin nanocomposite was shown to promote cell migration in a scratch wound assay and exhibited in vivo non-toxicity in a zebrafish embryo model.101
Ling et al. utilized gelatin methacrylate (GelMA) and chitin nanocrystal to develop a biomaterial ink for 3D bioprinting.102 The addition of 1% (w/v) chitin nanocrystal is sufficient to significantly enhanced the properties of 10% (w/v) GelMA. For example, in terms of mechanical properties, printability, cell adhesion and proliferation. Yet, the chitin nanocrystal introduction did not alter the porosity which is important for tissue engineering as the pores are necessary to facilitate nutrient transport, cell attachment, migration and proliferation.
In another study, Zhang et al. designed an in situ self-assembling bioink made of fumed silica (FS) and nanochitin for 3D printing. Nanochitin enabled the FS/nanochitin bioink to display the mechanical, viscoelastic, and rheological properties required for 3D printing. 3D objects printed with the optimal concentration of 5–8 wt% nanochitin in the bioink possessed high structural fidelity and the ability to support their own weight after extrusion. The 3D scaffolds printed using this FS/nanochitin bioink were elastic and could return to their original shape after being subject to multiple deformations.103
4.2 Nanochitin for photonic applications
Native photonic chitin structures can be found in natural occurring species such as crustaceans and insects.104 Due to the periodic ordered chitin exoskeleton, the chitin micro/nano-structure interacts with light selectively and displays bright iridescent colors.105,106 To mimic these photonic structures, scientists explored the use of the exoskeleton of these creatures as a template for replication (Fig. 8).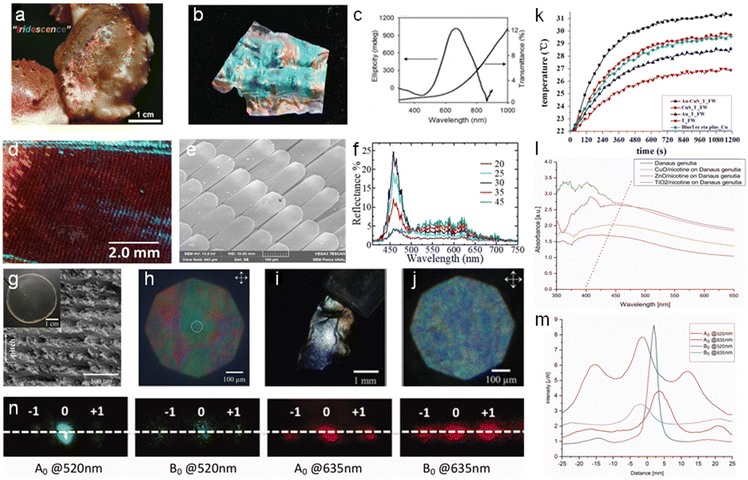 | ||
| Fig. 8 Nanochitin for photonic applications. (a) Optical image of a snow crab claw displaying iridescence. (b) Photograph of iridescent chitosan membrane derived from snow crab leg shells. (c) UV-vis and CD spectra of chitosan membrane. Reproduced with permission.112 Copyright 2019, John Wiley and Sons. Optical images of the M. cypris Colombian butterfly wing: (d) 1× magnification at 0° about the normal axis, viewed under the microscope. (e) SEM image at the scale of 100 μm. (f) Reflectance spectra of the M. cypris butterfly wing as a function of wavelength for the incidence angles of 20°, 25°, 30°, 35° and 45°. Reproduced with permission.113 Copyright 2020, Springer Nature Limited. (g) SEM image of the cross-section of fungal chitin nanocrystal film (f-ChNC) and the film optical image is shown in the inset. (h) Faint structural coloration observed for the f-ChNC film under the cross polarized microscopy. (i) Image showing strong blue/green coloration of f-ChNC film flake after alkaline treatment. (j) Microscopy image of f-ChNC film flake, highlighting the blueshift of the reflected color. Reproduced with permission.114 Copyright 2022, John Wiley and Sons. (k) T. helena forewings functioned as a biomimetic template to produce a photothermal conversion material under irradiation from a 980 nm laser. Reproduced with permission.109 Copyright 2015, Elsevier Ltd. (l) Changes in the absorbance of the butterfly wing scales due to the deposition of different metal oxide nanoparticles and nicotine mix. (m) Distribution of the electric field intensity of the butterfly wing scales illuminated by red and green light. (n) Far-field diffraction of butterfly wing scales upon exposure to red and green light. Adapted with permission.115 Copyright 2022, Informa UK Limited. | ||
Several studies have utilized butterfly wings as a bio-template for the in situ growth of metal nanoparticles. Boruah et al. deposited sliver ions (Ag+) in the chitin layers of a Pieris brassicae butterfly wing, and subsequently converted them into sliver (Ag) nanoparticles.107 The wings were soaked for varying durations and their photonic band gap was studied. The band gap opening was determined by the interactions between the localized surface plasmon of the Ag nanoparticles and homogenous air-hole structure on the butterfly wing. The photonic band gap could be tailored by adjusting the Ag adsorption time. An increase in the soaking time resulted in the reflectance peak maximum shifting from 335–355 nm to 680–730 nm. Mu et al. utilized the chitin/chitosan found on butterfly wings for reduction to synthesize gold (Au) nanoparticles in situ and presented their application as surface enhanced Raman spectroscopy (SERS) substrates.108 The butterfly wing from Morpho menelaus was employed to detect 4-ATP at the lowest concentration of 10−9 M and exhibited the lowest RSD among the samples. Using the forewing of Troides helena as a biomimetic template, Tian et al. modified the chitin from the forewing with amine moieties, and subsequently deposited Au and CuS nanoparticles in the structure.109 Subsequently, they demonstrated the ability of the obtained chitin to improve the infrared absorption, reduce the reflectance, and infrared (30.56%) and solar photothermal conversion ability. Additionally, it achieved a solar absorbance of up to 98% when fabricated into a solar absorber. These features are associated with the plasmon-to-exciton/plasmon coupling effect between the nanoparticles together with the favorable coupling between adjacent resonant systems in the sub-micrometer antireflection quasi-photonic structures of the forewing.
Chitin powder has also been explored as a starting material for photonic applications. A bio-ink derived from squid pen chitin functionalized with genetically produced amyloid proteins was developed by Wei et al.110 The bio-ink was proven to be applicable for multiple fabrication techniques such as airbrushing, electrospinning, and lithography. Particularly, soft lithography was used to produce ordered and freestanding structures at the micro-level. The potential photonic applications for the fabricated structures as light-guiding gratings include anti-reflection and photonic electrodes. To better understand the natural photonic observations, Liu et al. investigated the self-assembly of chitin nanocrystals in capillaries and found that the chitin nanocrystals form a continuous orderly anisotropic phase depending on the phase boundary growth.111 The air–liquid interface confined at the end of capillaries allowed the concurrent evaporation and deposition of chitin nanocrystals, which self-assemble into nested paraboloid Bouligand structures with a density gradient. Continuous birefringent layers were observed as a result of directional evaporation. This study provided insight into the biological self-assembly process of chitin nanocrystals found in living organisms.
The challenge with the fabrication of natural photonic structure arises from the difficulty in replicating the hierarchal structure without the use of a bio-template. Hence, many studies employed the forewings of butterflies or other species that have photonic architectures as a native photonic template.116–119 In addition, the stages of chitin biosynthesis that occur in living species require deeper understanding given that it ultimately influences the final morphologies.
