Operando characterization technique innovations in single-atom catalyst-derived electrochemical CO2 conversion
Syed Asim
Ali
ab,
Iqra
Sadiq
a and
Tokeer
Ahmad
 *a
*a
aNanochemistry Laboratory, Department of Chemistry, Jamia Millia Islamia, New Delhi 110025, India. E-mail: tahmad3@jmi.ac.in; Tel: +91-11-26981717 ext. 3261
bDepartment of Inorganic and Organic Chemistry, Inorganic Chemistry Section, University of Barcelona, Carrer de Martí iFranquès, 1-11, 08028 Barcelona, Spain
First published on 6th May 2025
Abstract
Single-atom catalysts (SACs) are the perfect epitome of cutting-edge innovation in catalysis, offering distinct active sites in the form of isolated, individual atoms. SACs amalgamate the advantages of homogeneous and heterogeneous catalysis, in turn enhancing the activity, selectivity, and stability during catalytic processes. The latest progressions in in situ/operando characterization techniques have simplified the understanding of the catalyst heterogeneity and structure sensitivity of SACs by enabling the real-time observation of SACs under a coexistent working environment to provide insights into their structure and catalytic efficiency. Operando techniques are the backbone for investigating the stability and activity of SACs during energy conversions. Techniques such as operando X-ray absorption spectroscopy (XAS), polarization-modulation infrared reflection absorption spectroscopy (PM-IRAS), and near-ambient-pressure X-ray photoelectron spectroscopy (NAP-XPS) have been employed to study SACs under different reaction conditions. Furthermore, in situ visualization microscopy techniques have made notable progress in the imaging of ongoing catalytic reactions on SACs and revealed enigmatic effects such as facet-resolved catalytic ignition and anisotropic surface oxidation. Therefore, in this review, we have compiled the developments in the significant operando characterization techniques for SAC-derived electrochemical carbon dioxide reduction reaction (eCO2RR).
Introduction
The last decade has witnessed an unprecedented pace in heterogeneous catalysis with the advent of a pioneering class of materials, notably transition metal carbonitrides (MXenes) and SACs. The research findings of MXenes are relatively more pronounced and significant knowledge of these two-dimensional (2D) materials has been harvested as compared to SACs.1,2 However, the fabrication of state-of-the-art MXene/MBene-based materials and their upscaling require a comprehensive mechanistic knowledge of the optimized synthetic protocols, etching/exfoliating parameters, and participatory intercalation/delamination steps.3 Conversely, the progress of SACs is incongruent with that of MXenes and is still in its embryonic stage. SACs have garnered considerable attention from researchers supported by the advancements in methodological developments, characterization tools, and machine learning. In contrast to homogeneous catalysis, wherein nearly one hundred percent metal atom harnessing can be achieved subject to the reaction conditions, heterogeneous catalysis has its limitations due to the aggregation of catalytically active sites, which in turn obscures the active centres. Catalytically active surface sites, such as apices and edge/corner/step-sites, of the catalyst surface only account for nearly 20 mol% of the available mass.4,5 Therefore, designing SACs externalizes the enduring objective of researchers to reach higher atom utilization efficiency. Reaching nearly unity atomic efficiency can bring about the predominance of homogeneous as well as heterogeneous catalysis. Nevertheless, the facile synthesis of SACs is hampered by the non-achievement of the enhanced single-atom loading on account of the Gibbs-Thomson limit, which highlights the challenges of reaching higher than 3 mol% loading.6The term SAC was first coined by Zhang and coworkers,7 manifesting the reasonably higher atom efficiency of single Pt atoms dispersed over FeOx, resulting in facilitated CO oxidation. The emergence of SAC-derived heterogeneous catalysis is eventuated due to the latest innovations in operando characterization techniques and robust computational modelling.8,9 The amalgamation of these two indispensable tools allows precise insights into the atoms' dispersion, structural evolution, and precise location during in situ dynamics. There are several well-known chemical transformations in which dispersed metal atoms are present over a supporting material, such as olefin chemisorption or metallic complexes over porous materials such as zeolite.10 Therefore, several studies in the past hinted at the superiority of isolated atomic sites in catalysis over a molecular catalyst ensemble of different molecules.11 However, SACs specifically differ from these earlier reactions and have no interactions with small organic groups or ligands.12 The synthesis of SACs via the metal coordination route has been realized very recently, thus paving the way for unconventional methodologies for the scalable synthesis of SACs.6 For instance, Duan et al.6 reported an ultrahigh metal loading as high as ∼16 mol% over a C3N4 support in a facile synthesis through one-pot metal coordination. The group exploited the accelerated complexation of ligand molecules/metal ions and their eventual in situ polymerization to form ultrahigh Cu-loaded SACs for CO2 photoconversion into formic acid. The scalable applicability of SACs is repeatedly questioned, ascribed to their susceptibility to aggregation before the activation and reaction steps. An ever-growing amount of research on SACs suggests the superior stability of metal atoms bonded to the support material through covalent bonding, in contrast to the nanoparticle analogue.13,14 The thorough examination of SACs in discrete environmental conditions via in situ techniques is significant for understanding their dynamics, and it led to the origination of the field of “dynamic single-atom catalysis” that focuses on the dynamic conversion of nanoparticles into SACs during catalytic transformations.15,16 Computational modelling has played an important role in understanding the dynamics in SACs. The agglomeration of Pt SACs into nanoclusters has been observed upon CO exposure, and their subsequent conversion into SACs has been documented after the removal of CO molecules.17 The stable (111) facets of Cu-SAC have been proved to undergo dynamism by etching and subsequent breaking into nanoparticles attributed to their interaction with CO molecules.18 These reports corroborate the effect of CO adsorption in the dynamics of the agglomeration of SACs into nanoparticles and necessitate the utilization of in situ and ex situ characterizations of SACs to determine their role during heterogeneous catalysis, specifically in CO2RR.
Low-metal-loaded catalysts dispersed on a supporting material have fascinated researchers for the past few decades. However, the lack of in situ/operando characterization tools limited the comprehensive understanding at that time and one could not probe the distinction between single-atom and cluster sites. Through the innovations in spectroscopic tools, it has now been well substantiated that decorating metal atoms on a supporting material efficiently tunes their catalytic performance in a certain way.19,20In situ operando techniques have been instrumental in advancing the understanding of catalytic processes involving SACs, offering valuable insights into their physicochemical characteristics and dynamic phase transitions. The development of a methodological framework for investigating the structural and electronic properties of SACs has ensured their efficient real-time monitoring.
Early reports have underscored the utilization of XAS to investigate the ability of SACs in heterogeneous catalysis.21 Researchers have exploited the extended X-ray absorption fine structure (EXAFS) technique to probe dispersed Pt atoms for enhanced propane combustion. This benchmark set the tone for successive studies by employing acid washing to preserve SACs in the catalytic system.22,23 However, the mechanistic scheme during catalytic reactions is vague, and researchers are stepping forward to examine the atomic lattice at better resolution via different characterization techniques. For instance, environmental transmission electron microscopy (ETEM), which aids in visualizing reaction steps, has been upgraded to environmental scanning TEM (ESTEM) to provide sub-angstrom resolution with precise analysis of SACs. ESTEM is an important operando tool for the real-time analysis of single atoms deposited over light support materials at higher temperature ranges.24,25Operando analysis provides significant insights into the different intermediate species formed during the SAC-derived CO2RR mechanism.
