Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
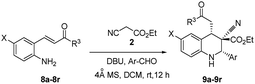
Synthesis of highly substituted tetrahydroquinolines using ethyl cyanoacetate via aza-Michael–Michael addition†
Arunan Palanimuthu b,
Chinpiao Chen
b,
Chinpiao Chen *ab and
Gene-Hsian Leec
*ab and
Gene-Hsian Leec
aDepartment of Nursing, Tzu Chi University of Science and Technology, Hualien 970, Taiwan. E-mail: chinpiao@ems.tcust.edu.tw; Fax: +886 3 856 1097; Tel: +886 3 857 2158 ext. 2624
bDepartment of Chemistry, National Dong Hwa University, Soufeng, Hualien 974, Taiwan
cInstrumentation Center, College of Science, National Taiwan University, Taipei 106, Taiwan
First published on 3rd April 2020
Abstract
A three-component cascade reaction involving 2-alkenyl aniline, aldehydes, and ethyl cyanoacetate in the presence of DBU to synthesize highly substituted 1,2,3,4-tetrahydroquinolines is reported. The reaction proceeded through the Knoevenagel condensation of ethyl cyanoacetate with aldehydes followed by the aza-Michael–Michael addition with 2-alkenyl anilines to prepare the tetrahydroquinoline scaffolds.
Introduction
Cascade or tandem reactions continue to be of interest because they offer a rapid and highly effective strategy for the synthesis of bioactive natural products1–5 and pharmaceutical agents.6–16 Tetrahydroquinolines have been targeted by many research groups because of their abundance in natural products and notable biological activity. Tetrahydroquinoline derivatives are used in pesticides, antioxidants, photosensitizers, and dyes in addition to pharmaceutical applications. Overall, the tetrahydroquinoline family has a wide range of applications and is a key structural motif in pharmaceutical agents; therefore, multiple strategies have been proposed for the synthesis of tetrahydroquinoline derivatives.17–22Cascade reactions are valuable for generating 1,2,3,4-tetrahydroquinoline skeletons with various substitution groups, and many new drugs have been designed on the basis of this process. Bunce et al. reported a tandem-reduction-reductive cyclization sequence in one pot of ozonolysis-reduction followed by a reductive amination reaction sequence provided by N-methyl-2-substituted-1,2,3,4-tetrahydroquinoline 4-carboxylic esters.23 Povaraov performed an acid catalyzed one-pot conversion of N-arylimines and electron-rich dienophiles to produce 1,2,3,4-tetrahydroquinoline, which is normally classified as an aza-Diels–Alder or imino Diels–Alder reaction.24 Menéndez et al. revealed that CAN catalyzed the one-pot diastereoselective synthesis of 4-alkoxy-2-ary-1,2,3,4-tetrahydroquinolines.25 Wang reported that earlier Mannich–Michael addition using malononitrile as a nucleophile toward 2-alkenyl substituted imines yielded optically enriched and highly substituted tetrahydroquinolines.26 Commercially available, inexpensive ethyl cyanoacetate has seldom been discussed in relation to the synthesis of tetrahydroquinolines.
Results and discussion
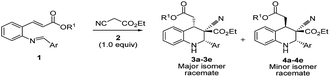
In this paper, it reports the simple one-pot economical preparation of highly substituted tetrahydroquinolines by using 2-alkenyl substituted aniline, aromatic aldehydes, and ethyl cyanoacetate; this method saves time during the workup procedure and purification of intermediates and yields minimal reagent waste.The DBU plays a dual role in the cascade conversion of the Knoevenagel condensation intermediate as well as in the aza-Michael–Michael addition to prepare 1,2,3,4-tetrahydroquinolines. Thus, the overall conversion was integrated irrespective of the Michael acceptors attached to aniline, and resulted in high diastereoselectivity up to 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7. Initial reaction conditions were tested with tert-butyl 2-alkenyl substituted imines (1) and ethyl cyanoacetate with bases including TEA, DIPEA, DABCO, and DBN (Table 1, entries 1–4) in DCM; however no characteristic reactions occurred.27 K2CO3 as a base in DMF and DMSO demonstrated reasonable conversion (Table 1, entries 5 and 6), and it was found that DBU in DCM enabled excellent conversion of (E)-tert-butyl-3-(2-((E)-4-nitrobenzylideneamino)phenyl)acrylate into tetrahydroquinolines 3a/4a at room temperature (Table 1, entries 10 and 11, 95%, racemate). DBU was deemed superior to the other bases.
7. Initial reaction conditions were tested with tert-butyl 2-alkenyl substituted imines (1) and ethyl cyanoacetate with bases including TEA, DIPEA, DABCO, and DBN (Table 1, entries 1–4) in DCM; however no characteristic reactions occurred.27 K2CO3 as a base in DMF and DMSO demonstrated reasonable conversion (Table 1, entries 5 and 6), and it was found that DBU in DCM enabled excellent conversion of (E)-tert-butyl-3-(2-((E)-4-nitrobenzylideneamino)phenyl)acrylate into tetrahydroquinolines 3a/4a at room temperature (Table 1, entries 10 and 11, 95%, racemate). DBU was deemed superior to the other bases.
| Entry | R1 | Ara | Base (mol%) | Solvent | T (h) | Yieldb (%) | Ratio 3/4c |
|---|---|---|---|---|---|---|---|
| a All reactions were performed in 30 to 50 mg scale.b Yield of isolated product is a mixture of diastereomers after column chromatography.c Determined by 1H NMR analysis of crude reaction mixture.d Reactions were completed at −10 °C to rt, 10 h. | |||||||
| 1 | t-Bu | 4-NO2Ph (3a/4a) | DABCO (100) | DCM | 24 | — | — |
| 2 | t-Bu | 4-NO2Ph (3a/4a) | DIPEA (200) | DCM | 24 | — | — |
| 3 | t-Bu | 4-NO2Ph (3a/4a) | DBN (100) | DCM | 12 | — | — |
| 4 | t-Bu | 4-NO2Ph (3a/4a) | TEA (100) | DCM | 24 | — | — |
| 5 | t-Bu | 4-NO2Ph (3a/4a) | K2CO3 (50) | DMF | 12 | 74 | 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
| 6 | t-Bu | 4-NO2Ph (3a/4a) | K2CO3 (50) | DMSO | 12 | 45 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
| 7 | t-Bu | 4-NO2Ph (3a/4a) | DBU (50) | DMF | 5 | 79 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
| 8 | t-Bu | 4-NO2Ph (3a/4a) | DBU (50) | MeOH | 8 | 31 | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49 49 |
| 9 | t-Bu | 4-NO2Ph (3a/4a) | DBU (50) | THF | 8 | 45 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46 46 |
| 10 | t-Bu | 4-NO2Ph (3a/4a) | DBU (200) | DCM | 3 | 95 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
| 11 | t-Bu | 4-NO2Ph (3a/4a) | DBU (50) | DCM | 3 | 95 | 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
| 12d | t-Bu | 4-NO2Ph (3a/4a) | DBU (50) | DCM | 10 | 62 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 48 |
| 13 | t-Bu | 4-OMePh (3b/4b) | DBU (50) | DCM | 12 | — | — |
| 14 | t-Bu | Ph (3c/4c) | DBU (50) | DCM | 12 | — | — |
| 15 | Me | 4-NO2Ph (3d/4d) | DBU (50) | DCM | 2 | 96 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
| 16 | Me | 2-OMePh (3e/4e) | DBU (50) | DCM | 12 | 67 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
| 17 | Me | 3,5-diOMePh (3f/4f) | DBU (50) | DCM | 48 | — | — |
| 18 | Me | 4-NO2Ph (3d/4d) | TEA (50) | DCM | 12 | — | — |
| 19 | Me | 4-NO2Ph (3d/4d) | DIPEA (100) | DCM | 12 | — | — |
The 3a/4a isomers were separated through column chromatography, were recrystallized in DCM, and underwent X-ray analysis (Fig. 1) to facilitate understanding of the relative configuration of the diastereomers. The groups of 1,3-cis-tetrahydroquinoline 3a (major isomer) with the distorted chair configuration of 4-NO2Ph, –CH2CO2-tBu preferred the same side of the ring; the opposite was observed for 4a (alternative, both hydrogens were 1,3 cis in the major diastereomer and in its opposite). To further evaluate diastereoselectivity, we measured the reaction temperature and catalyst loading; however, the results revealed a poor yield and no evident improvement in diastereoselectivity (Table 1, entries 10 and 12).
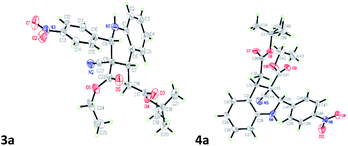 | ||
| Fig. 1 X-ray studies confirmed the relative isomeric structures of 3a (CCDC 1834300) and 4a (CCDC 1834305). | ||
Further investigations were conducted using solvents such as MeOH, THF, and DMF (Table 1, entries 7–9) with DBU as a base, but no significant improvements in diastereomeric ratio (dr) or yield were observed.
The combination of DCM and DBU was preferable to the other solvents. The mixture of diastereomers was inevitable, and it further experimented with the versatility of the reaction through the cascade addition. Methyl 2-alkenyl-substituted imine (Table 1, entries 15 and 16) yielded products with improved diastereoselectivity.
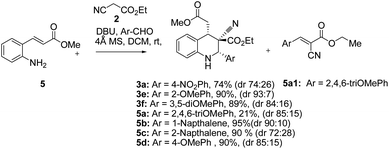
The synthesis and purification of Schiff bases were tedious in many cases; thus a complete tetrahydroquinoline conversion was attempted in a one-pot reaction. Reacting ethyl cyanoacetate, (E)-methyl 3-(2-aminophenyl)acrylate (5), and substituted aromatic aldehydes with DBU yielded 1,2,3,4-substituted tetrahydroquinolines effectively (Scheme 1).28 Electron rich aldehydes resulted in better conversion compared with the other aldehydes. The corresponding product 3e of 2-anisaldehyde demonstrated improved diastereoselectivity compared with the other substituted benzaldehydes (Scheme 1).
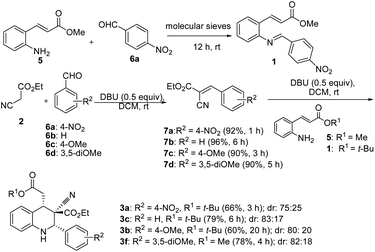
Tetrahydroquinolines obtained from 1-naphthaldehyde demonstrated improve yield and diastereoselectivity compared with those obtained from 2-naphthadehyde (5b and 5c Scheme 1). Unexpectedly, when DBU was used as the base, the synthesis of 3f (Scheme 1), was unsuccessful after the corresponding imine reacted with ethyl cyanoacetate (Table 1, entry 17). In addition, the synthesis of 5a (Scheme 1) produced a low yield, and we managed to isolate the intermediate 5a1, which altered our understanding regarding the mechanistic pathway of the cascade reaction and verified the formation of 1,2,3,4-tetrahydroquinolines through a Knoevenagel-condensation intermediate.