4.3 Nanochitin for intelligent food packaging
Nanochitin in the form of nanofibers or nanocrystals has been well exploited as a reinforcing agent in traditional food packaging because of its non-toxicity and hydrophilic nature for the ease of dispersion in various aqueous polymer matrices.120 It is also known to enhance the physical properties (e.g., mechanical, thermal, and barrier) of films. To advance traditional food packing for the better monitoring and preservation of food, intelligent food packaging capable of providing cues on food freshness is desirable. Anthocyanins are bioactive compounds that give rise to the different colors observed in flowers, fruits, and vegetables.121 Many studies exploited natural anthocyanins extracted from various plants as the source of pH sensitive pigments. In general, anthocyanins are incorporated into a polymer matrix compatible for food packaging, endowing them with an intelligent feature to change color under different pH environments.122Zheng et al. developed two colorimetric films for the evaluation of milk and pork freshness separately (Fig. 9a).123 The films consisted of a chitin whisker filler, anthocyanins extracted from black wolfberry, and sodium alginate or gelatin matrix. The films could exhibit visible color shifts between pH 3–12. The chitin whiskers could form hydrogen bonding and electrostatic interactions with the functional groups available in the other components, resulting in reduced water solubility, improved thermal stability, and enhanced anthocyanin binding. Depending on the coloration response to extreme acid (e.g., lactic acid) or alkaline (e.g., ammonia) conditions, the films were concluded to be suitable for detecting milk or pork spoilage. In another study, anthocyanin from red cabbage (RCAs) was added to a chitosan (CS)/oxidized-chitin nanocrystal (OCN) matrix by Chen et al. (Fig. 9b and c).124 The RCAs were successfully integrated with CS/OCN via hydrogen bonding and color-changing films were formed. The films exhibited a visible color change according to the surrounding pH. When tested in the presence of hairtail (Trichiurus lepturus) and shrimp (Penaeus vannamei), a color changed from reddish purple (fresh stage) to brown, and lastly yellow (spoiled stage) was observed across 48 h. The color change was strongly correlated with the seafood spoilage process (R2 > 0.90). Similarly, Sani et al. successfully entrapped red barberry anthocyanins (RBAs) in a composite chitin nanofiber (CNF) and methylcellulose (MC) matrix, thus forming a pH-sensitive colorimetric film.125 pH-Sensitive composite films were used to monitor the freshness of the fish fillets over a period of 72 h. The decomposition process released volatile ammonia and amines, which led to an increase in pH (6.3 to 8), and this was reflected by a colour change in the pH-sensitive film from reddish to pale pink.
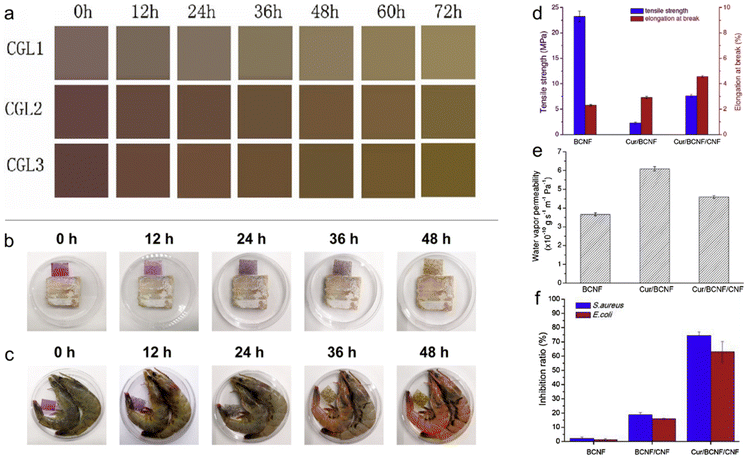 | ||
| Fig. 9 Nanochitin for food packaging. (a) Real-time images of the color change displayed by the colorimetric films developed with chitin whiskers, gelatin and anthocyanins, when stored with pork samples at 25 °C for 72 h. Reproduced with permission.123 Copyright 2022, Elsevier Ltd. Color indication captured by COR-1.2 films in response to the freshness of (b) hairtail and (c) shrimp during storage at 25 °C. Reproduced with permission.124 Copyright 2021, Elsevier B.V. (d) Mechanical strength, (e) water vapor permeability and (f) inhibitory effects of the film samples against bacteria determined using the CFU method. Reproduced with permission.126 Copyright 2019, Elsevier Ltd. | ||
Besides anthocyanins, compounds such as curcumin, which are pH sensitive, can also provide an observable colour indication for the monitoring of food spoilage. Curcumin (Cur) micro/nanoparticles were formed in situ in a bacterial cellulose nanofiber (BCNF)/chitin nanofiber (CNF) composite film, as reported by Yang et al. (Fig. 9d–f).126 With the incorporation of CNF, the Cur/BCNF/CNF film displayed improved mechanical and water barrier properties compared to the Cur/BCNF film. This is due to CNF acting as a reinforcement nanofiller and a hindrance to water diffusion. A colour change from yellow to reddish brown was observed when the pH increased from 1 to 13 when the Cur/BCNF/CNF films were immersed in solutions.
Another pH-sensitive compound that was investigated was elderberry extract. Cabrera-Barjas et al. obtained nanofibrous β-chitin from squid pen waste and added glycerol together with elderberry extract of varying concentrations to form intelligent films for fish freshness monitoring.127 Elderberry is considered a natural sensing pigment, which displays different colors in the presence of acid or alkaline surroundings. The films formed by the combination of nanofibrous β-chitin and elderberry extract exhibited improved tensile strength and elongation at break. As the pH changed from 2 to 12, the film changed color from pink to grey. The combination of these properties makes the film suitable for food monitoring. They tested the film by monitoring the freshness of Hake fish and found that the film transited initially from pink to purple, and subsequently, blue by day 6 due to the increasing basic environment caused by bacteria growth.
More recently, the incorporation of active ingredients to aid the preservation of food was explored by some researchers. These additives provide additional properties such as antioxidant and/or anti-bacteria behaviors. Fernández-Marín et al. investigated the addition of curcuma oil and anthocyanin extracted from Curcuma Longa L. and red cabbage, respectively, in chitosan/chitin nanocrystal composite films.128 Curcuma oil and anthocyanins were proven to be pH- and ammonia-sensitive components. The additional actives reduced the moisture content and water solubility and increased the ultraviolet light barrier and mechanical strength. Moreover, they endowed the film with antioxidant characteristics together with color sensitivity to pH and ammonia variations. In another study by Duan et al., pullulan/chitin nanofibers were electrospun with curcumin and anthocyanins as active ingredients.129 They successfully embedded curcumin and anthocyanins in the PCM substrate. The combination of both actives enhanced the antioxidant and antimicrobial performance of the film compared to the films only containing either component. The films with anthocyanins were proven to exhibit distinct color changes with a change in pH. The PCN/CR/ATH nanofiber composite was tested by monitoring the decay of Plectorhinchus cinctus and an observable color change was detected. Recently, natural red cabbage extracts (RCA) and nisin were immobilized in a chitin nanofiber reinforced pullulan/chitosan composite matrix by Wu et al.130 RCA and nisin were well integrated and dispersed and formed hydrogen bonding with the other components in the composite. The inclusion of these active compounds also improved the mechanical strength, thermal stability, water vapor and UV light barrier properties. The composite film exhibited antioxidant and antibacterial capabilities due to the presence of RCA and nisin, respectively. Fresh food monitoring was demonstrated with sea bass (Lateolabrax japonicus), whereby the nanocomposite films changed from red (pH 2) to blue (pH 12) over the decay duration.
In summary, nanochitin has been utilized as a reinforcing material to improve the natural limitations such as mechanical strength and barrier properties of polymeric films for food preservation. To provide a clear observable indication of food spoilage, plant-based anthocyanins were exploited as pH-sensitive pigments for intelligent food packaging. Therefore, the combination of both chitin-reinforcing agent and color-changing pH-responsive extracts led to the development of smart films for food monitoring. These films can provide visual indication of food spoilage rate, and simultaneously have suitable physical properties for the preservation of food. Furthermore, to expand the capabilities of these nanocomposite films, additional active chemical compounds can also be incorporated to provide bioactivities, which include antioxidant and antibacterial properties that aid with the preservation of food.
