Bis-resorcin[4]arene–bridged porphyrin conjugates: synthesis, fluorescence and binding studies†
Talal F. Al-Azemi*,
Mickey Vinodh and
Fatemeh H. Alipour
Chemistry Department, Kuwait University, P.O. Box 5969, Safat 13060, Kuwait. E-mail: t.alazemi@ku.edu.kw; Fax: +965-2481-6482; Tel: +965-2498-5540
First published on 8th August 2016
Abstract
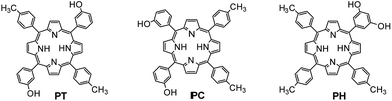
In this study, three bis-resorcin[4]arene–bridged porphyrin conjugates were synthesised from 5-15-di(3-hydroxyphenyl)-10,20-di(4-toluyl)porphyrin (PT), 5,10-di(3-hydroxyphenyl)-15,20-di(4-toluyl)porphyrin (PC) and 5-(3,5-dihydroxyphenyl)-10,15,20-tri(4-toluyl)porphyrin (PH), respectively; porphyrins PT, PC and PH were synthesised by the condensation of their corresponding aldehydes with pyrrole in propionic acid. In addition, the single-crystal X-ray diffraction analysis of the resorcin[4]arene host as well as the binding behaviour and fluorescence decay studies of the bisresorcin[4]arene–porphyrin conjugate are reported. Moreover, zinc derivatives of the three resorcin[4]arene–porphyrin conjugates, are synthesised. Binding studies conducted via UV-vis titration revealed that compared to the association constant (Ka) for free zinc-porphyrin (ZnTTP), those for bis-resorcin[4]arene–bridged porphyrin conjugates are higher, with an increase of approximately 50%. The fluorescence quenching of porphyrin is considerably enhanced by the attachment of resorcin[4]arene to the photoactive subunit; however, the efficiency of fluorescence quenching is affected by the structure type of porphyrin bis-conjugates.
Introduction
Macrocyclic oligomers, such as calixarenes,1 resorcinarenes,2 cyclodextrins3 and crown ethers,4 are attractive receptors possessing a multipoint recognition ability toward non-covalently bound guest molecules. Among the aforementioned oligomers, calixarenes and resorcinarenes have been extensively investigated because of their unique three-dimensional shapes, as well as their abilities to encapsulate non-covalently bound guest molecules.5,6 The functionality of systems can be extended via the combination of porphyrins with macrocyclic hosts, such as calix[4]arenes7–9 or resorcin[4]arenes,10–12 via covalent bonds, thereby broadening the prospects for the design of receptors via an increase in the stability, reactivity and selectivity of porphyrin fragments.On the other hand, the success of a macrocyclic receptor depends on its guest inclusion capability for ensuring effective molecular recognition. Porphyrin–calixarene tweezers, in which two porphyrin units are connected to a macrocyclic host, are common in host–guest chemistry.13,14 Such systems are interesting functional materials exhibiting remarkable electronic and encapsulation characteristics. Significant electronic and energy transitions from porphyrin or metalloporphyrins in such dimeric systems to various guest species are possible, which can be easily tuned by varying the characteristics of the porphyrin ring and the nature of the encapsulated guests.
On the other hand, the nature of the host cavitands is also observed to be a factor dominant for determining the characteristic properties of such functional materials. Appropriate structural modifications to the receptor entity are imperative for tuning the special characteristics of guest encapsulation and reactivity. Although a few studies have reported the synthesis of bis-calixarene–porphyrin conjugates and their use in guest complexation, to the best of our knowledge, bis-resorcinarene–porphyrin conjugates have not been reported.
Previously, we have reported the synthesis of a resorcin[4]arene–porphyrin conjugate and its effect on the quenching behaviour, binding and epoxidation of alkenes with the porphyrin fragment.15,16 In this study, we report the synthesis and characterisation of three resorcin[4]arene dimers, in which the porphyrin macrocycle is positioned as a bridging unit between the resorcin[4]arene hosts. The effect of the position of the resorcin[4]arene host relative to the porphyrin bridge on the fluorescence quenching and binding behaviour of the porphyrin fragment in the synthesised bis-resorcin[4]arene–bridged porphyrin conjugates is discussed in detail.
Experimental section
Material & methods
RO, ROBr, TTP and ROP, as well as the zinc-based metal derivative, have been synthesised according to previously reported procedures.15 Ultraviolet-visible (UV-vis) spectra were recorded on a spectrophotometer (Varian UV-vis cary-5 spectrophotometer, Agilent, USA). Fluorescence spectra were measured with spectrofluorometer (Horiba Jobin Vyon Fluoromax – 4, USA). Fast atom bombardment (FAB) mass analyses and high resolution mass spectrometry (HRMS) were performed using mass spectrometer (DFS High Resolution GC/MS – Thermo Scientific, Germany). Nuclear magnetic resonance (NMR) spectroscopy was done by two spectrometers (Avance II 600 MHz, Bruker, Germany and DPX 400, Burker, Germany). The single crystal data analyses were made on a diffractometer (R-AXIS RAPID II, Rigaku, Japan). The data were collected at a temperature of −123 °C (Oxford cryosystems, UK). Flash column chromatography was performed using silica gel (Silica gel 60, 230–400 mesh ASTM, EMD Millipore, Merck KGaA, Germany). DMF was dehydrated by vacuum distillation over calcium hydride. Pyrrole was vacuum distilled before each usage. Azobisisobutyronitrile (AIBN) was crystallized form hot ethanol. N-Bromosuccinimide (NBS) was crystallized prior use form boiling water. All other reagents and solvents were of reagent grade purity and used without further purification.Synthesis of di(3-hydroxyphenyl)-di(4-toluyl)porphyrins (PT and PC)
Porphyrins PT and PC were simultaneously synthesised by the condensation of mixed aldehydes in propionic acid using two equivalents of 3-hydroxybenzaldehyde, two equivalents of 4-tolualdehyde and four equivalents of pyrrole. First, 3-hydroxybenzaldehyde (12.20 g, 100 mmol) and 4-tolualdehyde (11.82 mL, 100 mmol) were added to 0.5 L of hot propionic acid and stirred for 10 min. As the mixture was about to boil, pyrrole (13.85 mL, 200 mmol) was added with vigorous mixing. Second, the reaction was refluxed for 45 min, allowed to cool to room temperature and kept in a refrigerator at 8–10 °C overnight. Finally, the precipitate formed was collected by filtration and washed with hot water until all of the propionic acid was removed. The crude reaction products were loaded onto a flash silica gel column by dissolving in a minimum quantity of dichloromethane and eluted with a solvent gradient from 100% CH2Cl2 to 99% CH2Cl2 and 1% CH3OH (v/v) for removing unwanted side products. When the solvent polarity was increased to 98% CH2Cl2 and 2% CH3OH (v/v), both trans and cis derivatives of porphyrins started to slowly elute. This fraction was collected and subjected to purification by another silica column chromatography run using 96% CH2Cl2 and 4% ethyl acetate (v/v). The trans isomer (PT) was eluted, followed by the cis isomer (PC). All these eluents were collected in 20 mL vials, and the purity of the components was checked by TLC. The initial vials containing pure PT were combined together. Similarly, the last vials containing only pure PC were also combined. The middle portion of the two fractions containing both PT and PC was separately combined and subjected to one more column separation run using the same solvent mixture. Pure PT or PC obtained from this column purification was then combined with the original PT or PC collected from the first column.meso-5,15-Di(3-hydroxyphenyl)-10,20-di(4-toluyl)porphyrin (PT)
Yield, 1.4 g (4%). UV-vis (CH2Cl2): λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 418 (5.30), 516 (4.17), 551 (3.90), 592 (3.64) and 646 (3.68); 1H NMR (400 MHz, CDCl3) δ: −2.81 (s, 2H), 2.70 (s, 6H), 7.22 (m, 2H), 7.57 (m, 6H), 7.66 (s, 2H), 7.78 (d, 2H, J = 7.2 Hz), 8.09 (d, 4H, J = 7.6 Hz), 8.86 (s, 8H); 13C NMR (150 MHz, DMSO) δ: 21.0, 115.1, 119.9, 119.9, 121.8, 125.7, 127.6, 127.8, 131.1, 134.2, 137.3, 138.3, 142.4, 155.8; ESI-MS: 674; HRMS for C46H34O2N4: 674.2676 (calcd); 674.2677 (found).