In situ visualization of SACs
In recent years, several operando techniques have been developed to elucidate the dynamics in SACs, probe their stability against aggregation, and determine the reaction mechanism of SAC-derived energy conversion processes.26,27In situ visualization of SACs plays a fundamental role in comprehending their structure, stability, and catalytic mechanisms under actual working conditions. Given the atomic-scale dispersion of active sites in SACs, advanced imaging and spectroscopy techniques are crucial to visualize and characterize them at the atomic level. For instance, aberration-corrected TEM, ETEM, and scanning transmission electron microscopy (STEM) have played a vital role in the visualization of SAC dynamics. For determining the active sites of SACs in heterogeneous catalysis, operando imaging is an important tool that elucidates the mechanism of particular transformations. In recent years, electron microscopic techniques have improved tremendously, thus enabling researchers to demonstrate the rapid reactions attributed to the accessibility of direct electron detectors and improvement in the image-taking technologies to capture more frames per second.26 Researchers have exploited in situ imaging techniques to analyse the dispersion of SACs over support materials. For instance, Wei et al.28 probed the thermal transformation of noble metal nanoparticles (NPs) to their corresponding SACs on a metal–organic framework (MOF)-based support material. The group successfully observed the accumulation of noble metal NPs during thermal heating (1000 °C) on account of the rearrangement of the NPs to lower the overall free energy. In situ visualization showcased the thermal mobility and high-impact collisions of the bigger NPs with the support material, facilitating Pd NPs' atomization. Based on these results, the authors corroborated the essential requirement of high-angle annular dark-field (HAADF)-STEM visualization to capture the collision between metal NPs and the interior of the support material and the different intermediary steps as TEM analysis was not able to prove the emergence of Pd single atoms owing to its phase contrast shortcomings. HAADF-STEM is instrumental in obtaining Z-contrast images in which the intensity of every atomic column is in proportion to its atomic number. Therefore, with this technique, direct imaging of monodispersed or aggregated single atoms on a support material is possible, allowing one to distinguish SACs from NPs. Therefore, this report highlighted the significance of HAADF-STEM in proving the formation of SACs and the conversion of NPs to SACs based on the heating profile. Recently, Wang et al.29 developed a unique negative pressure annealing (NPA) methodology to prepare exceptionally high-metal-loaded SAC@gC3N4 systems for a wide range of metals. Conventional fabrication routes usually struggle to attain metal loading above 10 wt% due to challenges like metal aggregation and high entropy of single atoms. In contrast, this study introduces an NPA strategy that successfully fabricates SACs with metal loadings ranging from 27.3 to 44.8 wt% over a gC3N4 array. For probing ultrahigh metal loading, the group exploited the operando aberration-corrected HAADF-STEM (AC-STEM) technique to determine the significant role of NPA in the removal rate of anionic species present in the metal sources and in boosting the interaction of single atoms with N-defect sites. The latter process underscores the strong bonding between the single atoms and the N-rich gC3N4 matrix. The group ascertained that increasing the Pt loading (41.8 wt%) over gC3N4 led to enhanced catalytic efficiency toward propane oxidation to yield higher carbon products. AC-STEM analysis was carried out to demonstrate the local environment of Pt single atoms as illustrated in Fig. 1(a) and (b). The decoration of monodispersed Pt atoms is represented by the bright spots in the dark background of gC3N4. Furthermore, the authors also examined the temperature-dependent AC-STEM images of ultrahigh Pt-loaded gC3N4 in vacuum and Ar conditions to elucidate the structural evolution of Pt precursor during the annealing operation as depicted in Fig. 1(c) and (d). Densely dispersed bright spots were evident at low temperatures, signifying the homogeneously distributed Pt source. Furthermore, cluster/particle formation was not observed even on increasing the temperature and the Pt SACs were not observed as revealed in Fig. 1(c). However, negative annealing under Ar flow resulted in the formation of Pt single atoms on increasing the temperature and the Pt atoms were well scattered at 400 °C as shown in Fig. 1(d). Therefore, the combined results of in situ analysis revealed the high metal loading of SACs over gC3N4. However, the NPA methodology should be tested for different support matrices such as TiO2 or MOF materials to shed light on the bonding between single atoms and defective-coordinated sites of a support material. It has been observed that annealing at ambient pressure gives rise to unwanted cluster formation of single atoms. Therefore, to tune the aggregation of single atoms via pressure lowering, Al-Hilfi et al.30 introduced a viable methodology to design high-density SACs for scalable production by manipulating metal diffusion during a pyrolysis process through pressure control. The group demonstrated the role of pressure reduction in enhancing metal diffusion, thus reducing the aggregation of atoms during pyrolysis.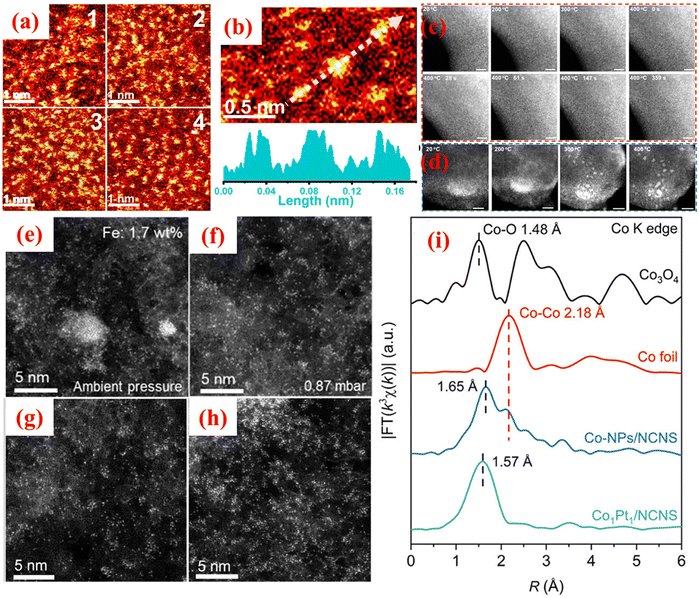 | ||
| Fig. 1 AC-STEM images of Pt@gC3N4 at (a) 1 nm scale and (b) 0.5 nm scale. Temperature-dependent operando AC-STEM images of (c) Pt SACs@gC3N4 and (d) Pt NPs@gC3N4. Reproduced with permission from ref. 29. Copyright 2024, Springer Nature. Morphological variations of Fe single atoms on N-graphitic carbon substrate (Fe-NC) at (e) ambient pressure and (f) 0.87 mbar. AC-HAADF-STEM images of (g) Fe0.1-NC0.87 and (h) Fe0.2-NC0.87. Reproduced with permission from ref. 30. Copyright 2024, American Chemical Society. (i) EXAFS of CoPt single atoms, Co-NPs, Co-foil, and Co3O4 at Co K edge. Reproduced with permission from ref. 31. Copyright 2021, Springer Nature. | ||
In theory, operating at high-vacuum or low-pressure conditions reduces the collision rate during the interaction of single atoms with the support material. This idea nurtured the exploitation of pressure-controlling by the group to enhance the loading of SACs on N-graphitic carbon substrate. Fig. 1(e) and (f) clearly distinguishes between the morphology of Fe single atoms at ambient and low-pressure conditions. The group exploited HAADF-STEM images to determine the role of pressure reduction in ultrahigh Fe loading and varied the pressure from 1.4 to 0.14 mbar. As shown in Fig. 1(g) and (h), significant Fe atom decoration can be seen at the optimized 0.87 mbar pressure conditions. The same experiments were repeated for different noble and non-precious metals to determine the effect of pressure-controlled metal diffusion. This study steps forward toward the scalable production and broader application of dense SACs in heterogeneous catalysis.