Two control experiments were performed and monitored by TLC. (E)-Methyl 3-(2-aminophenyl)acrylate (5) and p-nitrobenzaldehyde (6a) in CH2Cl2, combined with the application of molecular sieves (4 Å), were used to synthesize the corresponding imine. This reaction solution was stirred at room temperature for 1 h and monitored by TLC, which revealed extremely poor conversion. By contrast, ethyl cyanoacetate (2) reacted readily with p-nitrobenzaldehyde (6a) in the presence of DBU to produce a Knoevenagel condensation product (7a).29 Similarly, the other intermediates (7b, 7c, and 7d) were synthesized under optimized conditions (Scheme 2). Electron-rich aldehydes (2-anisaldehyde and 4-anisaldehyde) were converted to imine (1, Table 1) at high temperatures (110 °C) by using toluene as a solvent to describe the formation of 1,2,3,4-tetrahydroquinolines at room temperature through Knoevenagel-condensation intermediate. Tetrahydroquinolines (3c, 3b, and 3f) synthesis was successful when Knoevenagel intermediates (7b, 7c, and 7d) were used and mediated by DBU in a two-component approach (Scheme 2).
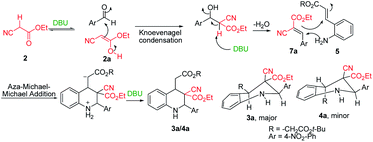
After the initial formation of enol intermediate 2a, the intermediate reacted with aldehyde to produce an aldol product that subsequently endured base-induced elimination to form 7a (Fig. 2). Reactions between Schiff base's and enol intermediate 2a (Mannich reaction) had failed in earlier experiments (Table 1, entries 13, 14, and 17) because the imines were mostly inert, and thus unable to react with ethyl cyanoacetate. It understand from the crystal structures 3a/4a (Fig. 1) that the initial aza-Michael addition to a Knoevenagel intermediate considerably increased the diastereoselectivity whereas subsequent Michael addition to α,β-unsaturated esters yielded a diastereomeric mixture. Thus, for the synthesis of 1,2,3,4-tetrahydroquinoline, it propose a plausible mechanism with a Knoevenagel intermediate that favours cascade transition through the aza-Michael–Michael addition.30
To determine the effective substrate scope of the reaction, it was reviewed systematic studies performed under optimized conditions (Table 2). In this study, 2-alkenyl-4-chloroanilines were efficiently converted to their corresponding tetrahydroquinolines 9a–9g (Table 2, entries 1–7). Regardless of the groups (X = Cl, H or CO2Me) present at 2-alkenylaniline, the yields of the tetrahydroquinolines primarily varied according to the reactivity of the aldehydes. Heteroaromatic aldehydes underwent one-pot conversion into 1,2,3,4-tetrahydroquinolines (9e–9g) with moderate yields (Table 2, entries 5–7). Aromatic aldehydes under the same conditions produced 9a, 9j, and 9n (Table 2, entries 1, 10, and 14) and demonstrated excellent yields compared with the other heteroaromatic aldehydes (Table 2, entries 5–7). In the synthesis of tetrahydroquinoline 9a up to 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, o-anisaldehyde exhibited the highest diastereoselectivity (Table 2, entry 1).
7, o-anisaldehyde exhibited the highest diastereoselectivity (Table 2, entry 1).
| Entry | Ara | X | R3 | Yieldb (%) | drc |
|---|---|---|---|---|---|
| a All reactions were performed in 50 mg scale at room temperature.b Yield of isolated product was a mixture of diastereomers after column chromatography.c Determined by 1H NMR analysis of the crude reaction mixture. | |||||
| 1 | 2-OMePh (9a) | Cl | OMe | 88 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 2 | 3,5-diOMePh (9b) | Cl | OMe | 89 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
| 3 | 2-Naphthyl (9c) | Cl | OMe | 92 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
| 4 | 1-Naphthyl (9d) | Cl | OMe | 90 | 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 9 |
| 5 | 2-Pyridyl (9e) | Cl | OMe | 54 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
| 6 | 3-Thiophenyl (9f) | Cl | OMe | 65 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
| 7 | 2-Thiophenyl (9g) | Cl | OMe | 63 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
| 8 | 2-Naphthyl (9h) | CO2Me | OMe | 85 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
| 9 | 1-Naphthyl (9i) | CO2Me | OMe | 88 | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 16 |
| 10 | 2-OMePh (9j) | CO2Me | OMe | 91 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 11 | 3,5-diOMePh (9k) | CO2Me | OMe | 86 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| 12 | 2-Naphthyl (9l) | H | Ph | 95 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25 |
| 13 | 2-OMePh (9m) | H | Ph | 90 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 14 | 1-Naphthyl (9n) | H | Ph | 92 | 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
| 15 | 2-Thiophenyl (9o) | H | Ph | 84 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
| 16 | 3,5-diOMePh (9p) | H | Ph | 81 | 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
| 17 | 4-NO2Ph (9q) | H | Ph | 83 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
| 18 | 2,4,6-triOMePh (9r) | H | Ph | 79 | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
Naphthaldehydes (Table 2, entries 3 and 4) were converted into 1,2,3,4-tetrahydroquinolines (9c and 9d) under optimized conditions, and 5-methoxycarbonylaniline analogues were converted into their corresponding tetrahydroquinolines (9h–9k) with good to moderate yields (Table 2, entries 8–11). In addition to examining the versatility of the reaction toward Michael acceptor α,β-unsaturated esters (Schemes 1 and 2), it was also examined that of the reaction toward α,β-unsaturated phenyl ketones in tetrahydroquinoline synthesis; the results demonstrated high efficiency. In one-pot, 2-amino substituted chalcones were converted into 1,2,3,4-tetrahydroquinolines with good to moderate yield; all yields were superior to those of the other analogues (Table 2, entries 12–18). No major differences in diastereoselectivity were caused by α,β-unsaturated phenyl ketones (Table 2, entries 13 and 14); however, better yields were obtained with high diastereoselectivity upto 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The stronger electron-withdrawing phenyl ketone group accelerated cascade conversion more easily than the other α,β-unsaturated esters. Separation of the diastereomers through column chromatography and preparative TLC failed in most cases; therefore, they were able to triturate the 1,3-cis isomer (major) separately from the mixture of diastereomers by using methanol.
10. The stronger electron-withdrawing phenyl ketone group accelerated cascade conversion more easily than the other α,β-unsaturated esters. Separation of the diastereomers through column chromatography and preparative TLC failed in most cases; therefore, they were able to triturate the 1,3-cis isomer (major) separately from the mixture of diastereomers by using methanol.
Conclusions
In summary, it was developed a simple DBU mediated cascade process to effectively synthesize a new class of highly substituted 1,2,3,4-tetarhydroquinolines by using ethyl cyanoacetate in one pot. The reaction mechanism was investigated through control experiments, namely three reactions involving Knoevenagel condensation followed by aza-Michael–Michael addition efficiently conducted at the room temperature with simple practicability.Experimental section
General methods
Melting points were recorded using a Yanagimoto Micro Melting Point Apparatus Model-S3 capillary melting point apparatus and are uncorrected. TLC analysis was carried out on silica gel 60 F254 precoated glass sheets and detected under UV light. 1H NMR spectra were obtained at 300, 400 or 500 MHz (as indicated), and 13C NMR spectra were obtained at 75.5, 100 or 125.6 MHz, using a Bruker NMR spectrometer. Chemical shifts (δ) are reported in parts per million (ppm) relative to CDCl3 (7.26 and 77.0 ppm), the coupling constants are reported in hertz (Hz) and the multiplicities are indicated as b = broad, s = singlet, d = doublet, dd = doublet of doublet, dt = doublet of triplet, t = triplet, m = multiplet. In each case proton NMR showed the presence of indicated solvent(s). Infrared spectra were recorded using PerkinElmer FT/IR spectrometer. Mass spectra were recorded on a Micromass Platform II or Finnigan/Thermo Quest MAT 95XL spectrometer. All reactions were carried out in anhydrous solvents. CH2Cl2, DMF, DMSO were distilled from Molecular Sieves. MeOH was distilled from Mg cake. All chemicals and solvents were purchased from Aldrich Chemical Co.A typical procedure for synthesis of ethyl 6-chloro-3-cyano-4-(2-methoxy-2-oxoethyl)-2-(2-methoxyphenyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 9a
A solution of (E)-3-(2-amino-5-chlorophenyl)acrylate (0.24 mmol), ethyl cyanoacetate (0.28 mmol), and 2-methoxybenzaldehyde (0.28 mmol) in CH2Cl2 (5 mL) with DBU (0.12 mmol) was stirred at room temperature, followed by the addition of molecular sieves (4 Å, 30 mg). The reaction mixture was stirred at room temperature for 12 h under N2 atmosphere, and the progress of the reaction was monitored by TLC (eluent: 20% EtOAc in hexane). The crude product was filtered through Celite and washed using CH2Cl2 (20 mL). The organic solvent was removed by a rotary evaporator under reduced pressure, and the obtained crude product was purified by column chromatography (100–200 mesh silica) using 30% ethyl acetate in hexane as an eluent. The mixture of diastereomers (94 mg, 88%) was stirred in anhydrous methanol, and the white precipitate that appeared was filtered and dried to yield major isomer 9a. Yield: 66.7% (71 mg); white solid; mp 164–166 °C; 1H NMR (400 MHz, CDCl3) 7.86 (1H, d, J = 7.8 Hz), 7.38–7.35 (1H, m), 7.08–7.02 (3H, m), 6.89–6.87 (1H, m), 6.58–6.56 (1H, m), 5.27 (1H, s), 4.40–4.38 (1H, m), 4.15 (1H, s), 4.03–3.96 (2H, m), 3.86 (1H, s), 3.80 (3H, b), 3.78 (3H, b), 2.93 (1H, dd, J = 7.9, 17.1 Hz), 2.71 (1H, dd, J = 3.4, 17.0 Hz), 0.97 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 172.0, 166.2, 157.1, 141.7, 130.5, 128.1, 127.3, 124.4, 123.8, 122.0, 121.3, 116.3, 115.8, 110.5, 62.9, 55.5, 54.2, 53.6, 52.5, 13.5; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3389, 2952, 2225, 1722, 1602, 1492, 1465, 1368, 1296, 1258, 1170, 1051, 1023, 862, 824, 755 cm−1; LRMS-EI+ (m/z) 465.40 ([M + Na]+100), 443.60 (43.69), 444.70 (16.67), 445.66 (13.54). HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C23H24ClN2O5 443.1374, found 443.1373.