ε): 418 (5.30), 516 (4.17), 551 (3.90), 592 (3.64) and 646 (3.68); 1H NMR (400 MHz, CDCl3) δ: −2.81 (s, 2H), 2.70 (s, 6H), 7.22 (m, 2H), 7.57 (m, 6H), 7.66 (s, 2H), 7.78 (d, 2H, J = 7.2 Hz), 8.09 (d, 4H, J = 7.6 Hz), 8.86 (s, 8H); 13C NMR (150 MHz, DMSO) δ: 21.0, 115.1, 119.9, 119.9, 121.8, 125.7, 127.6, 127.8, 131.1, 134.2, 137.3, 138.3, 142.4, 155.8; ESI-MS: 674; HRMS for C46H34O2N4: 674.2676 (calcd); 674.2677 (found).
meso-5,10-Di(3-hydroxyphenyl)-15,20-di(4-toluyl)porphyrin (PC)
Yield, 1.3 g (4%). UV-vis (CH2Cl2): λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 418 (5.31), 516 (4.16), 551 (3.90), 592 (3.64) and 645 (3.67); 1H NMR (400 MHz, CDCl3) δ: −2.73 (s, 2H), 2.67 (s, 6H), 7.03 (dd, 2H, J = 6.8 Hz and J = 7.6 Hz), 7.37 (m, 4H), 7.50 (d, 4H, J = 7.6 Hz), 7.67 (dd, 2H, J = 7.2 Hz and J = 7.2 Hz), 8.06 (d, 6H, J = 6.8 Hz), 8.76 (m, 8H); 13C NMR (150 MHz, CDCl3) δ: 21.6, 114.5, 114.6, 119.6, 120.8, 121.3, 121.6, 127.3, 127.5, 127.7, 131.3, 134.7, 137.5, 139.2, 143.0, 143.2, 145.8, 153.3, 153.5; ESI-MS: 674; HRMS for C46H34O2N4: 674.2676 (calcd); 674.2676 (found).
ε): 418 (5.31), 516 (4.16), 551 (3.90), 592 (3.64) and 645 (3.67); 1H NMR (400 MHz, CDCl3) δ: −2.73 (s, 2H), 2.67 (s, 6H), 7.03 (dd, 2H, J = 6.8 Hz and J = 7.6 Hz), 7.37 (m, 4H), 7.50 (d, 4H, J = 7.6 Hz), 7.67 (dd, 2H, J = 7.2 Hz and J = 7.2 Hz), 8.06 (d, 6H, J = 6.8 Hz), 8.76 (m, 8H); 13C NMR (150 MHz, CDCl3) δ: 21.6, 114.5, 114.6, 119.6, 120.8, 121.3, 121.6, 127.3, 127.5, 127.7, 131.3, 134.7, 137.5, 139.2, 143.0, 143.2, 145.8, 153.3, 153.5; ESI-MS: 674; HRMS for C46H34O2N4: 674.2676 (calcd); 674.2676 (found).
Synthesis of meso-5-(3,5-dihydroxyphenyl)-10,15,20-(4-toluyl)porphyrin (PH)
First, 3,5-dihydroxybenzaldehyde (2.76 g, 20 mmol) and 4-tolualdehyde (7.09 mL, 60 mmol) were added to 0.3 L of hot propionic acid and heated to boil. When the mixture was about to boil, pyrrole (5.54 mL, 80 mmol) was added with vigorous mixing. Second, the reaction was refluxed for 45 min, allowed to cool to room temperature and kept in the refrigerator at 8–10 °C overnight. Finally, the precipitate formed was collected by filtration and washed with hot water until all of the propionic acid was removed. Next, the crude products obtained from the reaction were loaded onto a flash silica gel column by dissolving in a minimum quantity of dichloromethane and eluted with a solvent mixture of 92% dichloromethane and 8% ethyl acetate (v/v). The major porphyrin band was collected and subjected to purification by another column run using the same solvent mixture to obtain PH in pure form, with a yield of 0.85 g (7%). UV-vis (CH2Cl2): λmax nm (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 419 (5.31), 515 (4.17), 551 (3.78), 591 (3.60) and 646 (3.65); 1H NMR (400 MHz, CDCl3) δ: −2.78 (s, 2H), 2.71 (m, 9H), 6.43 (s, 1H), 7.09 (s, 2H), 7.54 (m, 6H), 8.10 (m, 6H), 8.86 (m, 8H); 13C NMR (150 MHz, CDCl3) δ: 21.7, 21.7, 102.4, 115.5, 118.8, 120.4, 120.7, 127.6, 131.2, 134.7, 137.6, 139.4, 139.5, 144.7, 154.9; ESI-MS: 688.5; HRMS for C47H36O2N4: 688.2832 (calcd); 688.2832 (found).
ε): 419 (5.31), 515 (4.17), 551 (3.78), 591 (3.60) and 646 (3.65); 1H NMR (400 MHz, CDCl3) δ: −2.78 (s, 2H), 2.71 (m, 9H), 6.43 (s, 1H), 7.09 (s, 2H), 7.54 (m, 6H), 8.10 (m, 6H), 8.86 (m, 8H); 13C NMR (150 MHz, CDCl3) δ: 21.7, 21.7, 102.4, 115.5, 118.8, 120.4, 120.7, 127.6, 131.2, 134.7, 137.6, 139.4, 139.5, 144.7, 154.9; ESI-MS: 688.5; HRMS for C47H36O2N4: 688.2832 (calcd); 688.2832 (found).
Synthesis of bis-resorcin[4]arene–bridged porphyrin conjugates: general procedure
First, porphyrin (100 mg, 0.149 mmol for PC and PT; 0.145 mmol for PH) and K2CO3 (300 mg, 2.2 mmol) were dissolved in dry DMF (5 mL) in a sealed tube and stirred for 15 min. Second, ROBr (500 mg, 0.467 mmol) was added to this mixture, the tube was sealed and stirred in an oil bath at 80 °C for 2 days. Next, DMF was evaporated, and the solid residue was washed with water and dried. Finally, the crude product adsorbed on silica was loaded on a silica column and eluted with chloroform. The major band was collected, solvent was removed and the product was dried in a vacuum desiccator until constant weight.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 419 (5.46), 517 (4.12), 550 (3.75), 591 (3.57) and 645 (3.47); 1H NMR (400 MHz, CDCl3) δ: −2.73 (s, 2H), 0.86 (m, 24H), 1.29 (m, 64H), 1.89 (m, 16H), 2.11 (s, 12H), 2.18 (s, 6H), 2.75 (s, 6H), 3.42 (d, 24H, J = 10.4 Hz), 3.60 (s, 12H), 3.89 (s, 12H), 4.48 (t, 4H, J = 7.2 Hz), 4.54 (t, 4H, J = 7.2 Hz), 5.29 (s, 4H), 6.40 (s, 2H), 6.67 (s, 2H), 6.76 (s, 4H), 7.47 (d, 2H, J = 8.0 Hz), 7.60 (d, 4H, J = 7.6 Hz), 7.72 (t, 2H, J = 8.0 Hz), 7.91 (m, 4H), 8.14 (d, 4H, J = 7.2 Hz) and 8.93 (m, 8H). 13C NMR (100 MHz, CDCl3) δ: 10.1, 10.3, 14.1, 21.6, 22.8, 22.8, 28.5, 28.6, 29.6, 29.6, 31.9, 31.9, 35.7, 37.4, 37.5, 59.9, 59.9, 60.0, 61.8, 62.9, 114.1, 119.8, 120.3, 121.9, 123.1, 123.4, 123.6, 124.1, 124.1, 127.4, 127.6, 127.9, 128.0, 131.9, 132.4, 133.8, 134.5, 134.7, 137.4, 139.2, 143.6, 155.3, 155.8, 155.9, 156.6 and 157.4. FAB mass: 2659.2 [M + 1]+.