X-ray absorption spectroscopy (XAS)
The XAS technique has emerged as an element-definite approach to specifying the elemental properties of a wide range of advanced materials.19 As SACs consist of transition metals, in situ XAS can detect the elemental-specific properties at very low concentrations under actual electrochemical or photochemical CO2RR conditions. XAS's sensitivity to elements facilitates its application in determining the oxidation states and localized electronic properties of single metal atoms to elucidate the active centres during CO2 reduction. For adequately monitoring SACs during CO2RR, the XAS technique is a crucial method that can identify variations in oxidation states/coordination environments during the reaction process and provides better insights into product selectivity through the correlation of spectral features. XAS allows the precise analysis of active sites in SACs without interference from the support material, offering detailed information on the local atomic environment, including bond lengths and coordination numbers.5 XAS works excellently with non-crystalline/highly dispersed SACs where conventional crystallographic techniques such as X-ray diffraction fail. It permits the real-time monitoring of SAC behaviour during specific catalytic transformations and can be performed under certain reaction conditions concerning temperature, pressure, and gas environment. XAS complements other in situ characterization methods to comprehensively understand SAC properties. However, several challenges limit the large-scale applicability of XAS for SAC investigations. For instance, high-quality XAS measurements typically require expensive synchrotron radiation sources, and they provide an ensemble average of the atomic environment, which can obscure subtle differences in heterogeneous SAC systems.32 Prolonged exposure to hard X-rays can cause radiation-induced changes in SACs. EXAFS and X-ray absorption near edge structure (XANES) are subsets of XAS. These techniques are extremely important to confirm the monodisperse nature of SACs. As compared to EXAFS, XANES provides more consolidating evidence regarding the environment, distortion and electronic structure of single atoms due to its relatively superior signal-to-noise ratio, thus resulting in its suitability for in situ investigations.33 On the other hand, EXAFS findings are frequently affected by the lower metal loading or signal attenuations from the surroundings. Despite the extensive utilization of the XANES technique for operando analysis, its complicated fitting processes and time-intensiveness hinder its use in quantitative SAC characterization. Nevertheless, recent advancements in computational modelling taking into consideration XANES data have improved its feasibility for in situ studies.34,35 The basic distinction between XANES and EXAFS is that the former determines the electronic states of single atoms and emerges through the excitation of core electrons to the valence and conduction bands. In contrast, the latter is based on scattering effects between photoinduced electron carriers with the surrounding single atoms, and thus is useful for determining 3D geometry. The findings from XAS measurements have sharpened the understanding of SACs as corroborated by recent reports.36 For instance, Li et al.31 presented an innovative strategy for synthesizing Co/Fe/Ni-based single atoms with a pyrrolic-N4 coordination structure. This unique methodology utilized Pt atoms to catalyze the formation of Co/Fe/Ni-SACs coordinated with four pyrrolic N atoms, forming a stable pyrrolic-N4 structure as confirmed by XAS observations. The authors used the XANES simulations and EXAFS investigations to probe the M-coordinated pyrrolic-N4 geometry. The formation of Co single atoms was confirmed by EXAFS analysis which revealed the peak ascribed to the first shell of the Co-coordinated site with the heteroatom at 1.57 Å, thus implying the existence of Co-SACs as shown in Fig. 1(i). Moreover, the absence of a Co–Co peak bolstered the evidence for the formation of Co-SACs over NPs. In addition, to demonstrate the local surrounding structure of as-prepared SACs, the authors utilized XANES analysis that underscored the agreement with the XPS results, indicating the +2 oxidation state for Co single atoms. A schematic representation of the XAS characterisation setup in transmission, fluorescence, and total electron modes can be seen in Fig. 2(a)–(c). Fig. 3(a) illustrates the significant change in the XANES spectra of Co-SACs compared to Co foil and Co3O4 reference. This study provides an effective strategy for preparing pyrrolic-N4-type SACs for energy conversion applications, especially for HER/OER processes.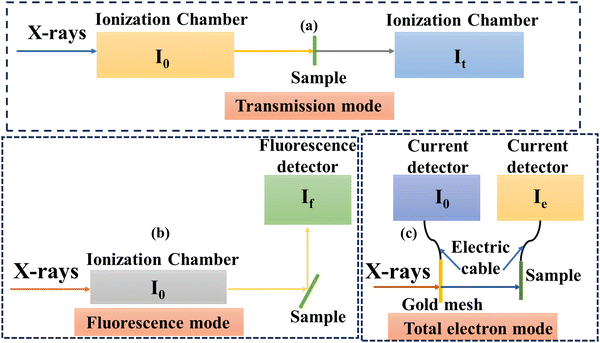 | ||
| Fig. 2 Schematic representation of XAS characterisation setup in (a) transmission, (b) fluorescence and (c) total electron modes. | ||
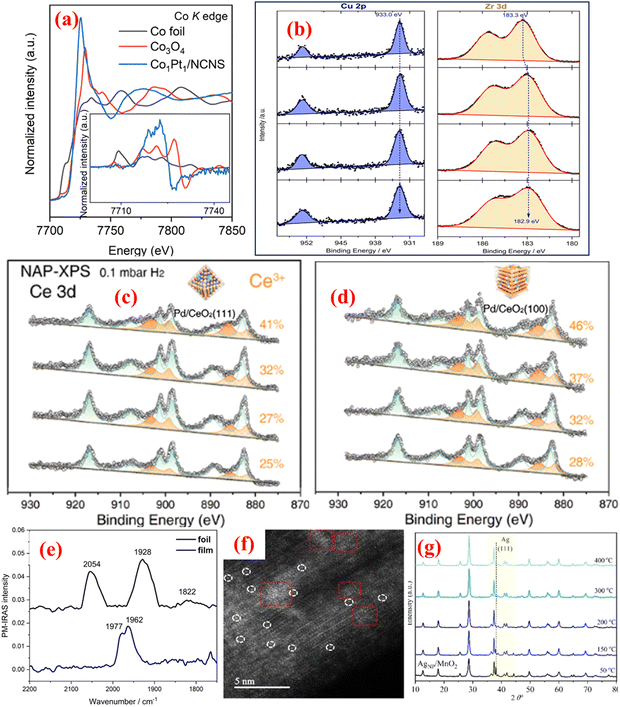 | ||
| Fig. 3 (a) XANES spectra and first derivative curves (inset) of Co1Pt1 single atoms and reference samples. Reproduced with permission from ref. 31. Copyright 2021, Springer Nature. (b) NAP-XPS of Cu 2p and Zr 3d at 250 °C and 2 mbar in different reaction conditions. Reproduced with permission from ref. 37. Copyright 2024, Royal Society of Chemistry. NAP-XPS of Ce 3d in (c) Pd/CeO2(111) and (d) Pd/CeO2(100). Reproduced with permission from ref. 38. Copyright 2022, John Wiley and Sons. (e) PM-IRAS spectroscopy of CO adsorption over a Pd LCC catalyst. Reproduced with permission from ref. 39. Copyright 2024, Springer Nature. (f) HAADF-STEM image of AgNP/MnO2-350. Isolated Ag atoms are circled in white, Ag clusters in red rectangles. (g) In situ XRD patterns of AgNP/MnO2 at distinct temperatures. Reproduced with permission from ref. 40. Copyright 2021, John Wiley and Sons. | ||
Near-ambient-pressure X-ray photoelectron spectroscopy (NAP-XPS)
NAP-XPS is an advanced variation of XPS that allows surface chemical analysis of SACs under near-realistic conditions, including gases, liquids, and elevated pressures. In contrast to traditional XPS that functions at ultrahigh-vacuum conditions, NAP-XPS can reach a pressure limit of up to a few tens of mbar, thus making it an important technique for in situ/operando studies and real-time reaction monitoring. NAP-XPS is capable of differentiating single-atom dispersions from NPs by investigating metal coordination and electronic states.41 Muravev et al.42 fabricated Pd single atoms decorated on CeO2 for CO oxidation investigations via an impregnation/spray pyrolysis methodology. The authors utilized in situ NAP-XPS to underscore the enhanced stability of Pd-SACs through significant Pd–CeO2 bonding. The NAP-XPS studies provided the surface electronic properties of Pd–CeO2via bonding elucidation through the 3d core-level spectrum of Pd. Two different Pd electronic states were observed at 50 °C during the CO oxidation process, showing the main peak of Pd–Ox–Ce type of bonding, whereas the low-intensity peak was ascribed to the minimal PdOx aggregation. These results corroborate the higher stability of Pd–CeO2 even at high temperature (300 °C) due to the peak retention. Impeng et al.37 constructed Cu single atoms on a UiO-66 MOF support and explored its catalytic efficiency toward preferential oxidation of CO in the presence of H2. In situ characterization techniques such as XANES and NAP-XPS revealed that increasing the temperature led to a higher proportion of Cu1+ single atoms and partial reduction of ZrOx nodes. NAP-XPS spectra of core line Cu 2p and Zr 3d at 250 °C are illustrated in Fig. 3(b), indicating the characteristic peaks of Cu1+ and Zr4+ from the Cu@UiO-66 system. This report elucidates the intricate balance between reaction temperature, oxidation states of Cu, and adsorption behaviours that govern the selectivity and efficiency of Cu@UiO-66 catalysts in preferential CO oxidation. Hu et al.38 investigated the role of different crystal facets of CeO2 in the dispersion of Pd single atoms and in turn in the catalytic efficiency of the Pd–CeO2 system N-alkylation reaction. The group exploited NAP-XPS to elucidate the interaction between Pd single atoms and CeO2 facets. The NAP-XPS analysis revealed that, under a reducing atmosphere, the CeO2(100) facet generated more oxygen vacancies compared to the CeO2(111) facet. These additional vacancies on the (100) facet effectively trapped Pd atoms, therefore promoting their atomic dispersion and preventing cluster formation. In contrast, on the (111) facet, Pd atoms were more prone to aggregation due to fewer available oxygen vacancies. This facet-dependent behaviour was directly observed through NAP-XPS (Fig. 3(c) and (d)), providing insights into the stabilization mechanisms of Pd on the CeO2 support. On increasing the temperature, the Ce3+ ratio in Pd–CeO2 increased from 25 to 41% for the (111) facet whereas for the (100) facet, the ratio increased from 28 to 46%.Polarization-modulation infrared reflection absorption spectroscopy (PM-IRAS)