4.4 Nanochitin for green catalysis
Nanochitin is created by breaking down chitin into nanoscale particles using various physical and chemical processes.1,131–133 Thus, nanochitin possesses a high surface area, together with biocompatibility, biodegradability and low toxicity, making it an attractive material for use in catalytic applications.134–136 Due to its unique surface chemistry, nanochitin is an attractive material and has been intensively investigated for potential use as a catalyst or as a support for catalytic materials.137,138 Nanochitin acts as the catalyst support for a wide range of catalysts, covering inorganic catalysts such as metal and metal oxides, as well as organic and biocatalyst such as organic molecules and enzymes.| Catalyst | Nanostructure | Reaction scheme | Performance and stability | Ref. |
|---|---|---|---|---|
| Pd-chitin nanocrystals | Chitin nanocrystals | Heck coupling | • Yield 100% | 139 |
| Au-chitin nanofibre membrane | Chitin nanofiber | Reduction of 4-nitrophenol | • As an indicator to show the successful recovery of Au NPs from Au3+ in the nanochitin matrix | 140 |
| Chitin nanofiber | Peroxidase substrate 3,3,5,5-tetramethylbenzidine (TMB) | • Useful in the accurate and rapid determination of H2O2 | ||
| • Potentially useful in food, pharmaceutical analysis | ||||
| Chitin nanofiber | Glucose oxidation | • Oxidize the glucose while generate H2O2 | ||
| • Important application for diagnosis of diabetes mellitus | ||||
| Pt-NP loaded macrofiber | Chitin nanofiber | Reduction of p-nitrophenol | • Achieved strain value of about 12% | 141 |
| • Work-of-fracture is around 10 MJ m−3 | ||||
| • Further loading with TiO2 could enable photocatalytic property | ||||
| Ag-NP/Au-NP/Pt-NP nanochitin aerogel | Nanofiber | Reduction of p-nitrophenol removing of organic dyes | • Highly stable | 142 |
| • The approach can be extended to other metal nanoparticle catalysts | ||||
| ZnO/chitin composite | Chitin/ZnO nanoparticle | Degrading NH4+-N under UV radiation | • 88.64% of NH4+-N removed in 2 h | 143 |
| • Achieving catalyst cyclic utilization remains as unsolved problem | ||||
| Cu2O-chitin/graphene oxide (GO) | Nanocomposite | Methyl orange (MO) degradation under sunlight | • Nanochitin acts as a template for Cu2O nanoparticle synthesis | 144 |
| • GO dramatically improves the photocatalytic performance of Cu2O via enhanced charge separation | ||||
| • Great potential for waste water treatment using solar energy | ||||
| Chitin-derived carbon/g-C3N4 heterojunction | Chitin-derived carbon nanoparticles | Rhodamine B degradation | • Non-metal photocatalyst is cost-effective and sustainable compared to metal based ones | 145 |
| • Chitin creates microstructural change for g-C3N4, leading to an increase in surface area |
Heterogeneous support for metal nanoparticles. Nanochitin has several advantages as a support material for metal catalysts, as follows:
(i) Its high surface area provides more active sites for loading metal catalysts and increases the accessibility of reactants to the catalyst.
(ii) Nanochitin is a biocompatible and biodegradable material, making it a sustainable and environmentally friendly alternative to synthetic support materials.
(iii) Nanochitin is highly chemically stable and can withstand harsh reaction conditions, making it a suitable support material for a wide range of metal catalysts.
(iv) Easy preparation: nanochitin can be easily prepared from natural chitin sources such as crustacean shells or fungal cell walls, making it a low-cost and readily available support material.
(v) Improved catalytic performance: nanochitin can improve the catalytic performance of metal catalysts due to its unique surface chemistry, which can enhance the adsorption of reactants and intermediates and increase the selectivity of the reaction.
The metal catalysts that can be supported by nanochitin include metal nanoparticles such as palladium, platinum, gold, and silver. Taking palladium nanoparticles (Pd NPs) as an example, nanochitin-supported Pd NPs have been used as catalysts for the Heck coupling reaction139 and waste water treatment.146 By directly reducing PdCl2 salt in a one-pot fashion, Pd NPs were deposited on the chitin nanocrystals and formed hetero-structured catalysts.139 The transmission electron microscopy images of the nanocomposites showed well-dispersed Pd NPs on the surface of the nanochitin crystals. The authors employed Heck coupling as the model reaction for testing the Pd NP-chitin catalyst, obtaining a full product yield under mild conditions, outperforming the use of other biomass-supported catalysts, such as cellulose nanocrystals.
Another highly efficient metal nanoparticle catalyst is platinum nanoparticles (Pt NPs), which are well known for their good catalytic performance in reactions such as hydrogenation, oxidation, and fuel cell reactions. Pt NPs exhibit high selectivity and stability even under harsh reaction conditions. However, Pt NPs easily aggregate, resulting in a reduction in the number of surface reactive sites when used alone. In this case, nanochitin is a great support substance for Pt NPs during the fabrication of the catalyst. Das et al. developed a recycled nanochitin hydrogel loaded with Pt NPs via the in situ reduction of H2PtCl6 salt in the hydrogel matrix.141 These organic/inorganic hybrid hydrogel fibers showed high activity in the catalytic reduction of p-nitrophenol in the presence of NaBH4. Moreover, these recyclable catalyst systems can be developed further with the addition of TiO2 nanoparticles to perform photocatalytic reactions.
Silver nanoparticles (Ag NPs) are another type of multifunction nanoparticles, which are widely utilized in antibacterial coatings, sensors, water treatment, solar devices and catalytic reactions. As shown in Fig. 10a, with the dopamine-activated nanochitin gel-like matrix, Ag+ ions were introduced and further reduced into Ag NPs, followed by freeze drying to obtain an aerogel of hybrid Ag-nanochitin.142 By controlling the activation of the nanochitin matrix via dopamine, the authors could control the storage capacity and the size distribution of metal nanoparticles precisely. Due to its high surface area, low density, tough mechanical strength and excellent catalytic activity, the hybrid aerogel reduced organic pollutants (such as methyl orange, methylene blue, p-nitrophenol and rhodamine B) in NaBH4 medium, and examples are displayed in Fig. 10b and c, respectively. This approach can be used for generating many other catalytic metal nanoparticles with nanochitin aerogels, such as Au and Pt, as demonstrated by the authors in this work.
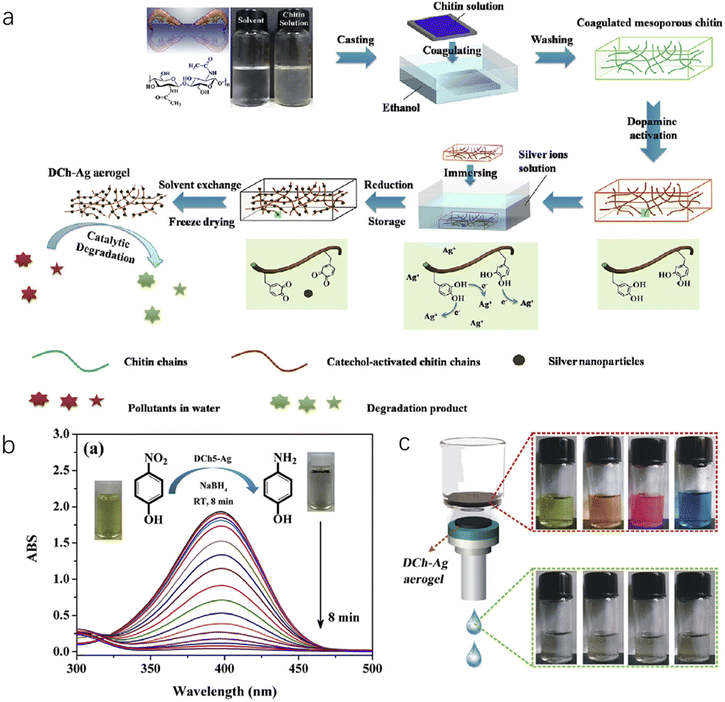 | ||
| Fig. 10 (a) Illustration of the fabrication of the ChNC-Ag aerogel. (b) Catalytic evaluation of ChNC-Ag NP aerogels with a typical UV–vis spectra of the conversion reaction. The inset photographs show the color change from yellow (before) to colorless (after). (c) ChNC-Ag aerogels as filtration membranes to remove organic pollutants, where four colored organic dyes become colorless after the filtration process. Reproduced with permission.142 Copyright 2017, Elsevier Inc. | ||
Finally, nanochitin can be chemically modified, for example, by introducing amino groups, chitin nanofibers can be used in filtration membranes, allowing the effective extraction of noble metal ions, such as Au3+, Ag+, Pt4+, and Pd2+.140 As shown in the schematic drawing in Fig. 11a, firstly the nanochitin fiber membranes absorb metal ions from the oil/water emulsion, then upon the in situ reduction of the absorbed metal ions, metal nanoparticle-loaded nanochitin fibers were obtained.140 Consequently, this nanochitin-supported metal catalyst is potentially useful for catalytic applications for biosensing and green catalyst production. Here, in this work, Au3+ was introduced to demonstrate the excellent catalytic property of the recovered Au NP-nanochitin fibers in the membrane form. The actual color of the recovered nanochitin membrane turned from yellow to red, and the TEM results demonstrated the appropriate size and crystallinity of the Au NPs. Two model reactions were chosen to test the Au NP-nanochitin fiber catalyst, with the results displayed in Fig. 11b and c, respectively. Employing the same approach, Ag, Pt and Pd nanoparticles could also be obtained for the metal nanoparticle-nanochitin fiber catalyst, although the authors did not report the details of this experiment. The successful recovery of the Au NP-chitin fiber membrane demonstrated its great catalytic activities and potential use in sensor and green catalysis systems.