ε): 419 (5.46), 517 (4.12), 550 (3.75), 591 (3.57) and 645 (3.47); 1H NMR (400 MHz, CDCl3) δ: −2.73 (s, 2H), 0.86 (m, 24H), 1.29 (m, 64H), 1.89 (m, 16H), 2.11 (s, 12H), 2.18 (s, 6H), 2.75 (s, 6H), 3.42 (d, 24H, J = 10.4 Hz), 3.60 (s, 12H), 3.89 (s, 12H), 4.48 (t, 4H, J = 7.2 Hz), 4.54 (t, 4H, J = 7.2 Hz), 5.29 (s, 4H), 6.40 (s, 2H), 6.67 (s, 2H), 6.76 (s, 4H), 7.47 (d, 2H, J = 8.0 Hz), 7.60 (d, 4H, J = 7.6 Hz), 7.72 (t, 2H, J = 8.0 Hz), 7.91 (m, 4H), 8.14 (d, 4H, J = 7.2 Hz) and 8.93 (m, 8H). 13C NMR (100 MHz, CDCl3) δ: 10.1, 10.3, 14.1, 21.6, 22.8, 22.8, 28.5, 28.6, 29.6, 29.6, 31.9, 31.9, 35.7, 37.4, 37.5, 59.9, 59.9, 60.0, 61.8, 62.9, 114.1, 119.8, 120.3, 121.9, 123.1, 123.4, 123.6, 124.1, 124.1, 127.4, 127.6, 127.9, 128.0, 131.9, 132.4, 133.8, 134.5, 134.7, 137.4, 139.2, 143.6, 155.3, 155.8, 155.9, 156.6 and 157.4. FAB mass: 2659.2 [M + 1]+.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 420 (5.42), 515 (4.13), 550 (3.72), 590 (3.52) and 646 (3.42); 1H NMR (400 MHz, CDCl3) δ: −2.73 (s, 2H), 0.86 (m, 24H), 1.30 (m, 64H), 1.88 (m, 16H), 2.09 (m, 12H), 2.20 (m, 6H), 2.74 (s, 6H), 3.41 (m, 24H), 3.64 (m, 12H), 3.90 (m, 12H), 4.51 (m, 8H), 5.30 (s, 4H), 6.39 (m, 2H), 6.66 (m, 2H), 6.78 (m, 4H), 7.48 (d, 2H, J = 7.6 Hz), 7.60 (d, 4H, J = 7.6 Hz), 7.72 (t, 2H, J = 8.0 Hz), 7.90 (m, 4H), 8.14 (d, 4H, J = 7.6 Hz), 8.95 (m, 8H). 13C NMR (100 MHz, CDCl3) δ: 10.2, 10.4, 14.1, 21.5, 22.9, 28.5, 28.6, 29.6, 29.6, 31.9, 31.9, 35.7, 37.4, 37.5, 59.9, 59.9, 60.0, 61.8, 62.9, 114.1, 119.6, 120.4, 123.1, 123.4, 123.6, 124.1, 127.4, 127.6, 128.0, 131.9, 132.4, 133.8, 134.5, 134.7, 137.4, 139.3, 143.7, 155.3, 155.8, 155.9, 156.6 and 157.4; FAB mass: 2658.2 [M]+.
ε): 420 (5.42), 515 (4.13), 550 (3.72), 590 (3.52) and 646 (3.42); 1H NMR (400 MHz, CDCl3) δ: −2.73 (s, 2H), 0.86 (m, 24H), 1.30 (m, 64H), 1.88 (m, 16H), 2.09 (m, 12H), 2.20 (m, 6H), 2.74 (s, 6H), 3.41 (m, 24H), 3.64 (m, 12H), 3.90 (m, 12H), 4.51 (m, 8H), 5.30 (s, 4H), 6.39 (m, 2H), 6.66 (m, 2H), 6.78 (m, 4H), 7.48 (d, 2H, J = 7.6 Hz), 7.60 (d, 4H, J = 7.6 Hz), 7.72 (t, 2H, J = 8.0 Hz), 7.90 (m, 4H), 8.14 (d, 4H, J = 7.6 Hz), 8.95 (m, 8H). 13C NMR (100 MHz, CDCl3) δ: 10.2, 10.4, 14.1, 21.5, 22.9, 28.5, 28.6, 29.6, 29.6, 31.9, 31.9, 35.7, 37.4, 37.5, 59.9, 59.9, 60.0, 61.8, 62.9, 114.1, 119.6, 120.4, 123.1, 123.4, 123.6, 124.1, 127.4, 127.6, 128.0, 131.9, 132.4, 133.8, 134.5, 134.7, 137.4, 139.3, 143.7, 155.3, 155.8, 155.9, 156.6 and 157.4; FAB mass: 2658.2 [M]+.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 419 (5.40), 516 (4.13), 551 (3.75), 590 (3.58) and 645 (3.46); 1H NMR (400 MHz, CDCl3) δ: −2.73 (s, 2H), 0.87 (m, 24H), 1.31 (m, 64H), 1.92 (m, 16H), 2.11 (s, 12H), 2.19 (s, 6H), 2.75 (m, 9H), 3.42 (d, 24H, J = 13.6 Hz), 3.63 (s, 12H), 3.94 (s, 12H), 4.82 (t, 4H, J = 7.6 Hz), 4.55 (t, 4H, J = 7.6 Hz), 5.31 (s, 4H), 6.38 (s, 2H), 6.65 (s, 2H), 6.80 (s, 4H), 7.16 (s, 1H), 7.60 (m, 8H), 8.15 (m, 6H), 8.92 (m, 6H), 9.08 (d, 2H, J = 4.8 Hz). 13C NMR (100 MHz, CDCl3) δ: 10.2, 10.3, 14.1, 21.5, 22.8, 28.5, 28.6, 29.6, 29.6, 29.7, 31.9, 31.9, 35.7, 37.4, 37.5, 59.9, 60.0, 60.0, 61.9, 62.9, 98.1, 115.4, 119.5, 120.2, 120.4, 123.0, 123.4, 123.6, 124.1, 127.4, 128.1, 131.7, 132.2, 133.9, 134.5, 134.9, 137.4, 139.3, 144.2, 155.2, 155.9, 155.9, 156.5, 158.3; FAB mass: 2673.8 [M + 1]+.