) 3389, 2952, 2225, 1722, 1602, 1492, 1465, 1368, 1296, 1258, 1170, 1051, 1023, 862, 824, 755 cm−1; LRMS-EI+ (m/z) 465.40 ([M + Na]+100), 443.60 (43.69), 444.70 (16.67), 445.66 (13.54). HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C23H24ClN2O5 443.1374, found 443.1373.
Ethyl 4-(2-(tert-butoxy)-2-oxoethyl)-3-cyano-2-(4-nitrophenyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate (major isomer), 3a
Yellow solid; mp 198–200 °C; 1H NMR (300 MHz, CDCl3) 8.26 (2H, d, J = 8 8 Hz), 7.80 (2H, d, J = 8.8 Hz), 7.20–7.01 (2H, m), 6.85 (1H, dd, J = 7.7, 7.7 Hz), 6.68 (1H, d, J = 8.0 Hz), 4.87 (1H, s), 4.38–4.01 (3H, m), 2.88 (1H, dd, J = 7.8, 17.2 Hz), 2.65 (1H, dd, J = 3.8, 17.3 Hz), 1.60–1.36 (9H, m), 1.05 (3H, t, J = 7.1 Hz); 13C NMR (75.5 MHz, CDCl3) 170.7, 166.5, 148.8, 143.5, 142.1, 129.3, 128.3, 127.5, 123.9, 120.6, 120.0, 115.3, 114.9, 81.7, 63.3, 61.4, 55.4, 41.2, 38.7, 41.2, 38.7, 28.0, 13.8; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3377, 2980, 2923, 2247, 1734, 1608, 1524, 1488, 1368, 1349, 1296, 1248, 1152, 1040, 858, 748 cm−1; LRMS-EI (m/z) 464.13 ([M − H]+, 100), 464.13 (28); HRMS-TOF-ES (m/z) [M − H]+ calcd for C25H26N3O6 464.1822, found 464.1825.
) 3377, 2980, 2923, 2247, 1734, 1608, 1524, 1488, 1368, 1349, 1296, 1248, 1152, 1040, 858, 748 cm−1; LRMS-EI (m/z) 464.13 ([M − H]+, 100), 464.13 (28); HRMS-TOF-ES (m/z) [M − H]+ calcd for C25H26N3O6 464.1822, found 464.1825.
Ethyl 4-(2-(tert-butoxy)-2-oxoethyl)-3-cyano-2-(4-nitrophenyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate (minor isomer), 4a
Yellow solid; mp 188–189 °C; 1H NMR (300 MHz, CDCl3) 8.26–8.21 (2H, d, J = 8.8 Hz), 7.91–7.86 (2H, d, J = 8.8 Hz), 7.22–7.07 (2H, m), 6.83–6.76 (1H, m), 6.65 (1H, d, J = 8.0 Hz), 4.97 (1H, s), 4.22–3.97 (3H, m), 2.91 (1H, dd, J = 7.7, 17.3 Hz), 2.57 (1H, dd, J = 5.2, 17.3 Hz), 1.46 (9H, m), 1.26 (1H, s), 1.15 (3H, t, J = 7.1 Hz); 13C NMR (75 MHz, CDCl3) 170.0, 166.0, 148.6, 144.4, 140.8, 130.3, 129.5, 128.8, 123.6, 119.7, 119.3, 116.5, 81.5, 63.2, 55.3, 50.7, 41.3, 40.8, 28.1, 13.7; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3392, 2918, 2319, 1739, 1605, 1525, 1490, 1349, 1246, 1155, 1041, 848 cm−1; LRMS-EI (m/z) 464.18 ([M − H]+, 100), 464.13 (28); HRMS-TOF-ES (m/z) [M − H]+ calcd for C25H26N3O6 464.1822, found 464.1821.
) 3392, 2918, 2319, 1739, 1605, 1525, 1490, 1349, 1246, 1155, 1041, 848 cm−1; LRMS-EI (m/z) 464.18 ([M − H]+, 100), 464.13 (28); HRMS-TOF-ES (m/z) [M − H]+ calcd for C25H26N3O6 464.1822, found 464.1821.
Ethyl 4-(2-(tert-butoxy)-2-oxoethyl)-3-cyano-2-(4-methoxyphenyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 3b
White solid; mp 138–140 °C; 1H NMR (400 MHz, CDCl3) 7.51–7.49 (2H, d, J = 8.6 Hz), 7.11–7.05 (2H, m), 6.92–6.90 (2H, d, J = 8.6 Hz), 6.78 (1H, t, J = 7.5 Hz), 6.64–6.60 (1H, m), 4.68 (1H, s), 4.26 (1H, dd, J = 2.7, 7.9 Hz), 4.06 (2H, q, J = 7.0 Hz), 3.82 (3H, s), 2.85 (1H, dd, J = 8.2, 17.0 Hz), 2.62 (1H, dd, J = 3.3, 16.9 Hz), 0.86 (1H, t, J = 7.1 Hz); 13C NMR (100 MHz, CDCl3) 170.9, 167.0, 160.6, 142.8, 129.1, 128.4, 128.1, 127.4, 120.49, 119.11, 115.67, 114.91, 114.15, 81.48, 62.86, 61.69, 55.6, 55.4, 41.1, 38.7, 28.0, 13.8; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 2979, 2247, 1738, 1610, 1585, 1514, 1485, 1368, 1299, 1152, 1034, 839, 748 cm−1; LRMS-EI+ (m/z) 473.24 ([M + Na]+, 100), 451.36 (13.48), 459.34 (69.06); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C26H30N2NaO5 473.2052, found 473.2054.
) 2979, 2247, 1738, 1610, 1585, 1514, 1485, 1368, 1299, 1152, 1034, 839, 748 cm−1; LRMS-EI+ (m/z) 473.24 ([M + Na]+, 100), 451.36 (13.48), 459.34 (69.06); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C26H30N2NaO5 473.2052, found 473.2054.
Ethyl4-(2-(tert-butoxy)-2-oxoethyl)-3-cyano-2-phenyl-1,2,3,4-tetrahydroquinoline-3-carboxylate, 3c
White solid; mp 146–148 °C; 1H NMR (400 MHz, CDCl3) 7.59–7.57 (2H, m), 7.41–7.40 (3H, m), 7.12–7.06 (2H, m), 6.80 (1H, t, J = 7.5 Hz), 6.64 (1H, d, J = 7.7 Hz), 4.73 (1H, s), 4.29 (1H, dd, J = 2.9, 7.8 Hz), 4.12–3.94 (2H, m), 2.90–2.82 (1H, dd, J = 8.24, 16.9 Hz), 2.63 (1H, dd, J = 3.3, 16.9 Hz), 1.51 (9H, s), 1.00–0.97 (1H, d, J = 7.1 Hz); 13C NMR (75 MHz, CDCl3) 170.9, 166.9, 142.7, 136.4, 129.7, 128.9, 128.1, 127.9, 127.5, 120.5, 119.2, 115.5, 115.0, 81.5, 62.9, 62.3, 55.4, 41.2, 38.8, 28.0, 13.7; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 2978, 2918, 2247, 1731, 1605, 1586, 1487, 1368, 1247, 1152, 988, 848, 747, 700 cm−1; LRMS-EI+ (m/z) 443.19 ([M + Na]+, 100), 421.19 (25.05); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C25H28N2NaO4 443.1947, found 443.1947.
) 2978, 2918, 2247, 1731, 1605, 1586, 1487, 1368, 1247, 1152, 988, 848, 747, 700 cm−1; LRMS-EI+ (m/z) 443.19 ([M + Na]+, 100), 421.19 (25.05); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C25H28N2NaO4 443.1947, found 443.1947.
Ethyl 3-cyano-4-(2-methoxy-2-oxoethyl)-2-(4-nitrophenyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 3d
Yellow solid; mp 81–83 °C; 1H NMR (400 MHz, CDCl3) 8.29–8.24 (2H, m), 7.83–7.78 (2H, m), 7.17–7.11 (1H, m), 7.07–7.02 (1H, m), 6.88–6.82 (1H, m), 6.7–6.66 (1H, m), 4.88 (1H, s), 4.35 (1H, m), 4.24 (1H, s), 4.11–4.02 (2H, m), 3.78 (3H, s), 3.00 (1H, dd, J = 7.5, 17.1 Hz), 2.78–2.70 (1H, m), 1.04 (3H, t, J = 7.0 Hz); 13C NMR (100 MHz, CDCl3) 172.0, 166.5, 148.8, 143.4, 142.1, 129.3, 128.5, 127.3, 124.0, 120.0, 115.4, 114.8, 63.4, 61.3, 55.2, 52.5, 41.1, 37.1, 13.8; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3375, 2955, 2247, 1739, 1608, 1524, 1488, 1350, 1295, 1247, 1247, 1161, 1041, 858, 750, 699 cm−1; LRMS-EI+ (m/z) 424.45 ([M + H]+, 100), 276.37 (4); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C22H22N3O6 424.1509, found 424.1508.
) 3375, 2955, 2247, 1739, 1608, 1524, 1488, 1350, 1295, 1247, 1247, 1161, 1041, 858, 750, 699 cm−1; LRMS-EI+ (m/z) 424.45 ([M + H]+, 100), 276.37 (4); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C22H22N3O6 424.1509, found 424.1508.
Ethyl 3-cyano-4-(2-methoxy-2-oxoethyl)-2-(4-nitrophenyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 4d
Yellow solid; mp 70–72 °C; 1H NMR (400 MHz, CDCl3) 8.23 (2H, d, J = 8.8 Hz), 7.88 (2H, d, J = 8.8 Hz), 7.22–7.07 (2H, m), 6.81 (1H, dd, J = 7.5, 7.5 Hz), 6.67 (1H, d, J = 7.7 Hz), 4.98 (1H, s), 4.21 (1H, s), 4.18–3.99 (3H, m), 3.69 (3H, s), 3.01 (1H, dd, J = 7.9, 17.4 Hz), 2.69 (1H, dd, J = 5.1, 17.2 Hz), 1.16 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 171.2, 166.0, 148.6, 144.2, 140.9, 130.2, 129.4, 129.0, 123.7, 119.3, 119.29, 116.3, 114.7, 63.5, 55.2, 52.1, 50.7, 41.46, 39.7, 13.7; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ): 3391, 2927, 2247, 1737, 1609, 1524, 1495, 1349, 1241, 1173.1, 1040.1, 857.0, 751.9 cm−1; LRMS-EI+ (m/z) 424.45 ([M + H]+, 100), 276.37 (4); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C22H22N3O6 424.1509, found 424.1508.