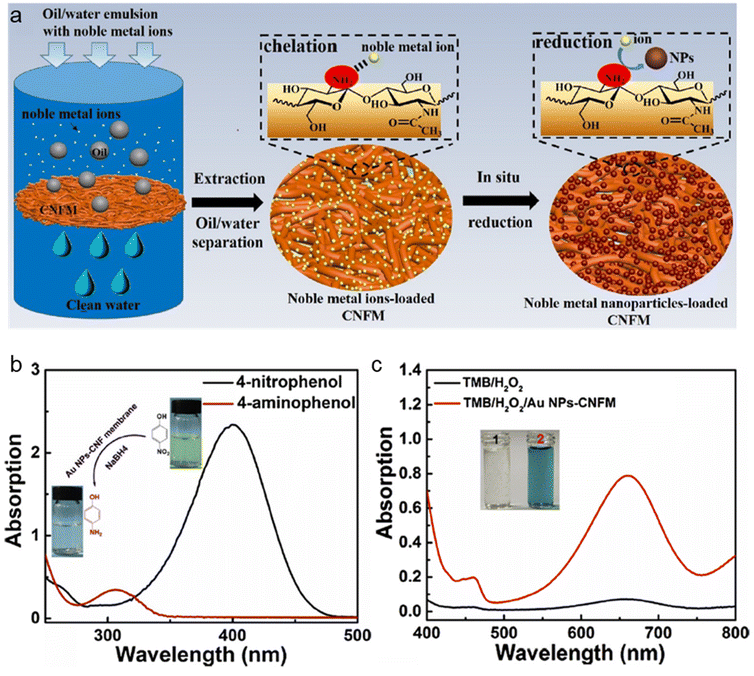 | ||
| Fig. 11 (a) Scheme illustration of the recovery of noble metal ions from oil/water emulsions by chitin fibres membranes. (b) UV-vis of the 4-nitrophenolate/NaBH4 before and after treatment using Au NP-nanochitin fibres. (c) UV–vis spectra of TMB/H2O2 before and after treatment by Au NP-nanochitin fibres. The insets are the photographs of TMB/H2O2 before (1) and after (2) treatment using Au NP-nanochitin fibres. Reproduced with permission.140 Copyright 2019, Elsevier Ltd. | ||
Support or additive for inorganic photocatalysts. Metal oxides and other inorganic nanoparticles, such as titanium dioxide (TiO2),147,148 zinc oxide (ZnO),143,149 copper oxide (Cu2O)144 and carbon nitride (C3N4),145 can be deposited on the surface of nanochitin and used as a catalyst for various reactions. The biodegradability of nanochitin is advantageous, given that it reduces the environmental impact of catalyst synthesis and use. Some of the reported works are included in Table 3 for these materials, demonstrating their attractive properties and good photocatalytic behaviour.
Instead of deposition on the surface of nanochitin, Nguyen et al. utilized a different strategy known as liquid crystal self-assembly to develop TiO2/graphene/chitin composite membranes.150 Liquid crystals of both graphene oxide nanosheets and chitin nanospindles supposedly self-assembled into a flexible nacre-mimicking membrane and peroxotitanate were incorporated in the structure during the co-assembly process. A subsequent reduction was performed to produce TiO2/graphene/chitin composite membranes. The absorption ability of this composite membrane was revealed to be significantly enhanced compared to graphene oxide (∼235 nm). In fact, the absorption range of the composite membrane was found to be extended to the visible light band (∼450 nm). Additionally, they showed that the developed membrane could photo-catalyze methylene blue solution to colorless within 30 min of UV-Vis irradiation, which was faster than the controls (graphene and Degussa P25). This was attributed to the synergistic semiconductor-graphene interactions, which were possible by the heterojunction of the TiO2 nanoparticle-localized graphene nanosheets supported onto nanochitin layers. Given that the surface chemistry of nanochitin can be modified to enhance its interaction with inorganic nanoparticles, together with the template effect, which facilitates the synthesis and distribution of the catalyst, nanochitin is an effective component that can improve the catalytic performance from several aspects.
Nanochitin serves as immobilization support for enzymes and other biocatalysts and is very useful in industrial biotechnology such as the production of pharmaceuticals, food processing, and biofuels. In general, the immobilization of enzymes on nanochitin can improve their stability, activity, and reusability, leading to more efficient and cost-effective enzyme-catalyzed processes.
(i) The high surface area of nanochitin allows for a greater number of active sites, leading to higher catalytic activity.
(ii) The biocompatibility of nanochitin also makes it a more sustainable and environmentally friendly alternative to synthetic organic catalysts.
(iii) Given that the amino and carboxyl functional groups in nanochitin can participate in the reaction mechanism, it can be more selective and achieve higher catalytic efficiency.
Tsutsumi et al. reported the synthesis of a highly porous nanochitin aerogel with C2-amine-functionalized chitin nanofibrils (ChNF), exhibiting high surface areas.159 The obtained nanochitin aerogel was employed as a catalyst for the aqueous Knoevenagel condensation reaction, achieving high efficiency due to the combination of active amine groups and the nanofibrous structure supporting continuous flow catalysis. Amino-functionalized nanochitin and chitosan have been shown to be an effective catalyst for the solvent-free synthesis of chalcones,160 self-condensation of linear aldehydes161 and production of biodiesel.162 Nanochitin can also be used as a bioreactor for various purposes, such as sulfate reducing for mining influenced water,163 metal removal and acid neutralization,164 as well as creating a multienzyme bioreactor.165 The high surface area of nanochitin allows for efficient cell attachment and growth, and its unique surface chemistry can be modified to enhance the interaction between cells and the supporting material.
However, although nanochitin has great potential for use in catalytic applications due to its unique properties and versatility, further research is needed to fully understand its catalytic activity and optimize its use in different applications.
In summary, the use of natural polysaccharides such as chitin as either a catalyst support or organocatalyst is an emerging trend in green and sustainable chemistry. However, there is still exciting scope that remains unexplored in utilizing natural polymers in sustainable chemistry. For instance, they can be explored as catalysts for the oxygen evolution reaction166,167 and high-kinetics oxygen reduction reaction.168
5 Conclusion and perspective
In this review, we summarized the different strategies to prepare nanochitin from chitin. We examined these strategies from a different viewpoint by identifying the comparably more sustainable methods from the traditional methods. For instance, researchers have explored the use of greener solvents and ways to optimize the process to be more environmentally friendly. It is recognized that chitin sources can be obtained from various biomass such as crustaceans, insects, and fungi. However, the efficiency of chitin isolation from these sources is different, even when the same technique is used due to the different compositions that it is found in. For example, chitin extraction from insect exoskeleton is likely to be easier than chitin isolation from crustacean shells, where the mineral content is higher. Not only can milder conditions be used for the former, but the yield can also be higher.At present, the chitin biorefinery is not as established as its cellulose counterpart. With reference to the market size of nanocellulose, it can be safely estimated that there will be a market for nanochitin. In fact, the market size of nanochitin will not be small and likely to be even bigger than nanocellulose. The reason for this is that nanochitin is the only natural polysaccharide that contains nitrogen. Although the actual extent of demand for nanochitin and its products is still unclear, all the above-mentioned studies demonstrated that nanochitin will be valuable as a sustainable and advanced materials for future manufacturing, consumer care, and even biomedicine.