ε): 419 (5.40), 516 (4.13), 551 (3.75), 590 (3.58) and 645 (3.46); 1H NMR (400 MHz, CDCl3) δ: −2.73 (s, 2H), 0.87 (m, 24H), 1.31 (m, 64H), 1.92 (m, 16H), 2.11 (s, 12H), 2.19 (s, 6H), 2.75 (m, 9H), 3.42 (d, 24H, J = 13.6 Hz), 3.63 (s, 12H), 3.94 (s, 12H), 4.82 (t, 4H, J = 7.6 Hz), 4.55 (t, 4H, J = 7.6 Hz), 5.31 (s, 4H), 6.38 (s, 2H), 6.65 (s, 2H), 6.80 (s, 4H), 7.16 (s, 1H), 7.60 (m, 8H), 8.15 (m, 6H), 8.92 (m, 6H), 9.08 (d, 2H, J = 4.8 Hz). 13C NMR (100 MHz, CDCl3) δ: 10.2, 10.3, 14.1, 21.5, 22.8, 28.5, 28.6, 29.6, 29.6, 29.7, 31.9, 31.9, 35.7, 37.4, 37.5, 59.9, 60.0, 60.0, 61.9, 62.9, 98.1, 115.4, 119.5, 120.2, 120.4, 123.0, 123.4, 123.6, 124.1, 127.4, 128.1, 131.7, 132.2, 133.9, 134.5, 134.9, 137.4, 139.3, 144.2, 155.2, 155.9, 155.9, 156.5, 158.3; FAB mass: 2673.8 [M + 1]+.General procedure for the synthesis of the zinc derivatives of porphyrin resorcin[4]arene conjugates: Zn(RO)2PT, Zn(RO)2PC and Zn(RO)2PH
First, the conjugate (30 mg) was dissolved in chloroform (10 mL), and zinc acetate (30 mg dissolved in methanol (5 mL)) was then added to the solution. Second, the mixture was refluxed until the UV-vis spectrum showed no peak at 650 nm. Finally, the solvent was evaporated, and the crude product was purified by silica-gel column chromatography using CH2Cl2 as the elution solvent. The conjugates were quantitatively obtained as a brownish-red solid.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 418 (5.67), 546 (4.25) and 586 (3.59); 1H NMR (600 MHz, CDCl3) δ: 0.88 (m, 24H), 1.29 (m, 64H), 1.88 (m, 16H), 2.08 (s, 12H), 2.15 (s, 6H), 2.74 (s, 6H), 3.41 (d, 24H, J = 13.8 Hz), 3.59 (s, 12H), 3.88 (s, 12H), 4.46 (t, 4H, J = 6.6 Hz), 4.51 (t, 4H, J = 6.6 Hz), 5.28 (s, 4H), 6.38 (s, 2H), 6.65 (s, 2H), 6.76 (s, 4H), 7.45 (m, 2H), 7.59 (d, 4H, J = 7.8 Hz), 7.71 (t, 2H, J = 8.4 Hz), 7.91 (m, 4H), 8.14 (d, 4H, J = 7.8 Hz), 9.03 (m, 8H). 13C NMR (150 MHz, CDCl3) δ: 10.4, 10.5, 14.3, 21.8, 23.0, 23.1, 28.7, 28.8, 29.6, 29.8, 29.8, 29.9, 32.0, 32.1, 35.9, 35.9, 37.6, 37.7, 60.0, 60.1, 60.2, 61.9, 63.1, 114.1, 121.0, 121.5, 122.0, 123.3, 123.6, 123.8, 124.3, 127.5, 127.7, 128.0, 128.2, 132.1, 132.3, 132.6, 134.6, 134.9, 137.4, 140.1, 144.5, 150.3, 150.6, 155.5, 156.1, 156.1, 156.8 and 157.5. FAB mass: 2720 [M − 1].
ε): 418 (5.67), 546 (4.25) and 586 (3.59); 1H NMR (600 MHz, CDCl3) δ: 0.88 (m, 24H), 1.29 (m, 64H), 1.88 (m, 16H), 2.08 (s, 12H), 2.15 (s, 6H), 2.74 (s, 6H), 3.41 (d, 24H, J = 13.8 Hz), 3.59 (s, 12H), 3.88 (s, 12H), 4.46 (t, 4H, J = 6.6 Hz), 4.51 (t, 4H, J = 6.6 Hz), 5.28 (s, 4H), 6.38 (s, 2H), 6.65 (s, 2H), 6.76 (s, 4H), 7.45 (m, 2H), 7.59 (d, 4H, J = 7.8 Hz), 7.71 (t, 2H, J = 8.4 Hz), 7.91 (m, 4H), 8.14 (d, 4H, J = 7.8 Hz), 9.03 (m, 8H). 13C NMR (150 MHz, CDCl3) δ: 10.4, 10.5, 14.3, 21.8, 23.0, 23.1, 28.7, 28.8, 29.6, 29.8, 29.8, 29.9, 32.0, 32.1, 35.9, 35.9, 37.6, 37.7, 60.0, 60.1, 60.2, 61.9, 63.1, 114.1, 121.0, 121.5, 122.0, 123.3, 123.6, 123.8, 124.3, 127.5, 127.7, 128.0, 128.2, 132.1, 132.3, 132.6, 134.6, 134.9, 137.4, 140.1, 144.5, 150.3, 150.6, 155.5, 156.1, 156.1, 156.8 and 157.5. FAB mass: 2720 [M − 1].![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 420 (5.66), 546 (4.25) and 586 (3.60); 1H NMR (400 MHz, CDCl3) δ: 0.84 (m, 24H), 1.28 (m, 64H), 1.89 (m, 16H), 2.08 (m, 12H), 2.19 (m, 6H), 2.74 (s, 6H), 3.39 (m, 24H), 3.62 (m, 12H), 3.90 (m, 12H), 4.50 (m, 8H), 5.29 (m, 4H), 6.37 (m, 2H), 6.64 (m, 2H), 6.77 (m, 4H), 7.46 (d, 2H, J = 8.4 Hz), 7.58 (d, 4H, J = 7.8 Hz), 7.71 (t, 2H, J = 7.8 Hz), 7.91 (m, 4H), 8.13 (m, 4H) and 9.05 (m, 8H). 13C NMR (150 MHz, CDCl3) δ: 10.4, 10.4, 10.5, 10.6, 11.2, 14.3, 14.3, 21.8, 22.9, 23.0, 23.0, 23.2, 24.0, 28.7, 28.8, 29.1, 29.6, 29.8, 29.9, 30.6, 32.1, 32.1, 34.2, 35.9, 37.6, 37.7, 39.0, 60.0, 60.1, 60.1, 60.2, 61.9, 63.1, 67.03, 114.1, 120.9, 121.6, 122.0, 123.3, 123.6, 123.8, 124.3, 127.5, 127.7, 128.0, 128.2, 132.0, 132.1, 132.2, 132.3, 134.1, 134.2, 134.6, 134.9, 137.4, 140.1, 144.5, 150.3, 150.3, 150.4, 150.6, 150.6, 155.4, 155.5, 156.1, 156.1, 156.8 and 157.5; FAB mass: 2744 [M + Na].