PM-IRAS is an important in situ technique for studying the surface chemistry, adsorption behaviour, and reaction mechanisms of SACs during heterogeneous catalysis. SACs feature higher atomic dispersion, so they exhibit more exposed active sites than other catalytic systems. PM-IRAS plays a crucial role in identifying molecular interactions and catalytic transformations at metal–support interfaces.43 SACs offer anomalous electronic structure and coordination geometry attributed to their isolated active sites. Conventional infrared techniques struggle to effectively elucidate these sites due to low signal intensities observed during the analysis. In contrast, PM-IRAS delivers enhanced surface sensitivity, which is significant for detecting molecular adsorption over SACs. The selective polarization modulation in PM-IRAS assists in distinguishing the surface-adsorbed species from the background signals. PM-IRAS is a remarkable technique that differentiates between linear CO (M–CO) and bridge-bonded CO (M2–CO), confirming atomic dispersion. PM-IRAS is useful in monitoring the formation of key intermediates (e.g., carbonate species) during oxidation processes and in understanding the stabilization mechanisms of SACs on different support matrices. Li et al.39 presented an innovative approach to designing a Pd catalyst for achieving high efficiency and selectivity in the semi-hydrogenation of acetylene to ethylene. The group proposed a unique, laterally condensed catalyst (LCC) in which a Pd layer is deposited over a specially designed SiO2 buffer layer. PM-IRAS analysis revealed the CO adsorption over the Pd LCC catalyst to be different from that over Pd foil (Fig. 3(e)). The spectra of Pd foil revealed the variation in the CO vibration, suggesting the strong CO binding on electron-rich Pd sites. In contrast, the Pd-based LCC showcased a single bridge-bonded CO vibration, implying the presence of defect sites on which CO adsorption had taken place.There has been substantial development in in situ techniques to comprehend SAC-derived CO2RR processes; however, several bottlenecks limit a comprehensive understanding of reaction mechanisms due to various factors. For instance, most in situ methods, such as XAS and Raman spectroscopy, do not provide adequate spatial resolution required for the precise resolution of single-atom sites, especially when embedded within an intricate support system. Furthermore, in situ XAS or XPS examine the bulk region properties that do not truly reveal the actual surface sites. This hinders their capability to reveal detailed mechanisms related to interfacial charge transfer and the adsorption of reaction intermediates, which are critical processes governing the CO2RR process. Nevertheless, in situ techniques have gradually become essential approaches for deciphering complex catalytic transformations and their concerted reaction mechanisms, particularly in energy conversion applications. Their ability to provide real-time, spatially resolved, and dynamic details of reaction pathways aligns well with the transition toward the optimization of materials for desired applications. Monitoring the variations in the physicochemical properties of active single atoms under operational conditions will allow researchers to optimize catalytic systems for large-scale energy conversion applications. A comparative overview categorizing different in situ characterization techniques used for SAC-derived CO2RR applications can be seen in Table 1.
| In situ characterization technique | Functionality in the CO2RR application |
|---|---|
| Aberration-corrected transmission electron microscopy (AC-TEM) | • AC-TEM enables direct visualization of single atoms, defects, and heteroatom dopants on a support material. |
| • Highly efficient to confirm the dispersion of SACs for structure–activity correlations in CO2RR. | |
| Environmental transmission electron microscopy (ETEM) | • ETEM allows for real-time observation of changes in SACs and nanoparticle structures, including surface reconstruction and atom movement during CO2RR. |
| • Highly efficient in investigating the surface interaction of SACs with CO2/CO and other reactants, providing valuable insight into adsorption, activation, and product formation during CO2RR. | |
| Scanning transmission electron microscopy (STEM) | • A highly effective technique for the study of CO2RR that provides atomic-scale resolution for both structural imaging and elemental analysis. |
| • By integrating methods such as energy-dispersive X-ray spectroscopy (EDS) and electron energy loss spectroscopy (EELS), STEM provides a detailed analysis of local chemical environments and elemental distributions in SACs. This facilitates the correlation between their structural characteristics, catalytic activity, and stability during CO2RR. | |
| Aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (AC-HAADF-STEM) | This method provides atomic-resolution imaging with Z-contrast sensitivity, making it instrumental for identifying isolated single atoms dispersed on light support materials. |
| X-ray absorption spectroscopy (XAS) | XAS is effective for analyzing atomically dispersed single metal atoms due to its element-specific sensitivity and capability to investigate local electronic and structural environments. |
| Near-ambient-pressure X-ray photoelectron spectroscopy (NAP-XPS) | Unlike XPS, which requires an ultra-high vacuum, NAP-XPS enables surface-sensitive analysis under reactive gases (CO2, H2O) at near-operational pressures. This is useful for SACs, as the metal atoms’ oxidation state and coordination can dynamically change during CO2RR. |
| Polarization-modulation infrared reflection absorption spectroscopy (PM-IRAS) | PM-IRAS can be employed to track the formation of reaction intermediates on SACs during CO2RR, showing a potential-dependent shift in the main reaction pathway and supporting the formation of products in working conditions. |
| In situ Raman spectroscopy | This provides molecular-level vibrational fingerprints and enables direct visualization of adsorbed intermediates such as *CO, *COOH, *HCOO, and carbonate species, which are crucial in determining product pathways and selectivity. |
| In situ FTIR spectroscopy | A powerful tool for investigating SAC-derived CO2RR under real-time conditions into surface intermediates and reaction pathways under operating conditions. When integrated with electrochemical measurements, it facilitates the evaluation of intermediate adsorption on SACs at various potentials. |
Single-atom catalyst-derived energy conversion applications
Anthropic actions related to technological development, economic advances, growth in population, and climatic conditions lead to a continuing increase in energy consumption. The renewable carbon cycle based on electrocatalysis is a favourable approach to concurrently alleviate the energy crisis and the greenhouse effect. However, the efficiency of energy conversion is constrained by insufficient carrier usage and deficient active sites in conventional catalysts.44–46 SACs exhibit remarkable activity in efficiently surpassing the abovementioned obstacles. Heterogeneous SACs, in which every single metal atom is completely revealed to the surroundings and accessible for contact with reactants, are highly efficient catalysts. SACs are cost-effective as they reduce the amount of metal needed to enhance a catalytic reaction and implement advanced functions during chemical transformations. For instance, SACs enable the stability of metallic elements in mixed spin and oxidation states, providing facilitated reactivity.47 Moreover, this characteristic is related to the exchange of noble and rare metals by earth-abundant ones, manifesting the lesser-known features that were previously enigmatic in their nanocatalyst counterparts.48 Electrochemical CO2RR propelled via renewable energy appears as a favourable technique to overcome environmental and energy-related issues by transforming CO2 into value-added chemicals.49 SACs consisting of isolated metal atoms with a support material exhibit tremendous activity for electrocatalytic CO2 reduction due to their robust single-atom support contacts, remarkable catalytic performance, and maximum utilization of metal. However, SACs experience particle agglomeration, problems in large-scale generation, and low metal loading. Furthermore, the substitute for single atom-based catalysts are molecular catalysts, comprising ligand molecules linked to metal ions, having almost the same metal–nitrogen (M–N) active centres as in metal–nitrogen–carbon (M–N–C) SACs, that are greatly active for electrochemical CO2 reduction because of their precise active sites and their alteration due to electronic and steric effects. However, molecular catalysts are confronted by moderate performance, stability and selectivity, aggregation, and poor conductivity. Several substrates have been exploited for fabricating SACs, specifically, carbon derivatives (carbon nanotubes, graphene oxide, MOF-derived nanocarbon, and g-C3N4),50–53 exhibiting advantages due to their low cost, excellent electrical conductivity, specific stability and tunable surface chemistry to synthesize metal–nitrogen–carbon (M–N–C) SACs for electrocatalytic CO2 reduction.54 Additionally, some metal complexes (MoS2,55 TiO2,56 CeO2,57 TiC58) are also established as efficient supports for fabricating effective SACs. For instance, Zhang et al.40 fabricated Ag1 single-atom catalyst (Ag1/MnO2) for which HAADF-STEM (Fig. 3(f)), in situ XRD (Fig. 3(g)), in situ ETEM, and density functional theory (DFT) were utilized to characterize the evolution of Ag NPs to single atoms. The as-synthesized Ag1/MnO2 displayed the highest current density in the potential range of −0.6 to −1.0 V and exhibited 95.7% faradaic efficiency (FE) for CO formation, showing excellent stability for electrocatalytic CO2 reduction with 169 mV dec−1 value of Tafel slope as shown in Fig. 4(a)–(d). DFT estimations revealed that single-atom Ag sites possessed high electronic density and served as the only active sites for CO2 reduction. Hence, the Ag1/MnO2 electrocatalyst exhibited noteworthy activity for CO2RR, better than that of a traditional Ag nanocatalyst (AgNP/MnO2). Cai et al.59 reported a novel carbon-dots-based SAC edged with special CuN2O2 for electrochemical CO2 reduction. The as-prepared electrocatalyst exhibited a remarkable 78% FE and 99% selectivity for CO2 reduction to CH4, showing a current density of 40 mA cm−2. The enhanced results were attributed to incorporating oxygen ligands, which surpassed those for most reported SACs. Moreover, theoretical simulations showed that the high activity and selectivity of CuN2O2 active sites were due to the surpassing of H2 and CH4 energy barriers and the tunable electronic framework of Cu active sites. Supported Ni catalysts are considered highly efficient for hydrogen evolution and CO2 activation among transition metals. For instance, Millet et al.60 synthesized Ni SACs with an amount of Ni up to 10 atom% and deduced it as the highest limit for the operation of SACs. STEM-HAADF images of Ni SACs before and after catalysis are depicted in Fig. 4(e)–(h). The as-synthesized Ni SACs were functioning for CO2 activation and showed stable activity during the reaction time, which revealed linear dependency on the concentration of Ni on the surface, as shown in Fig. 4(i) and (j). It was revealed that Ni clusters were required for the CO2RR to proceed as isolated Ni atoms were inaccessible for CO2 conversion to CH3OH or CH4. The change in selectivity for CH4 and the continuous increase of reaction rates with samples consisting of Ni clusters provided an insight into the distinct reaction durations.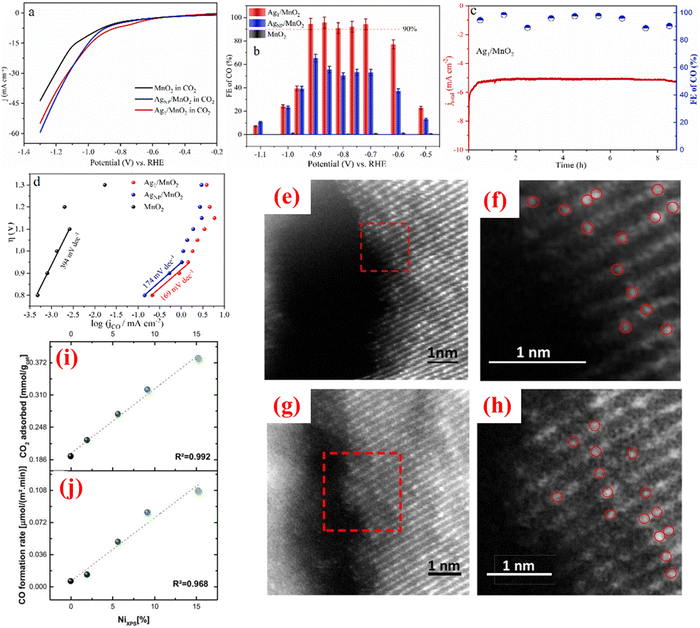 | ||
| Fig. 4 (a) LSV curves, (b) FE of CO, and (c) 8-hour electrocatalytic investigations of Ag1/MnO2 at −0.9 V. (d) Tafel plots of as prepared catalysts. Reproduced with permission from ref. 40. Copyright 2021, John Wiley and Sons. (e) STEM-HAADF image of Ni-10% before catalysis. (f) Magnified image. (g) STEM-HAADF image of Ni-10% after catalytic investigation. (h) Magnified image. (i) Examination of CO2 adsorbed versus concentration of surface nickel. (j) Investigation of CO production versus concentration of surface nickel. Reproduced with permission from ref. 60. Copyright 2019, American Chemical Society. | ||
Xi et al.61 prepared carbon-supported Ni SACs with a coordination of 80% N dopants with metal elements. The absence of unfavourable N species was credited for the remarkable activity of optimized Ni SACs in electrocatalytic CO2RR, which showed tremendous selectivity (∼92%) and stability for the formation of CO. Guo et al.62 fabricated Ni-SAC@NC for electrochemical CO2 conversion. As-prepared SACs achieved up to ∼95% FE for CO formation with a partial current density of 187.7 mA cm−2 at 2.7 V. The enhanced activity stemmed from defect-Ni-N3 structure that provided more active sites in Ni-SAC@NC for CO2 reduction.
Guo et al.63 fabricated an Sn SAC (Sn-NOC) consisting of an asymmetric SnN3O1 configuration, which proved to be an efficient CO2 to CO transformation electrocatalyst. The as-prepared Sn-NOC exhibited a partial current density as high as 13.9 mA cm−2 and 94% FE at −0.7 V for CO with improved stability as shown in Fig. 5(a)–(d). Additionally, a linear relationship was revealed between FECO and the amount of Sn–N, indicating that atomically distributed SnN3O1 coordination was a highly active site for CO2RR in Sn-NOC, as displayed in Fig. 5(e). The theoretical and experimental results revealed that the asymmetric SnN3O1 decreased the energy barrier for the formation of CO while increasing the energy for *OCO adsorption, which is considered the main step for HCOOH formation.
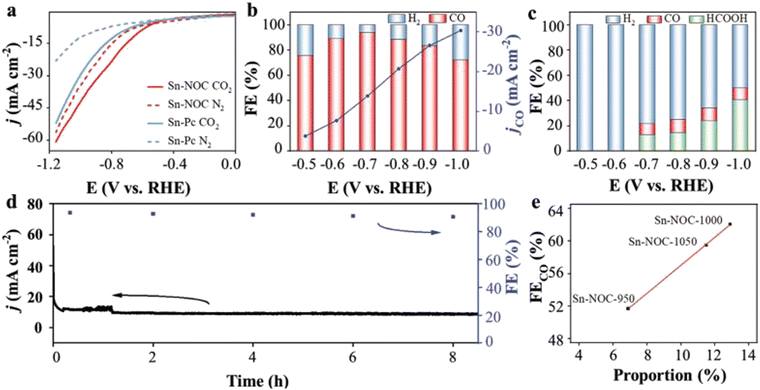 | ||
| Fig. 5 (a) LSV curves of Sn-Pc and Sn-NOC in CO2- and N2-based electrolytes. (b) Partial current densities and FE of Sn-NOC at different potentials. (c) FE of Sn-Pc at different potentials. (d) Stability test and FECO of Sn-NOC in CO2-based electrolyte. (e) The linear relationship between the concentration of Sn-N and FE of Sn-NOC catalysts synthesized at distinct temperatures. Reproduced with permission from ref. 63. Copyright 2021, John Wiley and Sons. | ||
Leverett et al.64 studied the Cu SAC coordination for both CO2RR and nitrate reduction reaction (NO3RR). DFT simulations indicated that Cu–N4 sites showed higher intrinsic performance for CO2RR, whereas both Cu–N4−x–Cx and Cu–N4 sites were active for NO3RR. These results assisted in merging CO2RR and NO3RR for the generation of urea on Cu SACs, attaining 28% FE with –27 mA cm−2 current density at –0.9 V. Li et al.65 fabricated two kinds of In SACs, one coordinated with four nitrogen atoms (In–N4) and the second one coordinated with three nitrogen atoms having one vacancy (In–N3–V) to compare the electrocatalytic CO2 activity. The In–N3–V site exhibited the highest FE of 95% for CO production in comparison to In–N4 (80% FECO). Theoretical calculations revealed that the structural tuning from In–N4 to In–N3–V brought the energies of In orbitals (s and pz) nearer to the Fermi energy. Hence, the improved electrocatalytic performance was attributed to these hybridized orbitals that decreased the energy barrier for the formation of the COOH* intermediate. Li et al.66 studied the atomic alteration of Fe SAC having phosphorus on carbon (Fe–N@P–C) for electrocatalytic CO2 reduction. The as-prepared Fe–N@P–C showed excellent activity for CO2 conversion to CO, achieving 98% FE and 508.8 h−1 turnover frequency (TOF) at 0.34 V. The results of XAS and DFT revealed that the alteration of P in Fe SAC reduced the energy barrier of *CO intermediate construction and decreased the oxidation state of the Fe centre, leading to enhanced electrochemical performance and high stability of Fe SAC. Chen et al.67 investigated a sulfur-doped SAC in FeN4 (Fe1NSC) for electrocatalytic CO2 reduction and characterized it via aberration-corrected STEM and XAS. Fe1NSC exhibited enhanced CO2RR activity compared with FeN4 in the absence of sulfur, achieving 98.6% FE for CO and 1197 h−1 TOF. The results of theoretical analysis and operando characterizations verified that sulfur doping expedited H2O activation and supplied enough protons for accelerating CO2 transformation to *COOH. Lakshmanan et al.68 reported Fe SAC based on three coordinated oxygens, Fe–(O)3, with carbon nanotubes (Fe-n-f-CNTs) for electrochemical CO2 reduction. The outcomes showed that Fe SACs attained 45% FE and a yield of 56.42 μmol cm−2 h−1 for C2H5OH formation. The catalytic characteristics decreased the reaction energy and caused the transfer of electrons to the intermediates *OCHO and *COOH, thus enhancing CO formation. The production rate of Fe SACs implied that the Fe single-atom sites generated a high amount of CO to assist the transformation of CO2 to C2 product. Zhang et al.69 developed Ga SACs coordinated with P, S atomic surroundings showing unique features, achieving 92% FE in electrocatalytic CO2 reduction. Theoretical calculations indicated that the versatile dynamic transition of Ga improved the adsorption energy of *COOH and upgraded the active sites over time, resulting in remarkable CO2RR stability and selectivity. The electrochemical efficiency of different SACs toward CO2RR application can be seen in Table 2.