It seems that several challenges need to be overcome in the process of establishing the chitin biorefinery. The first challenge is to isolate and obtain homogeneous sources of biomass waste. There is also the problem with resource complexity, whereby different sources have different chitin contents. Furthermore, currently, the nanochitin extraction methods are still not very eco-friendly with low yield, despite being energy intensive. The characterization, processing and modification methods are very limited at this stage. At present, nanochitin is mostly applied as an additive instead of bulk material and more studies will be needed to understand its interaction with the host matrix. There is also the question of the feasibility of scale up, which should be possible given that its predecessor is nanocellulose. However, the success of scaling up eventually depends on the techniques employed to extract chitin and convert it to nanochitin as well as the efficiency. A crucial and unavoidable issue will be the cost of nanochitin fabrication given that a high cost will deter its use for applications.
Besides the challenges encountered in the set-up of the chitin biorefinery, it is necessary to consider the environmental impact of the nanochitin fabrication from cradle to grave. It is important to reduce and valorize waste; however, there should not be more waste generated in the process of doing so. Thus, life cycle assessments will be a powerful tool to gauge the “real” environmental benefits of nanochitin synthesis from biomass waste.
Finally, the potential applications for nanochitin in advanced manufacturing that have been explored by researchers were presented, which range from 3D printing and photonics to packaging and catalysis. Indeed, nanochitin appears to have numerous advantages that can be imparted to the materials. However, in most cases nanochitin can only be used as additives or supportive materials. Thus, how to better explore its advantage and maximize its potential is something worthy to investigate to achieve a circular economy.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research is supported by the RIE2025 MTC Individual Research Grants (M22K2c0085) and Urban and Green Technology Horizontal Technology Seed Fund (C211718009), administered by the Agency of Science, Technology and Research (A*STAR), Singapore. This work was also supported by the National Medical Research Council (NMRC), Singapore, under its Clinician Scientist-Individual Research Grant (MOH-001357-00).References
- L. Bai, L. Liu, M. Esquivel, B. L. Tardy, S. Huan, X. Niu, S. Liu, G. Yang, Y. Fan and O. J. Rojas, Chem. Rev., 2022, 122, 11604–11674 CrossRef CAS PubMed
.
- H.-S. Jung, H. C. Kim and W. H. Park, Carbohydr. Polym., 2019, 213, 311–319 CrossRef CAS PubMed
.
- S. Olza, A. M. Salaberria, A. Alonso-Varona, A. Samanta and S. C. M. Fernandes, J. Mater. Chem. B, 2023, 11, 5630–5649 RSC
.
- T. Jin, T. Liu, E. Lam and A. Moores, Nanoscale Horiz., 2021, 6, 505–542 RSC
.
- H. Ma, L. Liu, J. Yu and Y. Fan, Biomacromolecules, 2021, 22, 4373–4382 CrossRef CAS PubMed
.
- X. Ma, G. Gözaydın, H. Yang, W. Ning, X. Han, N. Y. Poon, H. Liang, N. Yan and K. Zhou, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 7719–7728 CrossRef CAS PubMed
.
- I. Younes and M. Rinaudo, Mar. Drugs, 2015, 13, 1133–1174 CrossRef CAS PubMed
.
- B. T. Iber, N. A. Kasan, D. Torsabo and J. W. Omuwa, J. Renewable Mater., 2022, 10, 1097–1123 CAS
.
- X. Yang, J. Liu, Y. Pei, X. Zheng and K. Tang, Energy Environ. Mater., 2020, 3, 492–515 CrossRef
.
- E. Alabaraoye, M. Achilonu and R. Hester, J. Polym. Environ., 2017, 26, 2207–2218 CrossRef
.
-
C. Peniche, W. Argüelles-Monal and F. M. Goycoolea, in Monomers, Polymers and Composites from Renewable Resources, ed. M. N. Belgacem and A. Gandini, Elsevier, Amsterdam, 2008, pp. 517–542, DOI:10.1016/B978-0-08-045316-3.00025-9
.
- K. Mohan, T. Muralisankar, R. Jayakumar and C. Rajeevgandhi, Carbohydr. Polym. Technol. Appl., 2021, 2 DOI:10.1016/j.carpta.2021.100037
.
- A. van Huis, J. Insects Food Feed, 2020, 6, 27–44 Search PubMed
.
- G. Huet, C. Hadad, E. Husson, S. Laclef, V. Lambertyn, M. A. Farias, A. Jamali, M. Courty, R. Alayoubi, I. Gosselin, C. Sarazin and A. N. Van Nhien, Carbohydr. Polym., 2020, 228, 115382 CrossRef CAS PubMed
.
- L. Soetemans, M. Uyttebroek and L. Bastiaens, Int. J. Biol. Macromol., 2020, 165, 3206–3214 CrossRef CAS PubMed
.
- R. Chandran, L. Williams, A. Hung, K. Nowlin and D. LaJeunesse, Micron, 2016, 82, 74–85 CrossRef CAS PubMed
.
- K. P. Sambasevam, S. F. Sateria, S. N. A. Baharin, N. J. Azman, S. A. Wakid and S. Shahabuddin, Int. J. Biol. Macromol., 2023, 238, 124079 CrossRef CAS PubMed
.
- M. Jones, M. Kujundzic, S. John and A. Bismarck, Mar. Drugs, 2020, 18, 64 CrossRef CAS PubMed
.
- J. Sietsma and J. Wessels, Biochim. Biophys. Acta, Gen. Subj., 1977, 496, 225–239 CrossRef CAS PubMed
.
- J. Vetter, Food Chem., 2007, 102, 6–9 CrossRef CAS
.
- Y. Bamba, Y. Ogawa, T. Saito, L. A. Berglund and A. Isogai, Biomacromolecules, 2017, 18, 4405–4410 CrossRef CAS PubMed
.
- C. M. Stagg and M.
S. Feather, Biochim. Biophys. Acta, Gen. Subj., 1973, 320, 64–72 CrossRef CAS PubMed
.
- A. M. Salaberria, J. Labidi and S. C. Fernandes, Eur. Polym. J., 2015, 68, 503–515 CrossRef CAS
.
- R. N. Tharanathan and F. S. Kittur, Crit. Rev. Food Sci. Nutr., 2003, 43, 61–87 CrossRef CAS PubMed
.
- J. D. Goodrich and W. T. Winter, Biomacromolecules, 2007, 8, 252–257 CrossRef CAS PubMed
.
- S. Ling, D. L. Kaplan and M. J. Buehler, Nat. Rev. Mater., 2018, 3, 1–15 CrossRef
.
- H. O. Fabritius, C. Sachs, P. R. Triguero and D. Raabe, Adv. Mater., 2009, 21, 391–400 CrossRef CAS
.
- R. Marchessault, F. Morehead and N. Walter, Nature, 1959, 184, 632–633 CrossRef CAS
.
- J.-F. Revol and R. Marchessault, Int. J. Biol. Macromol., 1993, 15, 329–335 CrossRef CAS PubMed
.
- K. Kurita, K. Tomita, T. Tada, S. Ishii, S. I. Nishimura and K. Shimoda, J. Polym. Sci., Part A: Polym. Chem., 1993, 31, 485–491 CrossRef CAS
.
- A. Morin and A. Dufresne, Macromolecules, 2002, 35, 2190–2199 CrossRef CAS
.
- K. Gopalan Nair and A. Dufresne, Biomacromolecules, 2003, 4, 657–665 CrossRef PubMed
.
- K. Gopalan Nair, A. Dufresne, A. Gandini and M. N. Belgacem, Biomacromolecules, 2003, 4, 1835–1842 CrossRef PubMed
.
- J. Sriupayo, P. Supaphol, J. Blackwell and R. Rujiravanit, Carbohydr. Polym., 2005, 62, 130–136 CrossRef CAS
.
- P. Wongpanit, N. Sanchavanakit, P. Pavasant, T. Bunaprasert, Y. Tabata and R. Rujiravanit, Eur. Polym. J., 2007, 43, 4123–4135 CrossRef CAS
.
- Z. Li, H. Wang, S. An and X. Yin, J. Nanobiotechnol., 2021, 19, 1–13 CrossRef PubMed
.
- Y. Zhou, S. Jiang, Y. Jiao and H. Wang, Int. J. Biol. Macromol., 2017, 99, 205–212 CrossRef CAS PubMed
.
- Y. Qin, S. Zhang, J. Yu, J. Yang, L. Xiong and Q. Sun, Carbohydr. Polym., 2016, 147, 372–378 CrossRef CAS PubMed
.