ε): 420 (5.66), 546 (4.25) and 586 (3.60); 1H NMR (400 MHz, CDCl3) δ: 0.84 (m, 24H), 1.28 (m, 64H), 1.89 (m, 16H), 2.08 (m, 12H), 2.19 (m, 6H), 2.74 (s, 6H), 3.39 (m, 24H), 3.62 (m, 12H), 3.90 (m, 12H), 4.50 (m, 8H), 5.29 (m, 4H), 6.37 (m, 2H), 6.64 (m, 2H), 6.77 (m, 4H), 7.46 (d, 2H, J = 8.4 Hz), 7.58 (d, 4H, J = 7.8 Hz), 7.71 (t, 2H, J = 7.8 Hz), 7.91 (m, 4H), 8.13 (m, 4H) and 9.05 (m, 8H). 13C NMR (150 MHz, CDCl3) δ: 10.4, 10.4, 10.5, 10.6, 11.2, 14.3, 14.3, 21.8, 22.9, 23.0, 23.0, 23.2, 24.0, 28.7, 28.8, 29.1, 29.6, 29.8, 29.9, 30.6, 32.1, 32.1, 34.2, 35.9, 37.6, 37.7, 39.0, 60.0, 60.1, 60.1, 60.2, 61.9, 63.1, 67.03, 114.1, 120.9, 121.6, 122.0, 123.3, 123.6, 123.8, 124.3, 127.5, 127.7, 128.0, 128.2, 132.0, 132.1, 132.2, 132.3, 134.1, 134.2, 134.6, 134.9, 137.4, 140.1, 144.5, 150.3, 150.3, 150.4, 150.6, 150.6, 155.4, 155.5, 156.1, 156.1, 156.8 and 157.5; FAB mass: 2744 [M + Na].![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 418 (5.64), 546 (4.24) and 586 (3.59); 1H NMR (400 MHz, CDCl3) δ: 0.87 (m, 24H), 1.31 (m, 64H), 1.85 (m, 16H), 2.10 (s, 12H), 2.18 (s, 6H), 2.74 (m, 9H), 3.38 (d, 24H, J = 19.2 Hz), 3.61 (s, 12H), 3.92 (s, 12H), 4.46 (t, 4H, J = 7.2 Hz), 4.53 (t, 4H, J = 7.2 Hz), 5.28 (s, 4H), 6.35 (s, 2H), 6.62 (s, 2H), 6.79 (s, 4H), 7.13 (s, 1H), 7.59 (m, 8H), 8.13 (m, 6H), 8.99 (m, 6H) and 9.15 (d, 2H, J = 4.2 Hz). 13C NMR (150 MHz, CDCl3) δ: 10.2, 10.3, 14.1, 21.6, 22.8, 22.8, 28.5, 28.6, 29.6, 29.6, 31.9, 31.9, 35.7, 37.4, 37.5, 59.9, 60.0, 60.0, 62.9, 120.0, 121.1, 123.7, 124.1, 127.3, 128.0, 132.1, 134.4, 137.1, 149.6, 150.3, 155.2, 155.9, 156.0 and 156.5; FAB mass: 2758 [M + Na].
ε): 418 (5.64), 546 (4.24) and 586 (3.59); 1H NMR (400 MHz, CDCl3) δ: 0.87 (m, 24H), 1.31 (m, 64H), 1.85 (m, 16H), 2.10 (s, 12H), 2.18 (s, 6H), 2.74 (m, 9H), 3.38 (d, 24H, J = 19.2 Hz), 3.61 (s, 12H), 3.92 (s, 12H), 4.46 (t, 4H, J = 7.2 Hz), 4.53 (t, 4H, J = 7.2 Hz), 5.28 (s, 4H), 6.35 (s, 2H), 6.62 (s, 2H), 6.79 (s, 4H), 7.13 (s, 1H), 7.59 (m, 8H), 8.13 (m, 6H), 8.99 (m, 6H) and 9.15 (d, 2H, J = 4.2 Hz). 13C NMR (150 MHz, CDCl3) δ: 10.2, 10.3, 14.1, 21.6, 22.8, 22.8, 28.5, 28.6, 29.6, 29.6, 31.9, 31.9, 35.7, 37.4, 37.5, 59.9, 60.0, 60.0, 62.9, 120.0, 121.1, 123.7, 124.1, 127.3, 128.0, 132.1, 134.4, 137.1, 149.6, 150.3, 155.2, 155.9, 156.0 and 156.5; FAB mass: 2758 [M + Na].Single-crystal X-ray diffraction analysis
Single crystals of RO, ROBr and PT suitable for single-crystal X-ray diffraction were grown by solvent diffusion using ethyl acetate and hexane. The single crystal data collections were made on Rigaku R-AXIS RAPID diffractometer using filtered Mo-Kα radiation at −123 °C. The structure was solved by direct methods and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically. Hydrogen atoms were refined using the riding model. All calculations were performed using the Rigaku's ‘CrystalStructure’ crystallographic software package except for refinement, which was performed using SHELXL-97.UV-vis titration
A 25 mL sample of the porphyrin solution was prepared at a concentration of 8 μM using a spectroscopic-grade solvent (chloroform was dried over calcium chloride and basic alumina). A 10 mL sample of the ligand solution was prepared at a concentration of 0.004–0.2 M in a spectroscopic-grade solvent. All titration experiments were performed using a 5 mL receptor solution in a quartz cell at 298 K, and UV-vis spectra were recorded on the successive addition of aliquots of the stock solution of the appropriate ligands using a microsyringe. The UV-vis absorbance at three wavelengths was fitted to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherm by non-linear least-squares analysis using Microsoft Excel for determining the association constant Ka.6
1 binding isotherm by non-linear least-squares analysis using Microsoft Excel for determining the association constant Ka.6
Fluorescence decay study
First, 20 mL of a 0.0025 mM solution of porphyrin was prepared using dichloromethane and added into a vial. Second, a 10 mM solution of phenanthrenequinone (PAQ) was prepared. Using a micropipette, a predetermined amount of PAQ was added to the porphyrin solution, shaken well and fluorescence was measured at 648 nm (excitation wavelength was 418 nm). This procedure was repeated again until the fluorescence intensity at 648 nm became zero. The result was then expressed as a function of the decay of fluorescence intensity against the PAQ concentration.Results and discussion
Synthesis of bis-resorcin[4]arene–porphyrin conjugates
PT, PC and PH were synthesised by the condensation of aldehydes with pyrrole in propionic acid (Fig. 1). PT and PC were simultaneously prepared using 2 equiv. of 3-hydroxybenzaldehyde, 2 equiv. of p-tolualdehyde and 4 equiv. of pyrrole affording 4% yield for each isomer. The same procedure was used for preparing PH, with 7% yield obtained (Experimental section).Mass, NMR and UV-vis spectroscopy was employed for the characterisation of PT, PC and PH. The PT structure was further examined by single-crystal X-ray diffraction analysis; it is confirmed to be a typical mesophenyl-porphyrin with a planar porphyrin core, with the phenyl groups perpendicular to the macrocyclic core. As shown in Fig. 2, the hydroxyl functional groups on PT were observed at a position meta from the two alternate phenyl groups.
 | ||
| Fig. 2 Molecular structure of PT obtained from single-crystal X-ray diffraction analysis. Dark grey, light grey, red and blue denote carbon, hydrogen, oxygen and nitrogen, respectively. | ||
ROBr was treated with PT, PC and PH, affording bis-resorcin[4]arene–bridged porphyrins (RO)2PT, (RO)2PC and (RO)2PH, respectively, in greater than 65% yield (Fig. 3). The resulting conjugates were readily soluble in common organic solvents and were purified by silica-gel column chromatography using chloroform as the elution solvent. All of the conjugates thus synthesised were characterised by NMR, UV-vis, fluorescence and mass spectroscopic methods.
Crystal structure of the resorcin[4]arene host
Single crystals of both octamethoxymethylresorcin[4]arene (RO) and ROBr suitable for X-ray diffraction studies were grown by solvent diffusion. Fig. 4 shows the molecular structures of RO and ROBr derived from X-ray data; the crystal parameters and refinement strategies have been provided in the ESI.†The X-ray crystal structures clearly demonstrate characteristics, such as size and shape, for both RO and ROBr. In the crystal networks of RO and ROBr, the dimethoxyphenyl functionalities exhibited the boat conformation (adjacent units were right angles to each other, and alternate functionalities were in the same plane), providing a flexible upper rim to the host. Under solution conditions, RO was also observed to exhibit the boat conformation, as revealed by low-temperature NMR experiments (ESI†). The fast exchange spectrum at 297 K for RO shows singlet peaks corresponding to the methoxy, benzylic, and aromatic protons. The slow exchange 1H NMR spectrum at 220 K revealed two set of singlets for each resonance with equal intensity due to the present of the two different spatial orientations for the methoxy, benzylic, and phenyl protons corresponding to the boat (C2v) conformer. Notably, in the crystal network of ROBr, the bromine site exhibited severe positional disorder, attributed to the fact that its atomic mass is greater than those of the other constituent atoms. As a result of its positional disorder, the bromine atom appeared at all of the four apex methylene sites in the crystal structure of ROBr (ESI†). From the single-crystal X-ray diffraction analysis of ROBr, 60% of the bromine density was observed at C1, 5% was observed at C2, 30% was observed at C3 and 5% was observed at C4. However, the total site-occupancy factor of the bromine atom was unity, thereby confirming the molecular formula to be C90H100O10Br1. In this compound, the major contributor towards the bromine occupancy (60% + 30%) was observed in phenyl groups, which were at right angles to the direction of the resorcin[4]arene cavity. As we could not obtain crystals suitable for single-crystal X-ray diffraction analysis, a large porphyrin fragment of the synthesised conjugates is expected to be oriented in the same manner as that for bromine.