): 3391, 2927, 2247, 1737, 1609, 1524, 1495, 1349, 1241, 1173.1, 1040.1, 857.0, 751.9 cm−1; LRMS-EI+ (m/z) 424.45 ([M + H]+, 100), 276.37 (4); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C22H22N3O6 424.1509, found 424.1508.
Ethyl 3-cyano-4-(2-methoxy-2-oxoethyl)-2-(2-methoxyphenyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 3e
White solid; mp 118–120 °C; 1H NMR (400 MHz, CDCl3) 7.90 (1H, d, J = 6.8 Hz), 7.37–7.33 (1H, m), 7.11–7.03 (3H, m), 6.89–6.87 (1H, d, J = 8.0 Hz), 6.81–6.77 (1H, m), 6.63–6.62 (1H, d, J = 8.0 Hz), 5.30 (1H, s), 4.43 (1H, dd, J = 2.9, 7.7 Hz), 4.13 (1H, m), 4.02–3.98 (2H, m), 3.78 (6H, b), 2.96 (1H, dd, J = 8.0, 14.0 Hz), 2.72 (1H, dd, J = 3.3, 16.9 Hz), 0.98 (3H, t, J = 7.0 Hz); 13C NMR (100 MHz, CDCl3) 172.3, 166.5, 157.1, 143.1, 130.4, 128.2, 128.0, 127.3, 124.9, 121.3, 120.5, 119.2, 116., 116.1, 115.2, 110.5, 62.7, 55.5, 54.2, 54.1, 52.3, 41.1, 37.4, 13.5; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3381, 2953, 2840, 2236, 1737, 1606, 1492, 1439, 1368, 1291, 1249, 1161, 1025, 857, 755 cm−1; LRMS-EI+ (m/z) 431.17 ([M + Na]+, 100), 409.30 ([M + H]+, 38.94); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C23H25N2O5 409.1763, found 409.1762.
) 3381, 2953, 2840, 2236, 1737, 1606, 1492, 1439, 1368, 1291, 1249, 1161, 1025, 857, 755 cm−1; LRMS-EI+ (m/z) 431.17 ([M + Na]+, 100), 409.30 ([M + H]+, 38.94); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C23H25N2O5 409.1763, found 409.1762.
Ethyl 3-cyano-2-(3,5-dimethoxyphenyl)-4-(2-methoxy-2-oxoethyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 3f
White powder; mp 156–158 °C; 1H NMR (400 MHz, CDCl3) 7.11 (1H, t, J = 7.5 Hz), 7.03–7.01 (1H, d, J = 7.4 Hz), 6.79 (1H, t, J = 7.5 Hz), 6.73–6.72 (2H, m), 6.66–6.64 (1H, d, J = 8.0 Hz), 6.49–6.47 (1H, m), 4.65 (1H, s), 4.33 (1H, dd, J = 2.9, 7.7 Hz), 4.25 (1H, s), 4.14–4.02 (2H, m), 3.79 (6H, s), 3.78 (3H, s), 2.97 (1H, dd, J = 7.9, 17.1 Hz), 2.71 (1H, dd, J = 3.5, 17.1 Hz), 1.05 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 172.1, 166.96, 161.1, 142.5, 138.5, 128.2, 127.2, 120.6, 119.31, 115.5, 115.0, 105.7, 101.85, 63.0, 62.3, 55.5, 55.2, 52.3, 41.1, 37.1, 13.7; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3380, 2955, 2841, 2242, 1737, 1609, 1598, 1475, 1434, 1353, 1246, 1156, 1062, 851, 749 cm−1; LRMS-EI+ (m/z) 461.15 ([M + Na]+, 100), [M + H]+ 439.19 (12.92); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C24H27N2O6 439.1869, found 439.1884.
) 3380, 2955, 2841, 2242, 1737, 1609, 1598, 1475, 1434, 1353, 1246, 1156, 1062, 851, 749 cm−1; LRMS-EI+ (m/z) 461.15 ([M + Na]+, 100), [M + H]+ 439.19 (12.92); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C24H27N2O6 439.1869, found 439.1884.
Ethyl 3-cyano-4-(2-methoxy-2-oxoethyl)-2-(2,4,6-trimethoxyphenyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 5a
Low melting yellow solid; 1H NMR (400 MHz, CDCl3), 7.13–7.05 (2H, m), 6.87–6.76 (2H, s), 6.11 (2H, s), 5.25 (1H, d, J = 7.0 Hz), 4.79 (1H, d, J = 7.0 Hz), 4.03–3.09 (3H, m), 3.82–3.76 (12H, m), 2.95 (1H, dd, J = 8.4, 12.9 Hz), 2.69 (1H, dd, J = 2.8, 16.9 Hz), 0.96 (1H, t, J = 7.1 Hz); 13C NMR (100 MHz, CDCl3) 172.6, 167.3, 161.9, 160.1, 143.3, 127.8, 127.4, 123.1, 120.3, 118.3, 116.8, 104.4, 90.8, 62.4, 55.7, 55.6, 55.4, 52.3, 51.9, 43.0, 37.3, 13.6;. FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3396, 2940, 2841, 2236, 1734, 1606, 1468, 1333, 1202, 1156, 1122, 809, 749 cm−1; LRMS-EI+ (m/z) 469.37 [M + H]+ (100), 467.38 (4.39); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C25H29N2O7 469.1975, found 469.1972.
) 3396, 2940, 2841, 2236, 1734, 1606, 1468, 1333, 1202, 1156, 1122, 809, 749 cm−1; LRMS-EI+ (m/z) 469.37 [M + H]+ (100), 467.38 (4.39); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C25H29N2O7 469.1975, found 469.1972.
Ethyl 3-cyano-4-(2-methoxy-2-oxoethyl)-2-(naphthalen-1-yl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 5b
White powder; mp 138–140 °C; 1H NMR (400 MHz, CDCl3) 8.27 (1H, d, J = 8.0 Hz), 8.06 (1H, d, J = 8.0 Hz), 7.92–7.87 (2H, m), 7.62–7.51 (3H, m), 7.15–7.10 (2H, m), 6.86–6.83 (1H, m), 6.68–6.66 (1H, m), 5.68 (1H, b), 4.59–4.56 (1H, m), 4.24 (1H, m), 3.79 (3H, s), 3.70–3.52 (2H, m), 3.02 (1H, dd, J = 7.9, 17.1 Hz), 2.75 (1H, dd, J = 3.5, 17.1 Hz), 0.53–0.49 (3H, m); 13C NMR (100 MHz, CDCl3) 172.3, 166.8, 143.0, 133.8, 132.4, 131.1, 129.9, 129.0, 128.2, 127.5, 126.6, 126.0, 125.7, 125.4, 122.5, 120.3, 119.5, 115.2, 63.0, 55.9, 54.8, 52.4, 41.6, 37.4, 13.0; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3382, 2912, 2253, 1737, 1602, 1487, 1435, 1372, 1245, 1169, 1040, 856, 748 cm−1; LRMS-EI+ (m/z) 451.33 ([M + Na]+, 100), [M + H]+ 429.58 (12.92); HRMS-TOF-ES− (m/z) [M + H]+ calcd for C26H25N2O4 429.1814, found 429.1814.
) 3382, 2912, 2253, 1737, 1602, 1487, 1435, 1372, 1245, 1169, 1040, 856, 748 cm−1; LRMS-EI+ (m/z) 451.33 ([M + Na]+, 100), [M + H]+ 429.58 (12.92); HRMS-TOF-ES− (m/z) [M + H]+ calcd for C26H25N2O4 429.1814, found 429.1814.
Ethyl 3-cyano-4-(2-methoxy-2-oxoethyl)-2-(naphthalen-2-yl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 5c
White solid; mp 136–138 °C; 1H NMR (400 MHz, CDCl3) 8.04 (1H, s), 7.89–7.85 (3H, m), 7.71–7.69 (1H, d J = 8.1 Hz), 7.54–7.52 (2H, m), 7.15–7.12 (2H, dd, J = 7.4, 7.6 Hz), 7.06 (1H, d, J = 8.0 Hz), 6.82 (1H, dd, J = 7.7, 7.7 Hz), 6.68 (1H, d, J = 8.0 Hz), 4.90 (1H, s), 4.42–4.39 (1H, m), 4.34 (1H, s), 4.0–3.94 (2H, m), 3.79 (3H, s), 3.01 (1H, dd, J = 7.9, 17.1 Hz), 2.74 (1H, dd, J = 3.7, 17.2 Hz), m), 0.82 (3H, t, J = 7.9 Hz); 13C NMR (100 MHz, CDCl3) 172.2, 142.7, 133.9, 133.7, 1331, 128.8, 128.3, 127.7, 127.7, 127.3, 126.8, 126.6, 125.0, 120.1, 119.4, 115.1, 63.0, 62.4, 55.3, 52.4, 41.2, 37.2, 13.6; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3379, 2954, 2242, 1740, 1608, 1483, 1436, 1369, 241, 1160, 1042, 854, 781, 748 cm−1; LRMS-EI+ (m/z) 451.33 ([M + Na]+, 100), 443.52 (17.75), [M + H]+ 429.46 (12.92); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C26H25N2O4 429.1814, found 429.1812.
) 3379, 2954, 2242, 1740, 1608, 1483, 1436, 1369, 241, 1160, 1042, 854, 781, 748 cm−1; LRMS-EI+ (m/z) 451.33 ([M + Na]+, 100), 443.52 (17.75), [M + H]+ 429.46 (12.92); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C26H25N2O4 429.1814, found 429.1812.
Ethyl 3-cyano-4-(2-methoxy-2-oxoethyl)-2-(4-methoxyphenyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 5e
White powder; mp 128–130 °C; 1H NMR (400 MHz, CDCl3) 7.49 (2H, d, J = 8.8 Hz), 7.11 (1H, dd, J = 7.7, 7.7 Hz), 7.02 (1H, d, J = 7.7 Hz), 6.92 (2H, d, J = 8.8 Hz), 6.79 (1H, dd, J = 7.5, 7.5 Hz), 6.63 (1H, d, J = 7.7 Hz), 4.68 (1H, s), 4.33 (1H, m, J = 3.7, 7.7 Hz), 4.22 (1H, s), 4.04 (2H, q, J = 7.2 Hz), 3.82 (3H, m), 3.78 (3H, m), 2.97 (1H, dd, J = 7.9, 17.1 Hz), 2.71 (1H, dd, J = 3.7, 16.9 Hz), 1.02 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 172.2, 167.0, 160.6, 142.8, 129.1, 128.3, 128.2, 127.3, 112.0, 119.2, 115.0, 114.2, 63.0, 61.7, 55.5, 55.4, 52.4, 41.1, 37.2, 13.8; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3381, 2955, 2247, 1734, 1610, 1532, 1485, 1299, 1249, 1176, 1033, 841, 749 cm−1; LRMS-EI+ (m/z) 409.49 ([M + H]+, 100), 333.43 (13.60), 407.36 (5); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C23H25N2O5 409.1763, found 409.1764.