- Y. F. Aklog, M. Egusa, H. Kaminaka, H. Izawa, M. Morimoto, H. Saimoto and S. Ifuku, Int. J. Mol. Sci., 2016, 17, 1600 CrossRef PubMed
.
- Y. F. Aklog, T. Nagae, H. Izawa, M. Morimoto, H. Saimoto and S. Ifuku, J. Nanosci. Nanotechnol., 2017, 17, 5037–5041 CrossRef CAS
.
- P.-Y. Chen, A. Y.-M. Lin, J. McKittrick and M. A. Meyers, Acta Biomater., 2008, 4, 587–596 CrossRef PubMed
.
- K. Missoum, M. N. Belgacem and J. Bras, Materials, 2013, 6, 1745–1766 CrossRef PubMed
.
- S. Ifuku, M. Nogi, K. Abe, M. Yoshioka, M. Morimoto, H. Saimoto and H. Yano, Carbohydr. Polym., 2011, 84, 762–764 CrossRef CAS
.
- S. Ifuku, M. Nogi, M. Yoshioka, M. Morimoto, H. Yano and H. Saimoto, Carbohydr. Polym., 2010, 81, 134–139 CrossRef CAS
.
- J. H. Bang and K. S. Suslick, Adv. Mater., 2010, 22, 1039–1059 CrossRef CAS PubMed
.
- M. N. Islam, M. Zhang and B. Adhikari, Food Rev. Int., 2014, 30, 1–21 CrossRef CAS
.
- Y. Lu, Q. Sun, X. She, Y. Xia, Y. Liu, J. Li and D. Yang, Carbohydr. Polym., 2013, 98, 1497–1504 CrossRef CAS PubMed
.
- Y. Fan, T. Saito and A. Isogai, Biomacromolecules, 2008, 9, 192–198 CrossRef CAS PubMed
.
- S. Ifuku, T. Hori, H. Izawa, M. Morimoto and H. Saimoto, Carbohydr. Polym., 2015, 122, 1–4 CrossRef CAS PubMed
.
- Y. Fan, T. Saito and A. Isogai, Carbohydr. Polym., 2009, 77, 832–838 CrossRef CAS
.
- K. Pang, B. Ding, X. Liu, H. Wu, Y. Duan and J. Zhang, Green Chem., 2017, 19, 3665–3670 RSC
.
- J. Jiang, J. Yu, L. Liu, Z. Wang, Y. Fan and A. Isogai, J. Agric. Food Chem., 2018, 66, 11372–11379 CrossRef CAS PubMed
.
- Y. Fan, T. Saito and A. Isogai, Carbohydr. Polym., 2010, 79, 1046–1051 CrossRef CAS
.
- E. Belamie, P. Davidson and M. Giraud-Guille, J. Phys. Chem. B, 2004, 108, 14991–15000 CrossRef CAS
.
- J. Xu, X. Deng, Y. Dong, Z. Zhou, Y. Zhang, J. Yu, J. Cai and Y. Zhang, Carbohydr. Polym., 2020, 247, 116694 CrossRef CAS PubMed
.
- R. Nayak, R. Padhye, I. L. Kyratzis, Y. B. Truong and L. Arnold, Text. Res. J., 2012, 82, 129–147 CrossRef CAS
.
- P. S. Barber, C. S. Griggs, J. R. Bonner and R. D. Rogers, Green Chem., 2013, 15, 601–607 RSC
.
- T. Kida, S.-I. Sato, H. Yoshida, A. Teragaki and M. Akashi, Chem. Commun., 2014, 50, 14245–14248 RSC
.
-
R. M. Street, Electrospun Scaffolds for Spinal Cord Explant Cultures, Drexel University, 2018 Search PubMed
.
- B.-M. Min, S. W. Lee, J. N. Lim, Y. You, T. S. Lee, P. H. Kang and W. H. Park, Polymer, 2004, 45, 7137–7142 CrossRef CAS
.
- Y. Huang, Z. Zhong, B. Duan, L. Zhang, Z. Yang, Y. Wang and Q. Ye, J. Mater. Chem. B, 2014, 2, 3427–3432 RSC
.
- K. Zhu, H. Tu, P. Yang, C. Qiu, D. Zhang, A. Lu, L. Luo, F. Chen, X. Liu and L. Chen, Chem. Mater., 2019, 31, 2078–2087 CrossRef CAS
.
- H. Wu, G. R. Williams, J. Wu, J. Wu, S. Niu, H. Li, H. Wang and L. Zhu, Carbohydr. Polym., 2018, 180, 304–313 CrossRef CAS PubMed
.
- C. Zhong, A. Cooper, A. Kapetanovic, Z. Fang, M. Zhang and M. Rolandi, Soft Matter, 2010, 6, 5298–5301 RSC
.
- M. Rolandi and R. Rolandi, Adv. Colloid Interface Sci., 2014, 207, 216–222 CrossRef CAS PubMed
.
- B. Duan, Y. Huang, A. Lu and L. Zhang, Prog. Polym. Sci., 2018, 82, 1–33 CrossRef CAS
.
- M. A. Surati, S. Jauhari and K. Desai, Arch. Appl. Sci. Res., 2012, 4, 645–661 Search PubMed
.
- R. Fernández-Marín, F. Hernández-Ramos, A. M. Salaberria, M. Á. Andrés, J. Labidi and S. C. Fernandes, Int. J. Biol. Macromol., 2021, 186, 218–226 CrossRef PubMed
.
- C. M. Keck and R. H. Müller, Eur. J. Pharm. Biopharm., 2006, 62, 3–16 CrossRef CAS PubMed
.
- A. M. Salaberria, S. C. Fernandes, R. H. Diaz and J. Labidi, Carbohydr. Polym., 2015, 116, 286–291 CrossRef CAS PubMed
.
- W. Ye, S. Yokota, Y. Fan and T. Kondo, Cellulose, 2021, 28, 2167–2181 CrossRef CAS
.
- R. Kose and T. Kondo, Sen'i Gakkaishi, 2011, 67, 91–95 CrossRef CAS
.
- K. Ishida, S. Yokota and T. Kondo, J. Fiber Sci. Technol., 2021, 77, 203–212 CrossRef
.
- S. Ifuku, K. Yamada, M. Morimoto and H. Saimoto, J. Nanomater., 2012, 2012 DOI:10.1155/2012/645624
.
- S. Ifuku, K. Yamada, M. Morimoto and H. Saimoto, J. Nanomater., 2012, 2012, 645624 Search PubMed
.
- G. A. Baker, S. N. Baker, S. Pandey and F. V. Bright, Analyst, 2005, 130, 800–808 RSC
.
- J.-I. Kadokawa, A. Takegawa, S. Mine and K. Prasad, Carbohydr. Polym., 2011, 84, 1408–1412 CrossRef CAS
.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Chem. Rev., 2014, 114, 11060–11082 CrossRef CAS PubMed
.
- M. Sharma, C. Mukesh, D. Mondal and K. Prasad, RSC Adv., 2013, 3, 18149–18155 RSC
.
- C. Mukesh, D. Mondal, M. Sharma and K. Prasad, Carbohydr. Polym., 2014, 103, 466–471 CrossRef CAS PubMed
.
- Y. Yuan, S. Hong, H. Lian, K. Zhang and H. Liimatainen, Carbohydr. Polym., 2020, 236, 116095 CrossRef CAS PubMed
.
- S. Hong, Y. Yuan, Q. Yang, L. Chen, J. Deng, W. Chen, H. Lian, J. D. Mota-Morales and H. Liimatainen, Carbohydr. Polym., 2019, 220, 211–218 CrossRef CAS PubMed
.
- S. Hong, Y. Yuan, K. Zhang, H. Lian and H. Liimatainen, Nanomaterials, 2020, 10, 869 CrossRef CAS PubMed
.
- L. Barandiaran, B. Alonso-Lerma, A. Reifs, I. Larraza, R. Olmos-Juste, A. Fernandez-Calvo, Y. Jabalera, A. Eceiza and R. Perez-Jimenez, Commun. Mater., 2022, 3, 55 CrossRef CAS
.
- J. Lv, Y. Zhang, Y. Jin, D.-H. Oh and X. Fu, Int. J. Biol. Macromol., 2024, 254, 127662 CrossRef CAS PubMed
.