Binding studies
Recently, we have reported the crystal structure and binding study of a cavitand-bowl-shaped resorcin[4]arene–porphyrin conjugate ZnRCP. From the results obtained from UV titration, a 50% increase in the Ka value for Zn-porphyrin was observed in the presence of the resorcin[4]arene host with pyridine as the axial ligand. The bulky axial ligand 4-tert-butylpyridine was bound to Zn-porphyrin from the opposite side of cavitand resorcin[4]arene RC. In this binding study flexible with a boat confirmation host (RO) was used in synthesised bis-resorcin[4]arene–porphyrin conjugates. Zn(II)-metalloporphyrin conjugate derivatives were quantitatively prepared from the reaction between free base porphyrin and excess Zn(OAc)2 in CHCl3. Zn-porphyrin is known to bind to only one axial ligand, resulting in a five-coordinated zinc atom.17 Binding studies were performed via UV titration in CHCl3 using pyridine derivatives. For comparison, analogous experiments with Zn(II)-based octamethoxy–resorcin[4]arene (Zn(II)ROP) and Zn-tetratolylporphyrin (ZnTTP) were also performed.In the absorption spectra of ZnROP and bis-resorcin[4]arene–metalloporphyrin conjugates in chloroform, transitions similar to those in ZnRCP were observed, typical for Zn(II) porphyrins (Fig. 5a). Specifically, a symmetry-allowed transition centred at 422 nm, corresponding to the B-band or Soret band, was observed, as well as two transitions at 557 and 596 nm (Q-bands), respectively. The addition of pyridine or 4-tert-butylpyridine resulted in hyperchromic shifts in the absorption spectra. A typical example of UV titration is shown in Fig. 5b.
For characterising the effect of the large cavity of the resorcin[4]arene conjugate, exhibiting the boat conformation, on the binding affinity of Zn(II)ROP, UV-vis titration was performed using pyridine and 4-tert-butylpyridine guests in chloroform. The data thus obtained were compared to those of the binding studies reported previously for ZnRCP and ZnTTP.16 The data obtained for Zn(ROP) fitted well to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherm, and the binding constant was determined from non-linear least-squares fitting,6 utilising the change in absorbance at 430 nm. Table 1 summarises the results. The Ka of Zn(II)ROP containing a pyridine axial ligand in chloroform was calculated to be 1.09 × 103 M−1, which is approximately the same as that of Zn(II)TTP (1.06 × 103 M−1) within experimental error. The result indicates that the axial ligand (pyridine) with a larger cavity, exhibiting the boat conformation, of the resorcin[4]arene conjugate is not favourably encapsulated in Zn(II)ROP.
1 binding isotherm, and the binding constant was determined from non-linear least-squares fitting,6 utilising the change in absorbance at 430 nm. Table 1 summarises the results. The Ka of Zn(II)ROP containing a pyridine axial ligand in chloroform was calculated to be 1.09 × 103 M−1, which is approximately the same as that of Zn(II)TTP (1.06 × 103 M−1) within experimental error. The result indicates that the axial ligand (pyridine) with a larger cavity, exhibiting the boat conformation, of the resorcin[4]arene conjugate is not favourably encapsulated in Zn(II)ROP.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes measured by UV-vis titration in CHCl3 at 298 K (with percentage error)a
1 complexes measured by UV-vis titration in CHCl3 at 298 K (with percentage error)a
| Porphyrin | Ligand | Ka (M−1) |
|---|---|---|
a Titration data were fitted to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model, with an error of less than 7%. Ka was measured from the change in absorbance at 430 nm.b Data published previously (ref. 16). 1 binding model, with an error of less than 7%. Ka was measured from the change in absorbance at 430 nm.b Data published previously (ref. 16). |
||
| ZnTTPb | Pyridine | 1.06 × 103 (5%) |
| 4-tert-Butylpyridine | 1.67 × 103 (6%) | |
| ZnRCPb | Pyridine | 1.54 × 103 (6%) |
| 4-tert-Butylpyridine | 1.46 × 103 (5%) | |
| ZnROP | Pyridine | 1.09 × 103 (6%) |
| 4-tert-Butylpyridine | 1.84 × 103 (5%) | |
Similarly, the titration of Zn(II)ROP with 4-tert-butylpyridine resulted in a Ka value of 1.87 × 103 M−1, the value of which is 10% greater than that of Zn(II)TTP and 30% greater than that reported previously for the Zn(II)-porphyrin conjugate based on bowl-shaped resorcin[4]arene (Zn(II)RCP).16 From UV titration, the large cavity of the boat conformation of the resorcin[4]arene conjugate host could accommodate the bulky substituent of the 4-tert-butylpyridine axial ligand. In contrast, the large boat-shaped resorcin[4]arene cavity prevented the favourable accommodation of the pyridine axial ligand inside the resorcin[4]arene host. Fig. 6 shows the binding mode of 4-tert-butylpyridine with Zn(II)ROP. The results obtained from binding studies were in good agreement with those obtained from the single-X-ray crystal analysis of the octa-methylated methyl resorcin[4]arene host (RO).
For demonstrating the effect of the two resorcin[4]arenes and their positions relative to the porphyrin bridge on the binding affinity, UV-vis titration experiments were performed for the three Zn-metalled bis-resorcin[4]arene porphyrin conjugate systems with 4-tert-butylpyridine as the axial ligand. Similar to the results obtained for the mono-resorcin[4]arene porphyrin conjugate, the data fitted well to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherm. Table 2 summarises the results obtained. The Ka values measured from the UV-titration experiments were affected by the position of the resorcin[4]arene host relative to the porphyrin system. Ka calculated for the Zn(II) bis-resorcin[4]arene porphyrin conjugate based on PT was comparable to that of ZnROP, as the greater the distance from the hosts, the similar the behaviour to mono-conjugated ZnROP. As compared to that of free zinc-porphyrin (ZnTTP), those of Zn(RO)2PC and Zn(RO)2PH exhibited higher Ka values, with an approximately 50% increase. The close proximity of the two resorcin[4]arene hosts to each other in Zn(RO)2PC and Zn(RO)2PH forced one of the large substituents to face the porphyrin ring, resulting in the increase in the Ka value.