) 3381, 2955, 2247, 1734, 1610, 1532, 1485, 1299, 1249, 1176, 1033, 841, 749 cm−1; LRMS-EI+ (m/z) 409.49 ([M + H]+, 100), 333.43 (13.60), 407.36 (5); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C23H25N2O5 409.1763, found 409.1764.
Ethyl 6-chloro-3-cyano-4-(2-methoxy-2-oxoethyl)-2-(2-methoxyphenyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 9a
White solid; mp 164–166 °C; 1H NMR (400 MHz, CDCl3) 7.86 (1H, d, J = 7.8 Hz), 7.38–7.35 (1H, m), 7.08–7.02 (3H, m), 6.89–6.87 (1H, m), 6.58–6.56 (1H, m), 5.27 (1H, s), 4.40–4.38 (1H, m), 4.15 (1H, s), 4.03–3.96 (2H, m), 3.86 (1H, s), 3.80 (3H, b), 3.78 (3H, b), 2.93 (1H, dd, J = 7.9, 17.1 Hz), 2.71 (1H, dd, J = 3.4, 17.0 Hz), 0.97 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 172.0, 166.2, 157.1, 141.7, 130.5, 128.1, 127.31, 124.4, 123.8, 122.0, 121.3, 116.3, 115.8, 110.5, 62.9, 55.5, 54.2, 53.6, 52.5, 13.5; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3389, 2952, 2225, 1722, 1602, 1492, 1465, 1368, 1296, 1258, 1170, 1051, 1023, 862, 824, 755 cm−1; LRMS-EI+ (m/z) 465.40 ([M + Na]+, 100), 443.60 (43.69), 444.70 (16.67), 445.66 (13.54); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C23H24ClN2O5 443.1374, found 443.1373.
) 3389, 2952, 2225, 1722, 1602, 1492, 1465, 1368, 1296, 1258, 1170, 1051, 1023, 862, 824, 755 cm−1; LRMS-EI+ (m/z) 465.40 ([M + Na]+, 100), 443.60 (43.69), 444.70 (16.67), 445.66 (13.54); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C23H24ClN2O5 443.1374, found 443.1373.
Ethyl 6-chloro-3-cyano-2-(3,5-dimethoxyphenyl)-4-(2-methoxy-2-oxoethyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 9b
White powder; mp 179–181 °C; 1H NMR (400 MHz, CDCl3), 7.08–7.06 (1H, d, J = 8.0 Hz), 6.99 (1H, b), 6.69 (2H, b), 6.59 (1H, d, J = 8.8 Hz), 6.48 (1H, m), 4.62 (1H, s), 4.29–4.27 (2H, m), 4.11–4.05 (2H, m), 3.79 (9H, b), 2.94 (1H, dd, J = 8.1, 17.2 Hz), 2.71 (1H, dd, J = 3.7, 17.2 Hz), 1.04 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 171.8, 166.6, 161.1, 141.1, 138.0, 128.3, 127.2, 123.9, 121.5, 116.2, 115.2, 105.7, 101.8, 63.2, 62.3, 55.5, 54.8, 52.5, 41.0, 36.9, 13.7; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3379, 2955, 2242, 1734, 1599, 1493, 1470, 1353, 1300, 1245, 1156, 1060, 850, 696 cm−1; LRMS-EI+ (m/z) 495.47 ([M + Na]+, 100), 473.48 ([M + H]+, 13.13), 493.43 (15.24), 494.28 (15.19); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C24H26ClN2O6 473.1479, found 473.1475.
) 3379, 2955, 2242, 1734, 1599, 1493, 1470, 1353, 1300, 1245, 1156, 1060, 850, 696 cm−1; LRMS-EI+ (m/z) 495.47 ([M + Na]+, 100), 473.48 ([M + H]+, 13.13), 493.43 (15.24), 494.28 (15.19); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C24H26ClN2O6 473.1479, found 473.1475.
Ethyl 6-chloro-3-cyano-4-(2-methoxy-2-oxoethyl)-2-(naphthalen-2-yl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 9c
White powder; mp 172–174 °C; white powder; 1H NMR (400 MHz, CDCl3) 8.01 (1H, s), 7.91–7.84 (3H, m), 7.69–7.65 (1H, m), 7.55–7.51 (2H, m), 7.12–7.07 (1H, m), 7.03 (1H, s), 6.64–6.60 (1H, m), 4.87 (1H, s), 4.40–4.33 (2H, m), 4.00–3.91 (2H, m), 3.80 (3H, s), 2.97 (1H, dd, J = 8.1, 17.2 Hz), 2.74 (1H, dd, J = 3.7, 17.2 Hz), 0.81 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 171.8, 166.6, 161.1, 141.1, 138.1, 128.3, 127.2, 123.9, 121.5, 116.2, 115.2, 105.7, 101.8, 63.2, 62.3, 55.5, 54.8, 52.5, 41.0, 36.9, 13.7; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3376, 2953, 2247, 1737, 1605, 1493, 1369, 1311, 1245, 1169, 1048, 818, 758, 672 cm−1; LRMS-EI+ (m/z) 485.43 ([M + Na]+, 100), 463.54 (30.42). HR-MS-EI+ (m/z) [M]+ calcd for C26H23ClN2O4 462.1346, found 462.1347.
) 3376, 2953, 2247, 1737, 1605, 1493, 1369, 1311, 1245, 1169, 1048, 818, 758, 672 cm−1; LRMS-EI+ (m/z) 485.43 ([M + Na]+, 100), 463.54 (30.42). HR-MS-EI+ (m/z) [M]+ calcd for C26H23ClN2O4 462.1346, found 462.1347.
Ethyl 6-chloro-3-cyano-4-(2-methoxy-2-oxoethyl)-2-(naphthalen-1-yl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 9d
White solid; mp 230–232 °C; 1H NMR (400 MHz, CDCl3) 8.23 (1H, d, J = 7.3 Hz), 8.03 (1H, d, J = 8.1 Hz), 7.93–7.88 (2H, m), 7.62–7.49 (3H, m), 7.11–7.08 (2H, m), 6.62–6.59 (1H, m), 5.65 (1H, m), 4.54–4.51 (1H, m), 4.26 (1H, b), 3.80 (3H, s), 3.68–3.52 (2H, m), 2.99 (1H, dd, J = 7.9, 17.1 Hz), 2.74 (1H, dd, J = 3.2, 17.0 Hz), 1.29–1.24 (1H, m), 0.51 (3H, t, J = 7.0 Hz); 13C NMR (100 MHz, CDCl3) 171.9, 166.5, 141.6, 133.8, 132.0, 131.0, 130.1, 129.0, 128.3, 127.4, 126.7, 126.1, 125.7, 125.3, 124.1, 122.3, 121.8, 116.3, 115.6, 63.1, 55.8, 54.4, 52.5, 41.5, 37.2, 13.0; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3372, 2951, 2236, 1728, 1706, 1610, 1508, 1435, 1294, 1234, 1190, 1116, 1068, 784 cm−1; LRMS-EI+ (m/z) 485.22 ([M + Na]+, 100), 463.32 ([M + H]+, 15.47), 443.39 (18.56); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C26H24ClN2O5 463.1425, found 463.1427.
) 3372, 2951, 2236, 1728, 1706, 1610, 1508, 1435, 1294, 1234, 1190, 1116, 1068, 784 cm−1; LRMS-EI+ (m/z) 485.22 ([M + Na]+, 100), 463.32 ([M + H]+, 15.47), 443.39 (18.56); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C26H24ClN2O5 463.1425, found 463.1427.
Ethyl 6-chloro-3-cyano-4-(2-methoxy-2-oxoethyl)-2-(pyridin-2-yl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 9e
White solid; mp 136–138 °C; 1H NMR (400 MHz, CDCl3) 8.68 (1H, d, J = 4.7 Hz), 7.78–7.73 (1H, m), 7.44 (1H, d, J = 7.7 Hz), 7.35 (1H, dd, J = 5.0, 6.96 Hz), 7.11 (1H, dd, J = 1.9, 8.6 Hz), 7.03 (1H, s), 6.74 (1H, d, J = 8.8 Hz), 5.00 (1H, d, J = 4.2 Hz), 4.87 (1H, d, J = 4.2 Hz), 4.29 (1H, dd, J = 2.8, 7.9 Hz), 4.19–4.12 (2H, m), 3.81 (3H, s), 2.95 (1H, dd, J = 8.1, 17.2 Hz), 2.74 (1H, dd, J = 3.3, 17.2 Hz), 1.12 (3H, dd, J = 7.2, 7.2 Hz); 13C NMR (100 MHz, CDCl3) 171.9, 161.2, 154.5, 149.6, 140.5, 137.1, 128.4, 127.0, 124.6, 124.0, 122.7, 122.4, 118.1, 114.5, 63.2, 62.2, 52.8, 52.5, 41.1, 36.6, 13.8; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3368, 2954, 2242, 1737, 1590, 1493, 1471, 1437, 1243, 1170.3, 1049, 816, 753 cm−1; LRMS-EI+ (m/z) 436.20 ([M + Na]+, 100), 414.24 ([M + H]+ 93.14); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C21H25ClN3O4 414.1221, found 414.1215.
) 3368, 2954, 2242, 1737, 1590, 1493, 1471, 1437, 1243, 1170.3, 1049, 816, 753 cm−1; LRMS-EI+ (m/z) 436.20 ([M + Na]+, 100), 414.24 ([M + H]+ 93.14); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C21H25ClN3O4 414.1221, found 414.1215.