- A. M. Salaberria, R. H. Diaz, J. Labidi and S. C. Fernandes, Food Hydrocolloids, 2015, 46, 93–102 CrossRef CAS
.
- S. Shankar, J. P. Reddy, J.-W. Rhim and H.-Y. Kim, Carbohydr. Polym., 2015, 117, 468–475 CrossRef CAS PubMed
.
- H.-S. Jung, M. H. Kim and W. H. Park, ACS Biomater. Sci. Eng., 2019, 5, 1744–1752 CrossRef CAS PubMed
.
- C. Zhang, X. Zhuang, X. Li, W. Wang, B. Cheng, W. Kang, Z. Cai and M. Li, Carbohydr. Polym., 2016, 140, 195–201 CrossRef CAS PubMed
.
- H. K. Noh, S. W. Lee, J.-M. Kim, J.-E. Oh, K.-H. Kim, C.-P. Chung, S.-C. Choi, W. H. Park and B.-M. Min, Biomaterials, 2006, 27, 3934–3944 CrossRef CAS PubMed
.
- H. Zou, B. Lin, C. Xu, M. Lin and W. Zhan, Cellulose, 2018, 25, 999–1010 CrossRef CAS
.
- Y. Qin, X. Lu, N. Sun and R. D. Rogers, Green Chem., 2010, 12, 968–971 RSC
.
- N. Yang, W. Zhang, C. Ye, X. Chen and S. Ling, Biotechnol. J., 2019, 14, e1700754 CrossRef PubMed
.
- I. Muñoz, C. Rodríguez, D. Gillet and B. M. Moerschbacher, Int. J. Life Cycle Assess., 2017, 23, 1151–1160 CrossRef
.
- P. Cinelli, M. Coltelli, N. Mallegni, P. Morganti and A. Lazzeri, Chem. Eng. Trans., 2017, 60, 115–120 Search PubMed
.
- P. S. Gungor-Ozkerim, I. Inci, Y. S. Zhang, A. Khademhosseini and M. R. Dokmeci, Biomater. Sci., 2018, 6, 915–946 RSC
.
- X. Cui, J. Li, Y. Hartanto, M. Durham, J. Tang, H. Zhang, G. Hooper, K. Lim and T. Woodfield, Adv. Healthcare Mater., 2020, 9, 1901648 CrossRef CAS PubMed
.
- Y. Zhang, D. Zhou, J. Chen, X. Zhang, X. Li, W. Zhao and T. Xu, Mar. Drugs, 2019, 17, 555 CrossRef CAS PubMed
.
- B. Mahendiran, S. Muthusamy, S. Sampath, S. Jaisankar, K. C. Popat, R. Selvakumar and G. S. Krishnakumar, Int. J. Biol. Macromol., 2021, 183, 564–588 CrossRef CAS PubMed
.
- P. Karimipour-Fard, M. P. Jeffrey, H. JonesTaggart, R. Pop-Iliev and G. Rizvi, J. Mech. Behav. Biomed. Mater., 2021, 120, 104583 CrossRef CAS PubMed
.
- B. Sadhasivam, D. Ramamoorthy and R. Dhamodharan, Int. J. Biol. Macromol., 2020, 165, 3145–3155 CrossRef CAS PubMed
.
- Z. Ling, J. Zhao, S. Song, S. Xiao, P. Wang, Z. An, Z. Fu, J. Shao, Z. Zhang, W. Fu and S. Song, Regener. Biomater., 2023, 10, rbad058 CrossRef CAS PubMed
.
- R. Zhang, L. Deng, J. Guo, H. Yang, L. Zhang, X. Cao, A. Yu and B. Duan, ACS Nano, 2021, 15, 17790–17803 CrossRef CAS PubMed
.
- E. Lizundia, T.-D. Nguyen, R. J. Winnick and M. J. MacLachlan, J. Mater. Chem. C, 2021, 9, 796–817 RSC
.
- Y. Zhao, Z. Xie, H. Gu, C. Zhu and Z. Gu, Chem. Soc. Rev., 2012, 41, 3297–3317 RSC
.
- J. Hou, B. E. Aydemir and A. G. Dumanli, Philos. Trans. R. Soc., A, 2021, 379, 20200331 CrossRef CAS PubMed
.
- R. Boruah, P. Nath, D. Mohanta, G. A. Ahmed and A. Choudhury, Nanosci. Nanotechnol. Lett., 2011, 3, 458–462 CrossRef CAS
.
- Z. Mu, X. Zhao, Z. Xie, Y. Zhao, Q. Zhong, L. Bo and Z. Gu, J. Mater. Chem. B, 2013, 1, 1607–1613 RSC
.
- J. Tian, W. Zhang, J. Gu, T. Deng and D. Zhang, Nano Energy, 2015, 17, 52–62 CrossRef CAS
.
- S. Wei, Y. Li, K. Li, A. Kang, S. Zhang, T. Feng, H. Zhang and C. Zhong, Mater. Today Bio, 2022, 13, 100179 CrossRef CAS PubMed
.
- P. Liu, J. Wang, H. Qi, T. Koddenberg, D. Xu, S. Liu and K. Zhang, Nano Today, 2022, 43 DOI:10.1016/j.nantod.2022.101420
.
- T.-D. Nguyen and M. J. MacLachlan, Adv. Opt. Mater., 2019, 7, 1801275 CrossRef
.
- C. P. Barrera-Patiño, J. D. Vollet-Filho, R. G. Teixeira-Rosa, H. P. Quiroz, A. Dussan, N. M. Inada, V. S. Bagnato and R. R. Rey-González, Sci. Rep., 2020, 10, 5786 CrossRef PubMed
.
- A. Narkevicius, R. M. Parker, J. Ferrer-Orri, T. G. Parton, Z. Lu, G. T. van de Kerkhof, B. Frka-Petesic and S. Vignolini, Adv. Mater., 2022, 34, 2203300 CrossRef CAS PubMed
.
- T. Changcharoen, T. Apiphatnaphakul, W. Watjanavarreerat and K. Locharoenrat, Artif. Cells, Nanomed., Biotechnol., 2022, 50, 87–95 CrossRef CAS PubMed
.
- T. D. Nguyen, K. E. Shopsowitz and M. J. MacLachlan, Chemistry, 2013, 19, 15148–15154 CrossRef CAS PubMed
.
- T.-D. Nguyen and M. J. MacLachlan, Adv. Opt. Mater., 2014, 2, 1031–1037 CrossRef CAS
.
- C. Mille, E. C. Tyrode and R. W. Corkery, RSC Adv., 2013, 3, 3109–3117 RSC
.
- I. Zada, W. Zhang, Y. Li, P. Sun, N. Cai, J. Gu, Q. Liu, H. Su and D. Zhang, Appl. Phys. Lett., 2016, 109 DOI:10.1063/1.4962903
.
- S. Ngasotter, L. Sampath and K. A. M. Xavier, Carbohydr. Polym., 2022, 291, 119627 CrossRef CAS PubMed
.
- B. Yousuf, K. Gul, A. A. Wani and P. Singh, Crit. Rev. Food Sci. Nutr., 2016, 56, 2223–2230 CrossRef CAS PubMed
.
- N. Bhargava, V. S. Sharanagat, R. S. Mor and K. Kumar, Trends Food Sci. Technol., 2020, 105, 385–401 CrossRef CAS
.
- Y. Zheng, X. Li, Y. Huang, H. Li, L. Chen and X. Liu, Food Hydrocolloids, 2022, 127 DOI:10.1016/j.foodhyd.2022.107517
.
- M. Chen, T. Yan, J. Huang, Y. Zhou and Y. Hu, Int. J. Biol. Macromol., 2021, 179, 90–100 CrossRef CAS PubMed
.
- M. A. Sani, M. Tavassoli, H. Hamishehkar and D. J. McClements, Carbohydr. Polym., 2021, 255, 117488 CrossRef CAS PubMed
.
- Y.-N. Yang, K.-Y. Lu, P. Wang, Y.-C. Ho, M.-L. Tsai and F.-L. Mi, Carbohydr. Polym., 2020, 228, 115370 CrossRef PubMed
.