1 binding isotherm. Table 2 summarises the results obtained. The Ka values measured from the UV-titration experiments were affected by the position of the resorcin[4]arene host relative to the porphyrin system. Ka calculated for the Zn(II) bis-resorcin[4]arene porphyrin conjugate based on PT was comparable to that of ZnROP, as the greater the distance from the hosts, the similar the behaviour to mono-conjugated ZnROP. As compared to that of free zinc-porphyrin (ZnTTP), those of Zn(RO)2PC and Zn(RO)2PH exhibited higher Ka values, with an approximately 50% increase. The close proximity of the two resorcin[4]arene hosts to each other in Zn(RO)2PC and Zn(RO)2PH forced one of the large substituents to face the porphyrin ring, resulting in the increase in the Ka value.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes as measured by UV-vis titration in CHCl3 at 298 K (with percentage error)a
1 complexes as measured by UV-vis titration in CHCl3 at 298 K (with percentage error)a
| Entry | Porphyrin | Ligand | Ka (M−1) |
|---|---|---|---|
a Titration data were fitted to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model, with an error of less than 10%. Ka was measured from the change in absorbance at 430 nm. 1 binding model, with an error of less than 10%. Ka was measured from the change in absorbance at 430 nm. |
|||
| 1 | Zn(RO)2PC | 4-tert-Butylpyridine | 2.43 × 103 (6%) |
| 2 | Zn(RO)2TP | 4-tert-Butylpyridine | 1.99 × 103 (8%) |
| 3 | Zn(RO)2PH | 4-tert-Butylpyridine | 2.44 × 103 (9%) |
Fluorescence decay studies
Benzoquinone (BQ) is well known to quench the fluorescence of porphyrin systems, and the conjugation of porphyrin with a molecular trap, such as calix[n]arene, is well known to considerably enhance the quenching of fluorescence.15 In a previous study, the effect of the resorcin[4]arene conjugate towards the quenching behaviour of porphyrin was examined using structurally different quinones, such as BQ, 1,4-naphthoquinone (NQ), phenanthrenequinone (PAQ) and dichlorodicyanobenzoquinone (DDQ). The results obtained from that study suggested that the presence of the resorcin[4]arene host enhances the efficiency of fluorescence quenching of porphyrin. Particularly, PAQ exhibits high efficiency towards the quenching of the fluorescence of the porphyrin resorcin[4]arene conjugate RCP; therefore, PAQ is selected as a model compound for investigating the effect of the position of resorcin[4]arene relative to porphyrin in the synthesised bis-resorcin[4]arene–bridged porphyrin. The fluorescence decay of porphyrin was investigated by the addition of increasing amounts of PAQ to the solutions containing fixed concentration of the parent porphyrin TTP, ROP and porphyrin bis-resorcin[4]arene conjugates in dichloromethane.Even though the analysis of such a system is complicated by the possible competition between the quenching by free quinones and quenching by quinones encapsulated in the resorcin[4]arene cavity, the presence of resorcin[4]arenes conjugated to porphyrins typically enhances the efficiency of fluorescence quenching. Fig. 7 shows the fluorescence intensity at 648 nm as a function of the PAQ concentration for free porphyrin TTP, mono-conjugate ROP and bis-conjugates (RO)2PT. (RO)2PH and (RO)2PC. Typically, as compared to the unconjugated porphyrin TTP, all porphyrin–resorcin[4]arene conjugates exhibited enhanced fluorescence quenching. However, the fluorescence quenching efficiency was considerably affected by the structure of the porphyrin bis-resorcin[4]arene conjugates. Porphyrin bis-resorcin[4]arene conjugate based on trans-porphyrin ((RO)2TP) exhibited fluorescence quenching behaviour similar to that of the porphyrin–monoresorcin[4]arene conjugate (ROP). On the other hand, porphyrin bis-resorcin[4]arene conjugate based on cis-porphyrin (PC) and porphyrin (PH) exhibited similar and more efficient fluorescence quenching ability.
For example, after the addition of 4.0 × 10−5 M of PAQ, the fluorescence intensity noticeably decreased to 30% and 40% for (RO)2PC and (RO)2PH, respectively. No significant decrease was observed in the fluorescence intensities for TTP, ROP and (RO)2PT (>7%). Increasing the PAQ concentration to 6.5 × 10−5 M resulted in the severe decrease in the fluorescence of (RO)2CP and (RO)2PH by greater than 99%. The bis-conjugate based on trans-porphyrin exhibited a 39% reduction in fluorescence intensity, while ROP exhibited a 30% decrease. Free porphyrin TTP exhibited only a 10% decrease in fluorescence intensity. Further increase in the PAQ concentration to 9.0 × 10−5 M decreased fluorescence by 96% and 84% for (RO)2PT and ROP, respectively. On the other hand, a 15% decrease in the fluorescence intensity of TTP was observed. The results from the fluorescence quenching study demonstrate the effect of not only the resorcin[4]arene host, on the fluorescence quenching of porphyrin, but also the relative proximity of the bulky resorcin[4]arene conjugates to each other. As the structure of the porphyrin bridge in (RO)2PC and (RO)2PH force one of the resorcin[4]arene hosts to be positioned close to porphyrin, the fluorescence quenching efficiency increased (Fig. 7). This result was in agreement with those obtained from binding studies.
Conclusion
In this study, three bis-resorcin[4]arene porphyrin conjugates were synthesised, and their structures were confirmed by NMR and mass spectroscopic analyses. From the results obtained from UV-vis titrations of the Zn(II)-based metalloporphyrin conjugate, binding affinity is affected by the shape of the resorcin[4]arene host and the structure of the axial ligand. The large resorcin[4]arene host with the boat conformation exhibited no enhancement in the binding constant of Zn-porphyrin when pyridine was used as the axial ligand. For bis-resorcin[4]arene–bridged porphyrin conjugates, the binding constant is affected by structure of the conjugates. For example, when two resorcin[4]arene hosts were in close proximity to each other, the association constant (Ka) for Zn-porphyrin increased by 50% with 4-tert-butylpyridine as the axial ligand for Zn(RO)2PC and Zn(RO)2PH. Typically, for fluorescence decay studies, at low quinone concentration, the fluorescence quenching of porphyrin was considerably enhanced by the attachment of resorcin[4]arene to the photoactive subunit; however, fluorescence quenching efficiency was affected by the structural type of the porphyrin bis-conjugates. The data obtained from fluorescence decay studies were in agreement with those obtained from binding studies performed by UV-vis titration of porphyrin conjugates. These types of conjugates afford interesting opportunities for various applications. Currently, further studies and modification of the resorcinarene–porphyrin conjugates in terms of the increase in rigidly, stability and catalytic activity are underway in our laboratories.Acknowledgements
The support received from the University of Kuwait, made available through research grant no. SC01/12, and the facilities of RSP4 (grant no. GS01/01, GS03/01, GS01/03, GS01/10 and GS03/08) are gratefully acknowledged.Notes and references
-
(a) I. Ling, Y. Alias, A. N. Sobolev and C. L. Raston, Cryst. Growth Des., 2010, 10, 1312 CrossRef CAS
; (b) J. Luo, L. Shen and W. J. Chung, J. Org. Chem., 2010, 75, 464 CrossRef CAS PubMed
; (c) J. Liu and A. Wei, Chem. Commun., 2009, 4254 RSC
; (d) L. Nault, A. Cumbo, R. F. Pretot, M. A. Sciotti and P. Shahgaldian, Chem. Commun., 2010, 5581 RSC
; (e) C. Lynam and D. Diamond, J. Mater. Chem., 2005, 15, 307 RSC
; (f) L. Nault, A. Cumbo, R. F. Pretot, M. A. Sciotti and P. Shahgaldian, Chem. Commun., 2010, 5581 RSC
; (g) S. Sameni, C. Jeunesse, D. Matt and J. Harrowfield, Chem. Soc. Rev., 2009, 38, 2117 RSC
.
-
(a) Y. Kubo, K. Tsuruzoe, S. Okuyama, R. Nishiyabu and T. Fujihara, Chem. Commun., 2010, 3604 RSC
; (b) J. Nakazawa, J. Hagiwara, M. Mizuki, Y. Shimazaki, F. Tani and Y. Naruta, Angew. Chem., Int. Ed., 2005, 44, 3744 CrossRef CAS PubMed
; (c) K. Salorinne, D. P. Weimann, C. A. Schalley and M. Nissinen, Eur. J. Org. Chem., 2009, 35, 6151 CrossRef
; (d) B. Botta, M. Pierini, G. D. Monache, D. F. Subissati and S. G. Zappia, Synthesis, 2008, 13, 2110 CrossRef
; (e) E. Mileo, S. Yi, P. Bhattacharya and A. E. Kaifer, Angew. Chem., Int. Ed., 2009, 48, 5337 CrossRef CAS PubMed
; (f) E. Mann and J. Rebek, Tetrahedron, 2008, 64, 8484 CrossRef CAS
; (g) O. Hayashida and M. Uchiyama, J. Org. Chem., 2007, 72, 610 CrossRef CAS PubMed
; (h) M. R. Caira, T. Roex and L. R. Nassimbeni, CrystEngComm, 2006, 8, 275 RSC
; (i) M. Cacciarini, V. A. Azov, P. Seiler, H. Kuenzer and F. Diederich, Chem. Commun., 2005, 5269 RSC
.