| Electrocatalyst | Electrolyte | Faradaic efficiency | Selectivity | Current density | Ref. |
|---|---|---|---|---|---|
| CuN2O2 | 0.5 M KHCO3 | 78% (CH4) | 99% (CH4) | 40 mA cm−2 | 59 |
| Ni SACs | 0.1 M KHCO3 | >90% (CO) | ∼92% (CO) | 70 mA cm−2 | 61 |
| Ni-SAC@NC | 0.5 M KHCO3 | ∼95% (CO) | 187.7 mA cm−2 | 62 | |
| Sn-NOC | 0.1 M KHCO3 | 94% (CO) | ∼80% (CO) | 13.9 mA cm−2 | 63 |
| In–N3–V | 0.5 M KHCO3 | 95% (CO) | 6.7 mA cm−2 | 65 | |
| Fe–N@P–C | 0.5 M KHCO3 | 98% (CO) | 66 | ||
| Fe1NSC | 0.5 M KHCO3 | 98.6% (CO) | 40 mA cm−2 | 67 | |
| Fe-n-f-CNTs | 0.5 M KHCO3 | 45% (C2H5OH) | 68 | ||
| Ga SACs | 0.5 M KHCO3 | 92% (CO) | 90% (CO) | 69 |
Major bottlenecks and future outlook for SAC-based energy conversion
In the last few years, numerous efforts have been dedicated to single atom-based catalysts for CO2 reduction. SACs exhibit remarkable catalytic performance due to their robust single atom–support contacts, maximum atomic utilization and uniformity of metal atoms that ensure high faradaic efficiency and lower overpotential values during CO2RR as illustrated in Fig. 6. Fe and Ni SACs are exceptionally selective for the formation of CO with Ni-based SACs attaining higher FE, whereas Fe-based SACs show higher selectivity for CO at comparatively lower overpotentials. These are promising candidates for upcoming industrial CO2 reduction applications. Co-based SACs are also highly active for CO2 to CO transformation, with comparable results to those of the Fe- and Ni-based SACs. Notably, Cu-based SACs are specific materials to form products other than CO, though the generation of C2+ products is still in its nascent stage. Other metal-based SACs (Zn, Ag, Sn, Pb) are also potential candidates for the reduction of CO2. Despite the progress in research concerning SACs, numerous issues need to be solved for their advancements and applications. For example, CO2 reduction on an industrial scale is challenging due to the scarcity of efficient catalysts with notable activity and durability. Currently, the characterization of single atoms is chiefly realized by the amalgamation of XAFS, CO-DRIFT and HAADF-STEM operando techniques. Although these approaches provide a clear mechanistic sketch of the structure of the principal metal in a catalyst, not a single one offers the correct coordination environment and electronic structure of the single metal atoms with geometrical considerations in the working state. Hence, the current knowledge of the complex active sites of heterogeneous SACs and their mechanisms in catalytic reactions highly depends on information obtained from statistically averaged features. A promising solution for this obstacle is the utilization of single-atom electron spectroscopy, which would provide structural characterization of each metal in a microscopic range. This emerging strategy has been effectively utilized in revealing the localized electronic structure of single atoms; hence, it should be explored for gaining knowledge of the catalytic operation of isolated metal atoms.70 Other essential work concerning SACs is to find a method that provides imaging spectroscopically or microscopically of various metal species and, at the same time, distinguishes which ones are participating in the reaction and which ones are not. Generally, variously sized metals coincide in an operating heterogeneous catalyst, and the participation of all these metals in a reaction is difficult to untangle. A single-atom material, even itself, does not show identical catalytic and structural features. It should be taken into account that most catalytic reactions are linked to the heat effect and active sites introduce a considerable change of the surrounding temperature. Lately, phonons and plasmons have been utilized to investigate the temperature of nanosystems in electron microscopy.71 Building on these developments, a thermometry-microscopy system for catalytic applications can provide a reliable solution. As far as the CO2RR application of SACs is concerned, a high number of single atom-based catalysts displayed unsatisfactory CO2 reduction activity in real devices. The low solubility of CO2 (34 mmol L−1) in aqueous electrolytes and limited mass transfer of CO2 at high current densities are limiting factors for scalable CO2 reduction.72 To overcome such problems single atom-based catalysts have been merged with gas diffusion layers for utilization in membrane electrode assemblies and flow cells that assist in meeting industrial needs. There are some studies reported for M–N–C SACs’ exploitation at an industrial scale for CO generation in a flow cell with an excellent partial current density of more than 200 mA cm−2.73,74 Another shortcoming of SACs for CO2RR is their moderate selectivity for C2+ products. Longer carbon-chain products have a higher commercial value and possess significant energy densities in comparison to their C1 counterparts. However, the low selectivity on account of sluggish kinetics and rapid hydrogenation/oxidation of CO* before C–C coupling hampers the efficiency of C2+ product formation.75 Most of the transition metal SACs (Fe, Co, Ni SACs) are highly selective for CO formation, whereas only Cu SACs generate C2+ products (C2H4, C2H5OH, C3H7OH).76 CO2 conversion into stable C2+ products is quite an issue because of the multiple electron transfer (18 and 12) required for generating C2H4, C2H5OH and C3H7OH.77,78 The adjacent Cu sites can enhance CO2 reduction into distinct hydrocarbons.79 The two closest Cu–N2 sites can merge with *CO to originate C–C coupling, consequently enhancing selectivity for C2H4, whereas other sites react to form C1 products. Moreover, coupling Cu SACs to multiatomic sites or other metal SACs is favourable for enhancing the selectivity of C2+ products.Converting CO2 into C2+via two steps from CO2 to CO, followed by CO to C2+ can be efficient in improving the selectivity of C2+ due to the minimum formation of intermediates and less electron transfer. Additionally, CO2 reduction is a difficult reaction process that involves distinct reduction routes through multi-electron transfer, thus making it challenging to understand the concerted mechanisms. To comprehend CO2 reduction favourably with explicit active sites, aside from DFT simulations, operando characterization like HAADF-STEM is efficient in examining the distribution and position of single atoms on support materials, whereas Mössbauer spectroscopy, in situ XAS and XPS contribute in understanding the structural evolution of single atoms on a support material. Therefore, the state-of-the-art in situ characterizations, such as operando Fe Mössbauer, in situ Raman/FTIR/UV-Vis/XAS spectroscopy, and ETEM, are the pivotal instrumentation tools to monitor active sites and reaction pathways of CO2RR, which in turn are beneficial to understand activity and structural relationships in SAC-based CO2RR operations.
Conclusions
SACs have attracted significant attention for electrochemical CO2 conversion attributed to their high atom utilization efficiency, tunable electronic structure, and excellent activity and selectivity toward CO2RR products such as CO, formate, methanol, and ethanol. Operando techniques play a crucial role in understanding the structure-performance relationship of SACs during CO2RR by allowing the real-time monitoring of metal-atom evolution, reaction intermediates, and electronic structure changes under operating conditions, providing insights into CO2RR mechanisms and stability of SACs. Herein, we surveyed the progress in the in situ/operando characterization techniques in elucidating the catalytic efficiency of SAC-derived catalytic systems toward electrochemical CO2RR applications. Furthermore, we have discussed the challenges and future directions in upscaling C2+ products and enhancing the selectivity of SACs. We believe that combining the results of in situ techniques and machine learning/DFT-guided operando studies will pave the way for multi-faceted insights and robust catalyst design in CO2RR applications.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no financial competing interest.Acknowledgements
TA thanks SERB sponsored research scheme (CRG/2023/000480) for financial support.References
- S. A. Ali, M. Khanam, I. Sadiq, S. Shaheen and T. Ahmad, J. Colloid Interface Sci., 2025, 679, 1046–1075 CrossRef PubMed.