- G. Cabrera-Barjas, N. Radovanovic, G. B. Arrepol, A. F. de la Torre, O. Valdes and A. Nesic, Int. J. Biol. Macromol., 2021, 186, 92–99 CrossRef CAS PubMed
.
- R. Fernández-Marín, S. C. M. Fernandes, M. Á. A. Sánchez and J. Labidi, Food Hydrocolloids, 2022, 123 DOI:10.1016/j.foodhyd.2021.107119
.
- M. Duan, S. Yu, J. Sun, H. Jiang, J. Zhao, C. Tong, Y. Hu, J. Pang and C. Wu, Int. J. Biol. Macromol., 2021, 187, 332–340 CrossRef CAS PubMed
.
- C. Wu, H. Jiang, J. Zhao, M. Humayun, S. Wu, C. Wang, Z. Zhi and J. Pang, Food Hydrocolloids, 2022, 133 DOI:10.1016/j.foodhyd.2022.107998
.
- S. Lee, L. T. Hao, J. Park, D. X. Oh and D. S. Hwang, Adv. Mater., 2023, 35, 2203325 CrossRef CAS PubMed
.
- S. Ngasotter, L. Sampath and K. M. Xavier, Carbohydr. Polym., 2022, 119627 CrossRef CAS PubMed
.
- F. A. Yihun, Emergent Mater., 2022, 1–30 Search PubMed
.
- T. Jin, T. Liu, E. Lam and A. Moores, Nanoscale Horiz., 2021, 6, 505–542 RSC
.
- V. G. Matveeva and L. M. Bronstein, Prog. Mater. Sci., 2022, 100999 CrossRef CAS
.
- M. Dohendou, K. Pakzad, Z. Nezafat, M. Nasrollahzadeh and M. G. Dekamin, Int. J. Biol. Macromol., 2021, 192, 771–819 CrossRef CAS PubMed
.
-
N. Reddy, Y. Yang, N. Reddy and Y. Yang, Innovative Biofibers from Renewable Resources, 2015, pp. 449–450 Search PubMed
.
- P. T. A. Le, T. P. Vu, H. T. Le, D. Van Phan, C. X. Nguyen, T. D. Luong, N. T. T. Dang and T. D. Nguyen, J. Electron. Mater., 2020, 49, 3791–3803 CrossRef CAS
.
- T. Jin, M. Hicks, D. Kurdyla, S. Hrapovic, E. Lam and A. Moores, Beilstein J. Org. Chem., 2020, 16, 2477–2483 CrossRef CAS PubMed
.
- Z. Wang, P. Li, Y. Fang, L. Yan, W. Zhou, X. Fan and H. Liu, Carbohydr. Polym., 2019, 223, 115064 CrossRef CAS PubMed
.
- P. Das, T. Heuser, A. Wolf, B. Zhu, D. E. Demco, S. Ifuku and A. Walther, Biomacromolecules, 2012, 13, 4205–4212 CrossRef CAS PubMed
.
- Y. Wang, Q. Kong, B. Ding, Y. Chen, X. Yan, S. Wang, F. Chen, J. You and C. Li, J. Colloid Interface Sci., 2017, 505, 220–229 CrossRef CAS PubMed
.
- X. Lin, A. Yang, G. Huang, X. Zhou, Y. Zhai, X. Chen and E. McBean, Water, 2019, 11 DOI:10.3390/w11020310
.
- Y. Wang, Y. Pei, W. Xiong, T. Liu, J. Li, S. Liu and B. Li, Int. J. Biol. Macromol., 2015, 81, 477–482 CrossRef CAS PubMed
.
- B. He, M. Feng, X. Chen, D. Zhao and J. Sun, Appl. Surf. Sci., 2020, 527 DOI:10.1016/j.apsusc.2020.146737
.
- W. Wang, M. N. Nadagouda and S. M. Mukhopadhyay, Nanomaterials, 2022, 12, 3593 CrossRef CAS PubMed
.
- K. Mao, X. Wu, X. Min, Z. Huang, Y. G. Liu and M. Fang, Sci. Rep., 2019, 9, 16321 CrossRef PubMed
.
- S. Khaoulani, H. Chaker, C. Cadet, E. Bychkov, L. Cherif, A. Bengueddach and S. Fourmentin, C. R. Chim., 2015, 18, 23–31 CrossRef CAS
.
- X. Di, F. Guo, Z. Zhu, Z. Xu, Z. Qian and Q. Zhang, RSC Adv., 2019, 9, 41209–41217 RSC
.
- P. T. A. Le, T. P. Vu, H. T. Le, D. Van Phan, C. X. Nguyen, T. D. Luong, N. T. T. Dang and T. D. Nguyen, J. Electron. Mater., 2020, 49, 3791–3803 CrossRef CAS
.
- B. Thangaraj and P. R. Solomon, ChemBioEng Rev., 2019, 6, 167–194 CrossRef CAS
.
- M. L. Verma, S. Kumar, A. Das, J. S. Randhawa and M. Chamundeeswari, Environ. Chem. Lett., 2020, 18, 315–323 CrossRef CAS
.
- B. R. Facin, M. S. Melchiors, A. Valério, J. V. Oliveira and D. d. Oliveira, Ind. Eng. Chem. Res., 2019, 58, 5358–5378 CrossRef CAS
.
- M. Bilal, C. D. Fernandes, T. Mehmood, F. Nadeem, Q. Tabassam and L. F. R. Ferreira, Int. J. Biol. Macromol., 2021, 175, 108–122 CrossRef CAS PubMed
.
- W. Suginta, P. Khunkaewla and A. Schulte, Chem. Rev., 2013, 113, 5458–5479 CrossRef CAS PubMed
.
- T. Machałowski, K. Jankowska, K. Bachosz, W. Smułek, H. Ehrlich, E. Kaczorek, J. Zdarta and T. Jesionowski, Molecules, 2022, 27, 1354 CrossRef PubMed
.
- W. A. Mehdi, A. A. Mehde, M. Ozacar and Z. Ozacar, Int. J. Biol. Macromol., 2018, 117, 947–958 CrossRef CAS PubMed
.
- F. M. A. Manan, N. Attan, Z. Zakaria, A. S. A. Keyon and R. A. Wahab, Enzyme Microb. Technol., 2018, 108, 42–52 CrossRef PubMed
.
- Y. Tsutsumi, H. Koga, Z. D. Qi, T. Saito and A. Isogai, Biomacromolecules, 2014, 15, 4314–4319 CrossRef CAS PubMed
.
- Hernawan, B. Purwono, Triyono and M. Hanafi, J. Taiwan Inst. Chem. Eng., 2022, 134 DOI:10.1016/j.jtice.2022.104354
.
- T. Jose, N. Sudheesh and R. S. Shukla, J. Mol. Catal. A: Chem., 2010, 333, 158–166 CrossRef CAS
.
- B. Aghabarari, J. Renewable Energy Environ., 2016, 3, 57–62 Search PubMed
.
- S. R. Al-Abed, P. X. Pinto, J. McKernan, E. Feld-Cook and S. M. Lomnicki, Chem. Eng. J., 2017, 323, 270–277 CrossRef CAS PubMed
.
- Z. DiLoreto, P. Weber, W. Olds, J. Pope, D. Trumm, S. Chaganti, D. Heath and C. Weisener, J. Environ. Manage., 2016, 183, 601–612 CrossRef CAS PubMed
.
- E. Vlakh, E. Ponomareva and T. Tennikova, Appl. Biochem. Microbiol., 2014, 50, 441–446 CrossRef CAS
.
- D. Yu, Y. Hao, S. Han, S. Zhao, Q. Zhou, C.-H. Kuo, F. Hu, L. Li, H.-Y. Chen, J. Ren and S. Peng, ACS Nano, 2023, 17, 1701–1712 CrossRef CAS PubMed
.
- Y. Hao, S.-F. Hung, W.-J. Zeng, Y. Wang, C. Zhang, C.-H. Kuo, L. Wang, S. Zhao, Y. Zhang, H.-Y. Chen and S. Peng, J. Am. Chem. Soc., 2023, 145, 23659–23669 CrossRef CAS PubMed
.
- H. Huang, A. Huang, D. Liu, W. Han, C.-H. Kuo, H.-Y. Chen, L. Li, H. Pan and S. Peng, Adv. Mater., 2023, 35, 2303109 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2024 |