-
(a) W. Tang and S. Ng, Nat. Protoc., 2008, 3, 691 CrossRef CAS PubMed
; (b) M. Yu, Y. Chen, N. Zhang and Y. Liu, Org. Biomol. Chem., 2010, 8, 4148 RSC
; (c) H. Xu, E. A. Ermilov, B. Roeder and D. K. P. Ng, Phys. Chem. Chem. Phys., 2010, 12, 7366 RSC
; (d) S. Yu, X. Huang, L. Miao, J. Zhu, Y. Yin, Q. Luo, J. Xu, J. Shen and J. Liu, Bioorg. Chem., 2010, 38, 159 CrossRef CAS PubMed
; (e) Y. Zhang, Y. Chen, Y. Yang, P. Liu and L. Y. Yu, Chem.–Eur. J., 2009, 15, 11333 CrossRef CAS PubMed
; (f) W. Muderawan, T. Ong, W. Tang, D. J. Young, C. Ching and S. Ng, Tetrahedron Lett., 2005, 46, 1747 CrossRef
.
-
(a) A. Spaeth and B. Koenig, Tetrahedron, 2010, 66, 6019 CrossRef CAS
; (b) B. Martinez-Haya, P. Hurtado, A. R. Hortal, S. Hamad, J. D. Steill and J. Omens, J. Phys. Chem. A, 2010, 114, 7048 CrossRef CAS PubMed
; (c) A. Spaeth and B. Koenig, Tetrahedron, 2010, 66, 1859 CrossRef CAS
; (d) Z. Ge, J. Hu, F. Huang and S. Liu, Angew. Chem., Int. Ed., 2009, 48, 1798 CrossRef CAS PubMed
; (e) K. Zhu, S. Li, F. Wang and F. Huang, J. Org. Chem., 2009, 74, 1322 CrossRef CAS PubMed
; (f) A. Spaeth and B. Koenig, Tetrahedron, 2009, 65, 690 CrossRef CAS
; (g) T. G. Levitskaia, L. Maya, G. J. Van Erkel and B. A. Moyer, Inorg. Chem., 2007, 46, 261 CrossRef CAS PubMed
.
-
(a) G. M. Mamardashvili, Russ. J. Coord. Chem., 2006, 10, 756 CrossRef
; (b) A. Mulholland, P. Thordason, E. J. Mensforth and S. Langford, Org. Biomol. Chem., 2012, 10, 6045 RSC
; (c) O. Middel, W. Verboom and D. N. Reinhoudt, J. Org. Chem., 2001, 66, 3998 CrossRef CAS PubMed
.
- P. Thordarson, Chem. Soc. Rev., 2011, 40, 1305 RSC
.
- H. Iwamoto, Y. Yukimasa and Y. Fukazawa, Tetrahedron Lett., 2002, 43, 8191 CrossRef CAS
.
- N. Z. Mamardashvili and O. I. Koifman, Russ. J. Gen. Chem., 2005, 6, 787 CrossRef
.
- D. M. Rudkevich, W. Verboom and D. N. Reinhoudt, J. Org. Chem., 1995, 60, 6585 CrossRef CAS
.
-
(a) S. D. Starnes, D. M. Rudkevich and J. Rebek, Org. Lett., 2000, 14, 1995 CrossRef
; (b) J. Nakazawa, M. Mizuki, Y. Shimazaki, F. Tani and Y. Naruta, Org. Lett., 2006, 19, 4275 CrossRef PubMed
; (c) J. Nakazawa, Y. Sakae, M. Mizuk and Y. Naruta, J. Org. Chem., 2007, 72, 9448 CrossRef CAS PubMed
; (d) J. Nakazawa, J. Hagiwara, M. Mizuki, Y. Shimazaki, F. Tani and Y. Naruta, Angew. Chem., 2005, 117, 2810 CrossRef
.
- J. T. Groves and Y. Z. Han, Cytochrome P 450: Structure, Mechanism and Biochemistry, ed. P. R. Ortiz de Montellano, Plenum Press, New York, 2nd edn, 1995, pp. 3–48 Search PubMed
.
- D. Fankhauser, D. Kolarski, W. R. Gruning and F. Diederich, Eur. J. Org. Chem., 2014, 17, 3575 CrossRef CAS
; B. Botta, P. Ricciardi, C. Galeffi, M. Botta, A. Tafi, R. Pogni, R. I. Iacovino Garella, B. D. Blasio and G. D. Monache, Org. Biomol. Chem., 2003, 1, 3131 Search PubMed
.
-
(a) O. Kundrat, M. Kas, M. Tkadlecova, K. Lang, J. Cvacka, I. Stibor and P. Lhotak, Tetrahedron Lett., 2007, 48(38), 6620 CrossRef CAS
; (b) T. Arimura, T. Nashik, Y. Suga, S. Kumamoto, Y. Tsuchiya, T. Yamaguchi and M. Tachiya, J. Oleo Sci., 2007, 56(3), 155 CrossRef CAS PubMed
; (c) M. Dudic, P. Lhotak, H. Petrickova, I. Stibor, K. Lang and J. Sykora, Tetrahedron, 2003, 59(14), 2409 CrossRef CAS
; (d) T. Arimura, T. Nishioka, S. Ide, Y. Suga, H. Sugihara, S. Murata and M. Tachiya, J. Photochem. Photobiol., A, 2001, 145(1–2), 123 CrossRef CAS
; (e) G. M. Mamardashvili, S. V. Zvezdina and N. Z. Mamardashvili, Russ. J. Gen. Chem., 2011, 81(3), 594 CrossRef CAS
.
-
(a) G. Pognon, C. Boudon, K. J. Schenk, M. Bonin, B. Bach and J. Weiss, J. Am. Chem. Soc., 2006, 128(11), 3488 CrossRef CAS PubMed
; (b) D. Jokic, C. Boudon, G. Pognon, M. Bonin, K. J. Schenk, M. Gross and J. Weiss, Chem.–Eur. J., 2005, 11(14), 4199 CrossRef CAS PubMed
; (c) V. Valderrey, G. Aragay and P. Ballester, Coord. Chem. Rev., 2014, 258–259, 137 CrossRef CAS
; (d) G. M. Mamardashvili, N. V. Chizhova and N. Zh. Mamardashvili, Russ. J. Inorg. Chem., 2012, 57(3), 390 CrossRef CAS
; (e) N. Z. Mamardashvili and O. I. Koifman, Macroheterocycles, 2009, 2(1), 30 CrossRef CAS
.
- T. F. Al-Azemi and M. Vinodh, Tetrahedron, 2011, 67, 2585 CrossRef CAS
.
- T. F. Al-Azemi and M. Vinodh, RSC Adv., 2015, 5, 88154 RSC
.
- M. Hong, Y. Zhang and Y. Liu, J. Org. Chem., 2015, 80, 1849 CrossRef CAS PubMed
; S. Busi, H. Saxell, R. Frohlich and K. Rissanen, CrystEngComm, 2008, 10, 1803 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1481430 and 1481431. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra13963a |
| This journal is © The Royal Society of Chemistry 2016 |