- K. Liu, Z. Sun, W. Chen, X. Lang, X. Gao and P. Chen, Adv. Funct. Mater., 2024, 34, 2312589 CrossRef CAS.
- S. A. Ali and T. Ahmad, Langmuir, 2024, 40, 10835–10846 CrossRef PubMed.
- A. Wang, J. Li and T. Zhang, Nat. Rev. Chem., 2018, 2, 65–81 CrossRef CAS.
- B. B. Sarma, F. Maurer, D. E. Doronkin and J. D. Grunwaldt, Chem. Rev., 2022, 123, 379–444 CrossRef PubMed.
- Y. Duan, Y. Wang, W. Zhang, C. Ban, Y. Feng, X. Tao, A. Li, K. Wang, X. Zhang, X. Han, W. Fan, B. Zhang, H. Zou, L. Gan, G. Han and X. Zhou, Adv. Mater., 2024, 36, 2404900 CrossRef CAS PubMed.
- B. Qiao, A. Wang, X. Yang, L. F. Allard, Z. Jiang, Y. Cui, J. Liu, J. Li and T. Zhang, Nat. Chem., 2011, 3, 634–641 CrossRef CAS PubMed.
- X. Li, X. Yang, J. Zhang, Y. Huang and B. Liu, ACS Catal., 2019, 9, 2521–2531 CrossRef CAS.
- V. Muravev, G. Spezzati, Y. Q. Su, A. Parastaev, F. K. Chiang, A. Longo, C. Escudero, N. Kosinov and E. J. Hensen, Nat. Catal., 2021, 4, 469–478 CrossRef CAS.
- V. Vidal, A. Théolier, J. Thivolle-Cazat and J. M. Basset, Science, 1997, 276, 99–102 CrossRef CAS PubMed.
- W. O. Haag, R. M. Lago and P. B. Weisz, Nature, 1984, 309, 589–591 CrossRef CAS.
- E. Bayram, J. Lu, C. Aydin, N. D. Browning, S. Özkar, E. Finney, B. C. Gates and R. G. Finke, ACS Catal., 2015, 5, 3514–3527 CrossRef CAS.
- Z. Zhang, Y. Zhu, H. Asakura, B. Zhang, J. Zhang, M. Zhou, Y. Han, T. Tanaka, A. Wang, T. Zhang and N. Yan, Nat. Commun., 2017, 8, 16100 CrossRef CAS PubMed.
- L. Nie, D. Mei, H. Xiong, B. Peng, Z. Ren, X. I. P. Hernandez, A. DeLaRiva, M. Wang, M. H. Engelhard, L. Kovarik and A. K. Datye, Science, 2017, 358, 1419–1423 CrossRef CAS PubMed.
- J. C. Liu, Y. G. Wang and J. Li, J. Am. Chem. Soc., 2017, 139, 6190–6199 CrossRef CAS PubMed.
- Y. G. Wang, D. Mei, V. A. Glezakou, J. Li and R. Rousseau, Nat. Commun., 2015, 6, 6511 CrossRef CAS PubMed.
- R. Bliem, J. E. Van Der Hoeven, J. Hulva, J. Pavelec, O. Gamba, P. E. De Jongh, M. Schmid, P. Blaha, U. Diebold and G. S. Parkinson, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 8921–8926 CrossRef CAS PubMed.
- B. Eren, D. Zherebetskyy, L. L. Patera, C. H. Wu, H. Bluhm, C. Africh, L. W. Wang, G. A. Somorjai and M. Salmeron, Science, 2016, 351, 475–478 CrossRef CAS PubMed.
- Y. Liu, X. Su, J. Ding, J. Zhou, Z. Liu, X. Wei, H. B. Yang and B. Liu, Chem. Soc. Rev., 2024, 53, 11850–11887 RSC.
- Z. Jin, P. Li, Z. Fang and G. Yu, Acc. Chem. Res., 2022, 55, 759–769 CrossRef CAS PubMed.
- K. Asakura, H. Nagahiro, N. Ichikuni and Y. Iwasawa, Appl. Catal., A, 1999, 188, 313–324 CrossRef CAS.
- X. Zhang, H. Shi and B. Q. Xu, Angew. Chem., Int. Ed., 2005, 117, 7294–7297 CrossRef.
- Q. Fu, H. Saltsburg and M. Flytzani-Stephanopoulos, Science, 2003, 301, 935–938 CrossRef CAS PubMed.
- H. Zhao, Y. Zhu, H. Ye, Y. He, H. Li, Y. Sun, F. Yang and R. Wang, Adv. Mater., 2023, 35, 2206911 CrossRef CAS PubMed.
- E. D. Boyes, A. P. LaGrow, M. R. Ward, R. W. Mitchell and P. L. Gai, Acc. Chem. Res., 2020, 53, 390–399 CrossRef CAS PubMed.
- P. Tieu, X. Yan, M. Xu, P. Christopher and X. Pan, Small, 2021, 17, 2006482 CrossRef CAS PubMed.
- X. Li, H. Y. Wang, H. Yang, W. Cai, S. Liu and B. Liu, Small Methods, 2018, 2, 1700395 CrossRef.
- S. Wei, A. Li, J. C. Liu, Z. Li, W. Chen, Y. Gong, Q. Zhang, W. C. Cheong, Y. Wang, L. Zheng and H. Xiao, Nat. Nanotechnol., 2018, 13, 856–861 CrossRef CAS PubMed.
- Y. Wang, C. Li, X. Han, J. Bai, X. Wang, L. Zheng, C. Hong, Z. Li, J. Bai, K. Leng and Y. Lin, Nat. Commun., 2024, 15, 5675 CrossRef CAS PubMed.
- S. H. Al-Hilfi, X. Jiang, J. Heuer, S. Akula, K. Tammeveski, G. Hu, J. Yang, H. I. Wang, M. Bonn, K. Landfester and K. Müllen, J. Am. Chem. Soc., 2024, 146, 19886–19895 CrossRef CAS PubMed.
- J. Li, Y. F. Jiang, Q. Wang, C. Q. Xu, D. Wu, M. N. Banis, K. R. Adair, K. Doyle-Davis, D. M. Meira, Y. Z. Finfrock and W. Li, Nat. Commun., 2021, 12, 6806 CrossRef CAS PubMed.
- A. Martini, D. Hursán, J. Timoshenko, M. Rüscher, F. Haase, C. Rettenmaier, E. Ortega, A. Etxebarria and B. Roldan Cuenya, J. Am. Chem. Soc., 2023, 145, 17351–17366 CrossRef CAS PubMed.
- J. Timoshenko and B. Roldan Cuenya, Chem. Rev., 2020, 121, 882–961 CrossRef PubMed.
- J. Timoshenko and A. I. Frenkel, ACS Catal., 2019, 9, 10192–10211 CrossRef CAS.
- A. Zitolo, N. Ranjbar-Sahraie, T. Mineva, J. Li, Q. Jia, S. Stamatin, G. F. Harrington, S. M. Lyth, P. Krtil, S. Mukerjee and E. Fonda, Nat. Commun., 2017, 8, 957 CrossRef PubMed.
- Z. Chen, A. G. Walsh and P. Zhang, Acc. Chem. Res., 2024, 57, 521–532 CAS.
- S. Impeng, E. Salaya-Gerónimo, B. Kunkel, S. Bartling, K. Faungnawakij, B. Rungtaweevoranit and A. M. Abdel-Mageed, J. Mater. Chem. A, 2024, 12, 3084–3095 RSC.
- B. Hu, K. Sun, Z. Zhuang, Z. Chen, S. Liu, W. C. Cheong, C. Chen, M. Hu, X. Cao, J. Ma and R. Tu, Adv. Mater., 2022, 34, 2107721 CrossRef CAS PubMed.
- Z. Li, E. Öztuna, K. Skorupska, O. V. Vinogradova, A. Jamshaid, A. Steigert, C. Rohner, M. Dimitrakopoulou, M. J. Prieto, C. Kunkel and M. Stredansky, Nat. Commun., 2024, 15, 1–13 Search PubMed.
- N. Zhang, X. Zhang, L. Tao, P. Jiang, C. Ye, R. Lin, Z. Huang, A. Li, D. Pang, H. Yan and Y. Wang, Angew. Chem., Int. Ed., 2021, 60, 6170–6176 CrossRef CAS PubMed.