Ethyl 6-chloro-3-cyano-4-(2-methoxy-2-oxoethyl)-2-(thiophen-3-yl)-1,2,3,4-tetrahydroquinoline-3-carboxylate (9f)
White solid; mp 116–118 °C; 1H NMR (400 MHz, CDCl3) 7.86 (1H, d, J = 7.3 Hz), 7.36 (1H, t, J = 7.9 Hz), 7.09–7.01 (3H, m), 6.90–6.87 (1H, m), 6.58–6.54 (1H, m), 5.26 (1H, s), 4.38 (1H, m), 4.15 (1H, s), 4.00 (2H, q, J = 7.1 Hz), 3.79–3.80 (6H, b), 2.97–2.88 (1H, dd, J = 7.7, 16.9 Hz), 2.71 (1H, dd, J = 3.1, 17.1 Hz), 0.96 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 172.0, 166.2, 157.1, 141.7, 130.6, 128.1, 127.3, 124.5, 123.8, 122.0, 1213, 116.3, 115.8, 110.5, 62.9, 55.5, 55.2, 53.6, 52.5, 41.0, 37.2, 13.5; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3380, 2954, 2247, 1739, 1606, 1492, 1244, 1170, 1049, 814, 751, 606 cm−1; LRMS-EI+ (m/z) 441.33 ([M + Na]+, 100), 419.28 ([M + H]+ 7.96); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C20H20ClN2O4S 419.0832, found 419.0829.
) 3380, 2954, 2247, 1739, 1606, 1492, 1244, 1170, 1049, 814, 751, 606 cm−1; LRMS-EI+ (m/z) 441.33 ([M + Na]+, 100), 419.28 ([M + H]+ 7.96); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C20H20ClN2O4S 419.0832, found 419.0829.
Ethyl 6-chloro-3-cyano-4-(2-methoxy-2-oxoethyl)-2-(thiophen-2-yl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 9g
White solid; 1H NMR (400 MHz, CDCl3) 7.36 (1H, d, J = 5.0 Hz), 7.26 (1H, m), 7.08–7.01 (3H, m), 6.59 (1H, d, J = 8.4 Hz), 5.03 (1H, s), 4.38 (1H, s), 4.27 (1H, dd, J = 3.9, 7.5 Hz), 4.16–4.05 (2H, m), 3.78 (3H, m), 2.94 (1H, dd, J = 7.7, 17.2 Hz), 2.74 (1H, dd, J = 3.9, 17.3 Hz), 1.08 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 171.8, 166.6, 140.8, 138.0, 128.4, 127.4, 127.3, 127.0, 126.7, 124.3, 121.7, 116.4, 115.0, 63.4, 58.2, 55.8, 52.5, 40.8, 37.0, 13.7; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 2918, 2857, 2335, 1739, 1657, 1599, 1575, 1487, 1361, 1380, 1306, 1246, 1161, 1045, 815, 705 cm−1; LRMS-EI+ (m/z) 441.07 ([M + Na]+, 100), 419.03 ([M + H]+, 24.40). HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C20H20ClN2O4S 419.0832, found 419.0833.
) 2918, 2857, 2335, 1739, 1657, 1599, 1575, 1487, 1361, 1380, 1306, 1246, 1161, 1045, 815, 705 cm−1; LRMS-EI+ (m/z) 441.07 ([M + Na]+, 100), 419.03 ([M + H]+, 24.40). HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C20H20ClN2O4S 419.0832, found 419.0833.
3-Ethyl 6-methyl 3-cyano-4-(2-methoxy-2-oxoethyl)-2-(naphthalen-2-yl)-1,2,3,4-tetrahydroquinoline-3,6-dicarboxylate, 9h
White powder; mp 196–198 °C; 1H NMR (400 MHz, CDCl3) 8.02 (1H, s), 7.99–7.80 (6H, m), 7.68–7.66 (1H, d, J = 8.5 Hz), 7.62–7.45 (2H, m), 6.69 (1H, d, J = 8.4 Hz), 5.00 (1H, s), 4.79 (1H, s), 4.41–4.34 (1H, m), 4.05–3.94 (2H, m), 3.87 (3H, s), 3.83 (3H, s), 3.07 (1H, dd, J = 8.1, 16.9 Hz), 2.73 (1H, dd, J = 3.8, 16.9 Hz), 0.85 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 171.9, 166.8, 166.6, 146.5, 134.0, 133.0, 132.9, 130.2, 129.6, 129.0, 128.3, 127.7, 127.7, 127.1, 126.8, 124.70, 119.0, 115.0, 114.2, 63.3, 62.1, 54.5, 52.5, 51.8, 41.3, 36.5, 13.6; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3376, 2952, 2236, 1737, 1713, 1611, 1515, 1436, 1370, 1298, 1241, 11
) 3376, 2952, 2236, 1737, 1713, 1611, 1515, 1436, 1370, 1298, 1241, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 112, 1049, 855, 767 cm−1; LRMS-EI+ (m/z) 509.17 ([M + Na]+, 100), 487.26 ([M + H]+, 12.21), 443.31 (16.46); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C28H27N2O6 487.1869, found 487.1865.
112, 1049, 855, 767 cm−1; LRMS-EI+ (m/z) 509.17 ([M + Na]+, 100), 487.26 ([M + H]+, 12.21), 443.31 (16.46); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C28H27N2O6 487.1869, found 487.1865.
3-Ethyl 6-methyl 3-cyano-4-(2-methoxy-2-oxoethyl)-2-(naphthalen-1-yl)-1,2,3,4-tetrahydroquinoline-3,6-dicarboxylate, 9i
White solid; mp 230–232 °C; 1H NMR (400 MHz, CDCl3) 8.22 (1H, d, J = 7.3 Hz), 8.03 (1H, d, J = 8.2 Hz), 7.94–7.80 (4H, m), 7.62–7.52 (3H, m), 6.64 (1H, d, J = 8.3 Hz), 5.76 (1H, s), 4.70 (1H, s), 4.55 (1H, dd, J = 3.2, 7.7 Hz), 3.87 (3H, s), 3.82 (3H, s), 3.68–3.52 (2H, m), 3.06 (1H, dd, J = 8.1, 16.8 Hz), 2.73 (1H, dd, J = 3.4, 16.8 Hz), 0.52 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 171.98, 166.8, 166.4, 146.8, 133.8, 131.7, 130.9, 130.3, 130.2, 129.8, 129.1, 126.2, 125.7, 125.4, 122.3, 120.5, 119.1, 115.4, 114.2, 63.2, 55.5, 53.9, 52.5, 51.9, 41.6, 36.8, 13.0; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3372, 2951, 2236, 1728, 1707, 1610, 1508, 1435, 1334, 1293.9, 1234, 1190, 1116, 1068, 784 cm−1; LRMS-EI+ (m/z) 509.20 ([M + Na]+, 100), 487.25 ([M + H]+, 10.25), 443.23 (16.84); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C28H27N2O6 488.1869, found 487.1876.
) 3372, 2951, 2236, 1728, 1707, 1610, 1508, 1435, 1334, 1293.9, 1234, 1190, 1116, 1068, 784 cm−1; LRMS-EI+ (m/z) 509.20 ([M + Na]+, 100), 487.25 ([M + H]+, 10.25), 443.23 (16.84); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C28H27N2O6 488.1869, found 487.1876.
3-Ethyl 6-methyl 3-cyano-4-(2-methoxy-2-oxoethyl)-2-(2-methoxyphenyl)-1,2,3,4-tetrahydroquinoline-3,6-dicarboxylate, 9j
White solid; mp 212–215 °C; 1H NMR (400 MHz, CDCl3) 7.86 (1H, d, J = 8.8 Hz), 7.78–7.76 (2H, m), 7.37 (1H, dd, J = 7.7, 7.7 Hz), 7.06 (1H, dd, J = 7.5, 7.5 Hz), 6.89 (1H, d, J = 8.4 Hz), 6.60 (1H, d, J = 8.8 Hz), 5.39 (1H, s), 4.55 (1H, s), 4.38 (1H, dd, J = 2.8, 7.9 Hz), 4.11–3.92 (2H, m), 3.85 (3H, s), 3.82 (3H, s), 3.79 (3H, s), 3.01 (1H, dd, J = 8.1, 16.7 Hz), 2.70 (1H, dd, J = 3.3, 16.7 Hz), 0.99 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 172.0, 166.9, 166.1, 157.1, 147.0, 130.7, 130.0, 129.6, 128.0, 124.1, 121.3, 120.2, 119.2, 115.6, 114.5, 110.6, 63.0, 55.5, 53.8, 53.2, 52.4, 51.7, 41.1, 36.8, 13.5; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3374, 2945, 2396, 2242, 1739, 1709, 1611, 1436, 1298, 1241, 1113, 767 cm−1; LRMS-EI+ (m/z): 489.16 ([M + Na]+, 100), 467.23 ([M + H]+, 8.41); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C25H27N2O7 467.1818, found 467.1816.
) 3374, 2945, 2396, 2242, 1739, 1709, 1611, 1436, 1298, 1241, 1113, 767 cm−1; LRMS-EI+ (m/z): 489.16 ([M + Na]+, 100), 467.23 ([M + H]+, 8.41); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C25H27N2O7 467.1818, found 467.1816.
Ethyl 3-cyano-2-(3,5-dimethoxyphenyl)-4-(2-methoxy-2-oxoethyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 9k
White powder; mp 110–112 °C; 1H NMR (400 MHz, CDCl3) 7.81–7.78 (2H, m), 6.69 (2H, s), 6.64 (1H, d, J = 8.4 Hz), 6.48 (1H, s), 4.73 (1H, s), 4.31 (1H, dd, J = 3.0, 7.6 Hz), 4.18–4.04 (2H, m), 3.86–3.79 (12H, m), 3.05 (1H, dd, J = 8.0, 16.9 Hz), 2.72 (1H, dd, J = 3.6, 16.9 Hz), 1.12–1.06 (3H, t, J = 7.12 Hz); 13C NMR (100 MHz, CDCl3) 171.8, 166.8, 66.5, 161.2, 146.4, 137.7, 130.2, 129.6, 120.5, 118.9, 115.0, 114.2, 105.7, 101.9, 63.3, 62.0, 55.5, 54.3, 52.5, 51.8, 41.2, 36.5, 13.7; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 2954, 2841, 2242, 1737, 1709, 1610, 1514, 1466, 1436, 1299, 1240, 1205, 1113, 992, 850, 768 cm−1; LRMS-EI+ (m/z) 519.55 ([M + Na]+, 100), 577.00 (2.36), 581.12 (5.13). HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C26H29N2O8 497.1924, found 497.1921.
) 2954, 2841, 2242, 1737, 1709, 1610, 1514, 1466, 1436, 1299, 1240, 1205, 1113, 992, 850, 768 cm−1; LRMS-EI+ (m/z) 519.55 ([M + Na]+, 100), 577.00 (2.36), 581.12 (5.13). HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C26H29N2O8 497.1924, found 497.1921.
Ethyl 3-cyano-2-(naphthalen-2-yl)-4-(2-oxo-2-phenylethyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 9l
White solid; mp 196–198 °C; 1H NMR (400 MHz, CDCl3) 8.09–8.07 (3H, m), 7.90–7.85 (3H, m), 7.74–7.72 (1H, d, J = 8.2 Hz), 7.63 (1H, t, J = 7.3 Hz), 7.54–7.50 (4H, m), 7.11 (1H, t, J = 7.5 Hz), 6.87–6.85 (1H, d, J = 7.8 Hz), 6.72 (2H, dd, J = 8.1, 16.5 Hz), 4.98 (1H, s), 4.82–4.77 (1H, m), 4.39 (1H, s), 3.92 (2H, q, J = 7.2 Hz), 3.80 (1H, dd, J = 7.7, 18.3 Hz), 3.30 (1H, dd, J = 2.9, 18.3 Hz), 0.79 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 196.9, 166.9, 142.8, 136.1, 133.9, 133.8, 133.1, 128.9, 128.8, 128.4, 128.3, 128.1, 127.7, 126.8, 126.6, 125.1, 120.6, 119.3, 116.1, 115.1, 63.0, 62.3, 55.7, 42.0, 39.9, 13.6; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ): 3377, 3057, 2247, 1739, 1686, 1607, 1485, 1366, 1318, 12
): 3377, 3057, 2247, 1739, 1686, 1607, 1485, 1366, 1318, 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 445, 1052, 820, 751, 639 cm−1; LRMS-EI+ (m/z) 497.19 ([M + Na]+, 100), 475.21 ([M + H]+ 21.10); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C31H27N2O3 475.2022, found 475.2021.
445, 1052, 820, 751, 639 cm−1; LRMS-EI+ (m/z) 497.19 ([M + Na]+, 100), 475.21 ([M + H]+ 21.10); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C31H27N2O3 475.2022, found 475.2021.
Ethyl 3-cyano-2-(2-methoxyphenyl)-4-(2-oxo-2-phenylethyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 9m
White solid; mp 86–88 °C; 1H NMR (400 MHz, CDCl3) 8.07 (2H, m, J = 7.2 Hz), 7.93 (1H, d, J = 7.6 Hz), 7.62 (1H, t, J = 7.2 Hz), 7.52 (2H, m), 7.36 (1H, t, J = 7.9 Hz), 7.09–7.04 (2H, m), 6.86 (2H, dd, J = 8.1, 26.8 Hz), 6.69 (1H, t, J = 7.3 Hz), 6.63 (1H, d, J = 7.9 Hz), 5.39 (1H, s), 4.83 (1H, d, J = 7.7 Hz), 4.15 (1H, s), 4.02–3.88 (2H, m), 3.81–3.73 (4H, m), 3.25 (1H, dd, J = 2.2, 18.3 Hz), 0.93 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 196.9, 189.4, 166.5, 157.2, 143.2, 136.3, 133.6, 130.3, 128.8, 128.7, 128.4, 128.2, 127.7, 125.1, 121.3, 121.0, 119.2, 116.8, 115.2, 110.6, 62.7, 55.5, 54.4, 54.2, 42.2, 39.8, 13.5; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3382, 2923, 2346, 1740, 1687, 1602, 1493, 1289, 1248, 1052
) 3382, 2923, 2346, 1740, 1687, 1602, 1493, 1289, 1248, 1052![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 753 cm−1; LRMS-EI+ (m/z) 477.17 ([M + Na]+, 100), 455.30 ([M + H]+, 15.55); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C28H27N2O4 455.1971, found 455.1972.
753 cm−1; LRMS-EI+ (m/z) 477.17 ([M + Na]+, 100), 455.30 ([M + H]+, 15.55); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C28H27N2O4 455.1971, found 455.1972.
Ethyl 3-cyano-2-(4-nitrophenyl)-4-(2-oxo-2-phenylethyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate (major), 9n
Yellow crystal; mp 156–158 °C; 1H NMR (400 MHz, CDCl3) 8.31–8.29 (1H, d, J = 7.2 Hz), 8.12–8.09 (3H, m), 7.93–7.88 (2H, dd, J = 8.3, 11.6 Hz), 7.65–7.49 (6H, m), 7.13–7.09 (2H, m), 6.89 (1H, d, J = 7.6 Hz), 6.76–6.67 (2H, m), 5.78 (1H, b), 5.00 (1H, b), 4.27 (1H, b), 3.83 (1H, dd, J = 7.9, 18.3 Hz), 3.66–3.53 (2H, m), 3.30–3.26 (1H, m), 0.49 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 196.9, 166.8, 143.1, 136.1, 133.8, 132.6, 131.1, 129.9, 128.9, 128.0, 127.9, 126.7, 126.0, 125.7, 125.3, 122.6, 120.9, 119.5, 116.6, 115.2, 63.0, 55.9, 55.1, 42.2, 40.3, 13.0; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3382, 3056, 2242, 1737, 1686, 1597, 1481, 1365, 1240, 1052, 781, 752, 690 cm−1; LRMS-EI+ (m/z) 497.32 ([M + Na]+, 100), 475.38 ([M + H]+, 4.05); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C31H27N2O3 475.2022, found 475.2020.
) 3382, 3056, 2242, 1737, 1686, 1597, 1481, 1365, 1240, 1052, 781, 752, 690 cm−1; LRMS-EI+ (m/z) 497.32 ([M + Na]+, 100), 475.38 ([M + H]+, 4.05); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C31H27N2O3 475.2022, found 475.2020.
Ethyl 3-cyano-4-(2-oxo-2-phenylethyl)-2-(thiophen-2-yl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 9o
White powder; mp 170–172 °C; 1H NMR (400 MHz, CDCl3) 8.08–8.06 (2H, d, J = 7.5 Hz), 7.63 (1H, t, J = 7.3 Hz), 7.52 (2H, t, J = 7.5 Hz), 7.36–7.30 (2H, m), 7.11–7.03 (2H, m), 6.84–6.82 (1H, d, J = 7.7 Hz), 6.74–6.68 (2H, m), 5.15 (1H, s), 4.72 (1H, dd, J = 2.6, 7.0 Hz), 4.39 (1H, s), 4.14–4.02 (2H, m), 3.77 (1H, dd, J = 7.5, 18.5 Hz), 3.34 (1H, dd, J = 3.1, 18.5 Hz), 1.05 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 196.8, 166.8, 142.3, 138.8, 136.1, 133.7, 128.9, 128.4, 128.1, 127.6, 127.3, 126.9, 126.5, 120.7, 119.7, 115.9, 115.3, 63.1, 58.1, 56.6, 42.1, 39.5, 13.7; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3369, 2929, 2242, 1737, 1685, 1607, 1482, 1365, 1242, 1052, 971, 855, 751, 690 cm−1; LRMS-EI+ (m/z) 453.20 ([M + Na]+, 100), 451.36 (21.10), 431.33 ([M + H]+, 1.34); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C25H23N2O3S 431.1429, found 431.1414.
) 3369, 2929, 2242, 1737, 1685, 1607, 1482, 1365, 1242, 1052, 971, 855, 751, 690 cm−1; LRMS-EI+ (m/z) 453.20 ([M + Na]+, 100), 451.36 (21.10), 431.33 ([M + H]+, 1.34); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C25H23N2O3S 431.1429, found 431.1414.
Ethyl 3-cyano-2-(3,5-dimethoxyphenyl)-4-(2-oxo-2-phenylethyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 9p
White solid; mp 130–132 °C; 1H NMR (400 MHz, CDCl3) 8.07 (2H, d, J = 7.3 Hz), 7.63 (1H, t, J = 7.3 Hz), 7.52 (2H, t, J = 7.7 Hz), 7.09 (1H, t, J = 7.5 Hz), 6.82–6.65 (5H, m), 6.48 (1H, t, J = 2.0 Hz), 4.74 (1H, b), 4.29 (1H, s), 4.10–3.98 (2H, m), 3.80 (6H, m), 3.76 (1H, dd, J = 3.3, 10.5 Hz), 3.25 (1H, dd, J = 2.8, 18.5 Hz), 1.01 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 196.8, 166.9, 161.0, 142.6, 138.7, 136.1, 133.8, 128.9, 128.0, 127.7, 120.5, 119.3, 116.1, 115.0, 105.7, 101.7, 63.0, 62.2, 55.5, 41.9, 39.8, 13.8; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3374, 2923, 1597, 2835, 2247, 1737, 1685, 1597, 1473, 1347, 1202, 1243, 1158, 1059, 987, 933, 749, 694, 634
) 3374, 2923, 1597, 2835, 2247, 1737, 1685, 1597, 1473, 1347, 1202, 1243, 1158, 1059, 987, 933, 749, 694, 634![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 535 cm−1; LRMS-EI+ (m/z) 507.48 ([M + Na]+, 100), 485.52, ([M + H]+, 4.80); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C29H29N2O5 485.2076, found 485.2075.
535 cm−1; LRMS-EI+ (m/z) 507.48 ([M + Na]+, 100), 485.52, ([M + H]+, 4.80); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C29H29N2O5 485.2076, found 485.2075.
Ethyl 3-cyano-2-(4-nitrophenyl)-4-(2-oxo-2-phenylethyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 9q
Yellow crystal; mp 198–200 °C; 1H NMR (400 MHz, CDCl3) 8.27 (2H, d, J = 8.6 Hz), 8.06 (2H, d, J = 7.3 Hz), 7.84 (2H, d, J = 8.6 Hz), 7.63 (1H, t, J = 7.2 Hz), 7.54–7.50 (2H, m), 7.11 (1H, t, J = 7.3 Hz), 6.87–6.85 (1H, m), 6.78–6.68 (2H, m), 4.96 (1H, s), 4.76–4.72 (1H, m), 4.26 (1H, s), 4.01 (2H, q, J = 7.1 Hz), 3.77 (1H, dd, J = 7.3, 18.7 Hz), 3.38–3.30 (1H, dd, J = 3.2, 18.6 Hz), 0.97 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 196.6, 166.5, 148.8, 143.5, 142.2, 135.9, 133.9, 129.3, 128.9, 128.4, 128.3, 127.7, 124.0, 120.6, 120.0, 115.4, 115.4, 63.4, 61.2, 55.5, 42.0, 39.7, 13.8; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3376, 2929, 2242, 1739, 1686, 1608, 1524, 1488, 1347, 1246, 1109, 659, 750, 690 cm−1; LRMS-EI+ (m/z) 492.50 ([M + Na]+, 100), 470.56 ([M + H]+, 15.16), 443.7 (11.79); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C27H24N3O5 470.1716, found 470.1719.
) 3376, 2929, 2242, 1739, 1686, 1608, 1524, 1488, 1347, 1246, 1109, 659, 750, 690 cm−1; LRMS-EI+ (m/z) 492.50 ([M + Na]+, 100), 470.56 ([M + H]+, 15.16), 443.7 (11.79); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C27H24N3O5 470.1716, found 470.1719.
Ethyl 3-cyano-4-(2-oxo-2-phenylethyl)-2-(2,4,6-trimethoxyphenyl)-1,2,3,4-tetrahydroquinoline-3-carboxylate, 9r
White powder; mp 188–190 °C; 1H NMR (400 MHz, CDCl3) 8.13–8.11 (2H, m), 7.66–7.52 (3H, m), 7.13–7.09 (1H, m), 6.88–6.75 (3H, m), 6.15 (2H, b), 5.36 (1H, d, J = 7.7 Hz), 4.91–4.70 (2H, m), 4.06–3.78 (12H, m), 3.25 (1H, dd, J = 1.8, 16.6 Hz), 0.94 (3H, t, J = 7.2 Hz); 13C NMR (100 MHz, CDCl3) 197.3; 167.4, 161.8, 160.1, 143.4, 136.4, 133.6, 128.8, 128.4, 127.7, 127.6, 123.5, 120.2, 118.2, 117.5, 104.5, 90.7, 62.3, 55.7, 55.4, 52.2, 42.1, 41.8, 13.6; FT-IR (KBr,![[small nu, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e0e1.gif) ) 3392, 2938, 2236, 1737, 1686, 1602, 1583, 1468, 1231, 1155, 1137, 1106, 972, 816, 748, 690 cm−1; LRMS-EI+ (m/z) 514.55 ([M + H]+, 100), 537.15 ([M + Na]+, 55.28), 538.1 (29.63); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C30H31N2O6 515.2182, found 515.2181.
) 3392, 2938, 2236, 1737, 1686, 1602, 1583, 1468, 1231, 1155, 1137, 1106, 972, 816, 748, 690 cm−1; LRMS-EI+ (m/z) 514.55 ([M + H]+, 100), 537.15 ([M + Na]+, 55.28), 538.1 (29.63); HRMS-TOF-ES+ (m/z) [M + H]+ calcd for C30H31N2O6 515.2182, found 515.2181.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Ms. L. M. Hsu, at the Instruments Center, National Chung Hsing University, for her help in obtaining mass spectral data, and the Ministry of Science and Technology, Taiwan, for financially supporting this research under the contract MOST 107-2113-M-277-001.Notes and references
- K. C. Nicolaou, D. J. Edmonds and P. G. Bulger, Angew. Chem., Int. Ed., 2006, 45, 7134 CrossRef CAS PubMed.
- K. M. Witherup, R. W. Ransom, A. C. Graham, A. M. Bernard, M. J. Salvatore, W. C. Lumma, P. S. Anderson, S. M. Pitzenberger and S. L. Varga, J. Am. Chem. Soc., 1995, 117, 6682 CrossRef CAS.
- J. H. Rakotoson, N. Fabre, I. Jacquemond-Collet, S. Hannedouche, I. Fourasté and C. Moulis, Planta Med., 1998, 64, 762 CrossRef CAS PubMed.
- I. Jacquemond-Collet, F. Benoit-Vical, M. K. A. Mustofa, A. Valentin, E. Stanislas, M. Mallié and I. Fourasté, Planta Med., 2002, 68, 68 CrossRef CAS PubMed.
- G. Satyanarayana, D. Pflästerer and G. Helmchen, Eur. J. Org. Chem., 2011, 6877 CrossRef CAS.
- M. C. Maillard, M. E. Perlman, O. Amitay, D. Baxter, D. Berlove, S. Connaughton, J. B. Fischer, J. Q. Guo, L.-Y. Hu, R. N. McBurney, P. I. Nagy, K. Subbarao, E. A. Yost, L. Zhang and G. J. Durant, J. Med. Chem., 1998, 41, 3048 CrossRef CAS PubMed.
- V. K. Gore, V. V. Ma, R. Yin, J. Ligutti, D. Immke, E. M. Doherty and M. H. Norman, Bioorg. Med. Chem. Lett., 2010, 20, 3573 CrossRef CAS PubMed.
- J. H. Grønlien, M. Haakerud, H. Wenn, K. Thorin-Hagene, C. A. Briggs, M. Gopalakrishnan and J. Malysz, Mol. Pharmacol., 2007, 72, 715 CrossRef PubMed.
- R. Faghih, M. Gopalakrishnan and C. A. Briggs, J. Med. Chem., 2008, 51, 701 CrossRef CAS PubMed.
- C. G. Bologa, C. M. Revankar, S. M. Young, B. S. Edwards, J. B. Arterburn, A. S. Kiselyov, M. A. Parker, S. E. Tkachenko, N. P. Savchuck, L. A. Sklar, T. I. Oprea and E. R. Prossnitz, Nat. Chem. Biol., 2006, 2, 207 CrossRef CAS PubMed.
- D. S. Su, J. J. Lim, E. Tinney, B. L. Wan, M. B. Young, K. D. Anderson, D. Rudd, V. Munshi, C. Bahnck, P. J. Felock, M. Lu, M. T. Lai, S. Touch, G. Moyer, D. J. DiStefano, J. A. Flynn, Y. Liang, R. Sanchez, S. Prasad, Y. Yan, R. Perlow-Poehnelt, M. Torrent, M. Miller, J. P. Vacca, T. M. Williams and N. J. Anthony, Bioorg. Med. Chem. Lett., 2009, 19, 5119 CrossRef CAS PubMed.
- T. Asberom, T. A. Bara, J. W. Clader, W. J. Greenlee, H. S. Guzik, H. B. Josien, W. Li, E. M. Parker, D. A. Pissarnitski, L. Song, L. Zhang and Z. Zhao, Bioorg. Med. Chem. Lett., 2007, 17, 205 CrossRef CAS PubMed.
- L. Nallan, K. D. Bauer, P. Bendale, K. Rivas, K. Yokoyama, C. P. Hornéy, P. R. Pendyala, D. Floyd, L. J. Lombardo, D. K. Williams, A. Hamilton, S. Sebti, W. T. Windsor, P. C. Weber, F. S. Buckner, D. Chakrabarti, M. H. Gelb and W. C. Van Voorhis, J. Med. Chem., 2005, 48, 3704 CrossRef CAS PubMed.
- X.-F. Wang, S.-B. Wang, E. Ohkoshi, L.-T. Wang, E. Hamel, K. Qian, S. L. Morris-Natschke, K.-H. Lee and L. Xie, Eur. J. Med. Chem., 2013, 67, 196 CrossRef CAS PubMed.
- A. Escribano, A. I. Mateo, E. M. Martin de la Nava, D. R. Mayhugh, S. L. Cockerham, T. P. Beyer, R. J. Schmidt, G. Cao, Y. Zhang, T. M. Jones, A. G. Borel, S. A. Sweetana, E. A. Cannady and N. B. Mantlo, Bioorg. Med. Chem. Lett., 2012, 22, 3671 CrossRef CAS PubMed.
- P. D. Leeson, R. W. Carling, K. W. Moore, A. M. Moseley, J. D. Smith, G. Stevenson, T. Chan, R. Baker and A. C. Foster, J. Med. Chem., 1992, 35, 1954 CrossRef CAS PubMed.
- B. B. Snider, Y. Ahn and S. M. O'Hare, Org. Lett., 2001, 3, 4217 CrossRef CAS.
- V. Sridharan, P. A. Suryavanshi and J. C. Menéndez, Chem. Rev., 2011, 111, 7157 CrossRef CAS.
- B. Nammalwar and R. Bunce, Molecules, 2014, 19, 204 CrossRef.
- A. R. Katritzky, S. Rachwal and B. Rachwal, Tetrahedron, 1996, 52, 15031 CrossRef CAS.
- I. Muthukrishnan, V. Sridharan and J. C. Menéndez, Chem. Rev., 2019, 119, 5057 CrossRef CAS PubMed.
- H. Hayashi, T. Nakatani, Y. Inoue, M. Nakayama and H. Nozaki, Biosci., Biotechnol., Biochem., 1997, 61, 914 CrossRef CAS PubMed.
- R. A. Bunce, D. M. Herron, L. B. Johnson and S. V. Kotturi, J. Org. Chem., 2001, 66, 2822 CrossRef CAS.
- (a) L. S. Povarov, Russ. Chem. Rev., 1967, 36, 656 CrossRef; (b) W. Yang, H.-x. He, Y. Gao and D.-m. Du, Adv. Synth. Catal., 2013, 355, 3670 CrossRef CAS; (c) J. Jiang, X. Ma, C. Ji, Z. Guo, T. Shi, S. Liu and W. Hu, Chem.–Eur. J., 2014, 20, 1505 CrossRef CAS PubMed; (d) H. Mao, A. Lin, Y. Tang, Y. Shi, H. Hu, Y. Cheng and C. Zhu, Org. Lett., 2013, 15, 4062 CrossRef CAS PubMed; (e) L. L. Taylor, F. W. Goldberg and K. K. Hii, Org. Biomol. Chem., 2012, 10, 4424 RSC; (f) R. Adepu, A. RAjitha, D. Ahuja, A. K. Sharma, B. Ramudu, R. Kapavarapu, K. V. L. Parsa and M. Pal, Org. Biomol. Chem., 2014, 12, 2514 RSC; (g) S. Kim, K.-T. Kang and S.-G. Kim, Tetrahedron, 2014, 70, 5114 CrossRef CAS; (h) A. Kumar, S. Srivastava, G. Gupta, V. Chaturvedi, S. Sinha and R. Srivastava, ACS Comb. Sci., 2011, 13, 65 CrossRef CAS PubMed.
- V. Sridharan, C. Avendaño and J. C. Menéndez, Synthesis, 2008, 1039 CAS.
- T. H. Rui, N. H. Fen, C. Jun and W. Jian, Chem.–Eur. J., 2012, 18, 3865 CrossRef PubMed.
- A. Patra, S. Mukherjee, T. K. Das, S. Jain, R. G. Gonnade and A. T. Biju, Angew. Chem., Int. Ed., 2017, 56, 2730 CrossRef CAS PubMed.
- S. A. I. Sharif, E. D. D. Calder, F. G. Delolo and A. Sutherland, J. Org. Chem., 2016, 81, 6697 CrossRef CAS PubMed.
- A.-g. Ying, L. Liu, G.-f. Wu, X.-z. Chen, W.-d. Ye, J.-h. Chen and K.-y. Zhang, Chem. Res. Chin. Univ., 2008, 25, 876 Search PubMed.
- C.-E. Yeom, M. J. Kim and B. M. Kim, Tetrahedron, 2007, 63, 904 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1834300 and 1834305. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0ra01264e |
| This journal is © The Royal Society of Chemistry 2020 |