- Z. Lang, X. Wang, S. Jabeen, Y. Cheng, N. Liu, Z. Liu, T. Gan, Z. Zhuang, H. Li and D. Wang, Adv. Mater., 2025, 2418942 CrossRef CAS PubMed.
- V. Muravev, G. Spezzati, Y. Q. Su, A. Parastaev, F. K. Chiang, A. Longo, C. Escudero, N. Kosinov and E. J. Hensen, Nat. Catal., 2021, 4, 469–478 CrossRef CAS.
- H. Bühlmeyer, T. Talwar, R. Eschenbacher, J. Barreto, J. Hauner, L. Knörr, H. P. Steinrück, F. Maier and J. Libuda, ACS Appl. Mater. Interfaces, 2024, 16, 24063–24074 Search PubMed.
- S. A. Ali and T. Ahmad, Small, 2024, 20, 2403401 CrossRef CAS PubMed.
- S. A. Ali, I. Sadiq, M. Estrader and T. Ahmad, ChemCatChem, 2025, e202500032 CrossRef.
- S. A. Ali, I. Sadiq and T. Ahmad, Nanotechnology, 2025, 36, 122001 CrossRef CAS PubMed.
- J. Li, H. Huang, W. Xue, K. Sun, X. Song, C. Wu, L. Nie, Y. Li, C. Liu, Y. Pan, H.-L. Jiang, D. Mei and C. Zhong, Nat. Catal., 2021, 4, 719–729 CrossRef CAS.
- Y. Zhang, J. Zhao, H. Wang, B. Xiao, W. Zhang, X. Zhao, T. Lv, M. Thangamuthu, J. Zhang, Y. Guo, J. Ma, J. Tang, R. Huang and Q. Liu, Nat. Commun., 2022, 13, 58 CrossRef CAS PubMed.
- S. A. Ali, I. Sadiq and T. Ahmad, Langmuir, 2024, 40, 10414–10432 CrossRef CAS PubMed.
- A. Han, B. Wang, A. Kumar, Y. Qin, J. Jin, X. Wang, C. Yang, B. Dong, Y. Jia, J. Liu and X. Sun, Small Methods, 2019, 3, 1800471 CrossRef.
- Y. Cheng, S. Zhao, H. Li, S. He, J. P. Veder, B. Johannessen, J. Xiao, S. Lu, J. Pan, M. Chisholm, S. Yang, C. Liu, J. G. Chen and S. Jiang, Appl. Catal., B, 2019, 243, 294–303 CrossRef CAS.
- T. Wang, Q. Zhao, Y. Fu, C. Lei, B. Yang, Z. Li, L. Lei, G. Wu and Y. Hou, Small Methods, 2019, 3, 1900210 CrossRef CAS.
- J. G. Chen, Joule, 2018, 2, 587–589 CrossRef CAS.
- T. Wang, Q. Zhao, Y. Fu, C. Lei, B. Yang, Z. Li, L. Lei, G. Wu and Y. Hou, Small Methods, 2019, 3, 1900210 CrossRef CAS.
- R. K. Biroju, D. Das, R. Sharma, S. Pal, L. P. Mawlong, K. Bhorkar, P. Giri, A. K. Singh and T. N. Narayanan, ACS Energy Lett., 2017, 2, 1355–1361 CrossRef CAS.
- J. Wan, W. Chen, C. Jia, L. Zheng, J. Dong, X. Zheng, Y. Wang, W. Yan, C. Chen, Q. Peng, D. Wang and Y. Li, Adv. Mater., 2018, 30, 1705369 CrossRef PubMed.
- Y. Wang, Z. Chen, P. Han, Y. Du, Z. Gu, X. Xu and G. Zheng, ACS Catal., 2018, 8, 7113–7119 CrossRef CAS.
- S. Back and Y. Jung, ACS Energy Lett., 2017, 2, 969–975 CrossRef CAS.
- Y. Cai, J. Fu, Y. Zhou, Y. C. Chang, Q. Min, J. J. Zhu, Y. Lin and W. Zhu, Nat. Commun., 2021, 12, 586 CrossRef CAS PubMed.
- M. M. Millet, G. Algara-Siller, S. Wrabetz, A. Mazheika, F. Girgsdies, D. Teschner, F. Seitz, A. Tarasov, S. V. Levchenko, R. Schlögl and E. Frei, J. Am. Chem. Soc., 2019, 141, 2451–2461 CrossRef CAS PubMed.
- D. Xi, J. Li, J. Low, K. Mao, R. Long, J. Li, Z. Dai, T. Shao, Y. Zhong, Y. Li and Z. Li, Adv. Mater., 2022, 34, 2104090 CrossRef CAS PubMed.
- Y. Guo, S. Yao, Y. Xue, X. Hu, H. Cui and Z. Zhou, Appl. Catal., B, 2022, 304, 120997 CrossRef CAS.
- J. Guo, W. Zhang, L. H. Zhang, D. Chen, J. Zhan, X. Wang, N. R. Shiju and F. Yu, Adv. Sci., 2021, 8, 2102884 CrossRef CAS PubMed.
- J. Leverett, T. Tran-Phu, J. A. Yuwono, P. Kumar, C. Kim, Q. Zhai, C. Han, J. Qu, J. Cairney, A. N. Simonov and R. K. Hocking, Adv. Energy Mater., 2022, 12, 2201500 CrossRef CAS.
- S. Li, X. Lu, S. Zhao, M. Ceccato, X. M. Hu, A. Roldan, M. Liu and K. Daasbjerg, ACS Catal., 2022, 12, 7386–7395 CrossRef CAS.
- K. Li, S. Zhang, X. Zhang, S. Liu, H. Jiang, T. Jiang, C. Shen, Y. Yu and W. Chen, Nano Lett., 2022, 22, 1557–1565 CrossRef CAS PubMed.
- S. Chen, X. Li, C. W. Kao, T. Luo, K. Chen, J. Fu, C. Ma, H. Li, M. Li, T. S. Chan and M. Liu, Angew. Chem., Int. Ed., 2022, 61, e202206233 CrossRef CAS PubMed.
- K. Lakshmanan, W. H. Huang, S. A. Chala, B. W. Taklu, E. A. Moges, J. F. Lee, P. Y. Huang, Y. C. Lee, M. C. Tsai, W. N. Su and B. J. Hwang, Adv. Funct. Mater., 2022, 32, 2109310 CrossRef CAS.
- Z. Zhang, J. Zhu, S. Chen, W. Sun and D. Wang, Angew. Chem., 2023, 135, e202215136 CrossRef.
- Y. C. Lin, P. Y. Teng, P. W. Chiu and K. Suenaga, Phys. Rev. Lett., 2015, 115, 206803 CrossRef PubMed.
- M. J. Lagos and P. E. Batson, Nano Lett., 2018, 18, 4556–4563 CrossRef CAS PubMed.
- J. Zhang, W. Cai, F. X. Hu, H. Yang and B. Liu, Chem. Sci., 2021, 12, 6800–6819 RSC.
- Z. Chen, X. Zhang, W. Liu, M. Jiao, K. Mou, X. Zhang and L. Liu, Energy Environ. Sci., 2021, 14, 2349–2356 RSC.
- Q. Wang, K. Liu, J. Fu, C. Cai, H. Li, Y. Long, S. Chen, B. Liu, H. Li, W. Li, X. Qiu, N. Zhang, J. Hu, H. Pan and M. Liu, Angew. Chem., Int. Ed., 2021, 60, 25241–25245 CrossRef CAS PubMed.
- P. Zhu and H. Wang, Nat. Catal., 2021, 4, 943–951 CrossRef CAS.
- A. Guan, Z. Chen, Y. Quan, C. Peng, Z. Wang, T. K. Sham, C. Yang, Y. Ji, L. Qian, X. Xu and G. Zheng, ACS Energy Lett., 2020, 5, 1044–1053 CrossRef CAS.
- S. A. Ali, I. Sadiq and T. Ahmad, Mater. Today Sustain., 2023, 24, 100587 Search PubMed.
- I. Sadiq, S. A. Ali, S. Shaheen, I. Fatima and T. Ahmad, Int. J. Hydrogen Energy, 2025, 120, 146–180 CrossRef CAS.
- A. Guan, Z. Chen, Y. Quan, C. Peng, Z. Wang, T. K. Sham, C. Yang, Y. Ji, L. Qian, X. Xu and G. Zheng, ACS Energy Lett., 2020, 5, 1044–1053 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |