Synthesis and characterization of semiaromatic polyamides with dicyclohexane units
Guang-ming Yana,
Gang Zhang*a,
Hao-hao Rena,
Yan Lia and
Jie Yang*ab
aInstitute of Materials Science & Technology, Analytical & Testing Center, Sichuan University, Chengdu 610064, P. R. China. E-mail: gangzhang@scu.edu.cn
bState Key Laboratory of Polymer Materials Engineering (Sichuan University), Chengdu, 610065, P. R. China. E-mail: ppsf@scu.edu.cn
First published on 8th August 2016
Abstract
Two new difluorobenzamide monomers, N,N′-bis(4-fluorobenzamide)dicyclohexyl methane and N,N′-bis(4-fluorobenzamide)-3,3′-dimethyl dicyclohexyl methane were synthesized from 4,4′-diamino dicyclohexyl methane and bis-(3-methyl-4-aminocyclohexyl)-methane with 4-fluorobenzoyl chloride through a facile interfacial reaction. Both of the monomers were used to prepare semiaromatic polyamides by a nucleophilic polycondensation reaction with 1,1-bis(4-hydroxyphenyl)-1-phenylethane (BHPPE). The resultant semiaromatic polyamides were characterization by FT-IR, differential scanning calorimetry, thermogravimetric analysis, dynamic thermomechanical analysis etc. These semiaromatic polyamides were found to have excellent thermal properties: the glass transition temperature (Tg) of Ph-DCM and Ph-MCM was 217.2 °C and 236.9 °C, and the 5% weight-loss temperature (T5%) was 442.3 °C and 429.8 °C, respectively. The tensile test and dynamic thermo-mechanical analysis indicates that the resultant polyamides have good mechanical properties. The rheological testing showed that when the temperature increased from 290 °C to 350 °C, the complex viscosities of Ph-DCM decreased from 8.7 to 2.9 kPa s and the complex viscosities of Ph-MCM dropped from 20.8 to 0.9 kPa s. The results indicate that these semiaromatic polyamides have good melt flowability and are suitable for the melting processing. In particular, the polyamide with pendent methyl groups was found to have lower complex viscosities than that with no pendent methyl groups.
Introduction
Aromatic polyamides are a type of high-performance polymeric materials that have been widely used at elevated temperatures for their excellent mechanical property, chemical stability, and superior thermal stability.1–3 However, their poor processability (poor solubility in common organic solvents and high melt or glass transition temperature) limits their widely applications in industrial and some other fields. The reasons for the high melt and glass transition temperatures are their inherent rigidity of aromatic rings and strong inter-chain forces (such as hydrogen bonding). In view of that, it is necessary to prepare good processability polyamides. Usually, the approaches to improve the processability of aromatic polyamides without deteriorating their excellent properties include incorporating flexible linkages,4–6 noncoplanar structure,7–9 as well as bulky pendants10–12 into the polymer backbones. Aliphatic polyamides such as the homopolymer of poly(ε-caprolactam) (PA6) and PA66 are one of the most important engineering thermoplastic, have been more commercial over the past several decades.13 However, with the development of industry, aliphatic polyamides can't achieve the stringent requirements because of their poor thermal properties and dimensional stability. The incorporation of aromatic rings into the backbones is an effective method to improve the heat resistance and mechanical properties.14–16 Accordingly, by taking advantages of aromatic polyamides and aliphatic polyamides, semiaromatic polyamides have attracted a lot of attention and have broad application prospects in the fields of electric/electronic products.5,17–19 Although semiaromatic polyamides have better thermal stability and chemical stability than aliphatic polyamide but still a few semiaromatic polyamides have been successful commercialization. Semiaromatic polyamides with short aliphatic diamines can't be produced by melt processing because they will decompose (their melting point is higher than thermal decomposition temperature). Therefore, it is necessary to develop novel and processable semiaromatic polyamides. Similarly, the methods of improving the semiaromatic processability contained the introduction of flexible segments, bulky pendant groups, unsymmetrical units, noncoplanar biphenylene moieties and so on into the polymer backbone.20–23 Generally speaking, the incorporation of flexible segments into semiaromatic polyamides backbones is an effective method to yield high performance polyamides which have good processability.24,25 However, the commercial semiaromatic polyamides such as PA6T, PA9T and PA10T containing linear aliphatic linkage have low glass transition temperature, and their thermal-oxidation resistance at high temperature was found to be not well.In the last few years, our research group has been focusing on incorporating aliphatic linkages and thioether units into semiaromatic polyamide main chain as flexible segments to improve the processability.20,26–30 The resultant semiaromatic polyamides were found to have excellent thermal properties, superior mechanical properties and good processability. However, there were only a few reports about the semiaromatic polyamides containing cyclohexane. According to our recent study, we had found that the semiaromatic polyamide which containing cyclohexane rings had better thermal, mechanical and anti-oxidation properties than that of semiaromatic polyamides containing linear aliphatic linkages.28 Therefore we expected to incorporate high content cyclohexane units and bulky pendent methyl group into the polymer chain to improve the polymer thermal properties and melt processability, respectively. So, in this work, we synthesized two kinds of difluorobenzamides monomers containing two cyclohexane units with a facile interfacial reaction. Then they were conducted to react with 1,1-bis(4-hydroxyphenyl)-1-phenylethane (BHPPE) through nucleophilic polycondensation reaction to afford two kinds of semiaromatic polyamides containing two cyclohexane units. The effects of the cyclohexane and pendent methyl group on the properties of these semiaromatic polyamides such as thermal properties and rheological properties were studied in detail.
Experimental
Materials
4-Fluorobenzoyl chloride (4-FBC) (99.5%, Lanning Chemical Company Limited), 1,1-bis(4-hydroxyphenyl)-1-phenylethane (BHPPE) was synthesized as reported by our group.29 4,4′-Diaminodicyclohexylmethane (DDCM), bis-(3-methyl-4-aminocyclohexyl)-methane (MACM), sodium hydroxide (NaOH) (AR, SiChuan ChengDu ChangLian Chemical Reagent Company), N-methyl-2-pyrrolidone (NMP) (JiangSu NanJing JinLong Chemical Industry Company), toluene (AR, SiChuan ChengDu KeLong Chemical Reagent Company), other reagents and solvents were obtained commercially.Monomer synthesis (Scheme 1)
Polymer synthesis (Scheme 2)
In a representative procedure of synthesis of Ph-DCM, to a 500 mL three-neck round bottom flask equipped with mechanical stirrer, Dean–Stark trap and thermometer were added BHPPE (29 g, 0.1 mol), BFDCM (45.6 g, 0.1 mol), NMP (100 mL), toluene (20 mL) and potassium carbonate (26.4 g, 0.2 mol). The reaction solution was heated to 150 °C, refluxed for 2 h under the protection of nitrogen to remove the toluene and byproduct water. Then the reaction mixture was heated up to 200 °C and kept for another 8 h under stirring and N2 atmosphere. After that, the reaction solution was poured into water with stirring. The crude product was washed with hot water several times, and then the threadlike solid was pulverized into powder, washed with deionized water and ethanol three times, respectively. Then the product was dried under vacuum at 100 °C for 12 h to yield Ph-DCM (64.6 g, yield: 91.5%).Ph-MCM was prepared by a similar procedure as that of Ph-DCM (67.6 g, yield: 92.1%).
Characterization
Intrinsic viscosity analysis: the intrinsic viscosity ηint of the Ph-DCM and Ph-MCM was measured by a Cannon–Ubbelodhe viscometer at 30 ± 0.1 °C. Polymer solution was prepared by dissolving 0.5 g of polymer into 100 mL of NMP. The results were obtained by the one-point method (or Solomon–Ciuta equation) as follows:where ηr = η/η0, ηsp = η/η0 − 1.
The 1H NMR (400 MHz) spectra was measured on a Bruker Avance-400 NMR spectrometer with dimethyl sulfoxide-d6 (DMSO-d6) as the solvent. FT-IR spectra was performed on a NEXUS670 FT-IR instrument. Differential Scanning Calorimeter (DSC) was measured on a TA Q20 thermal analyzer at a heating rate of 10 °C min−1 under a nitrogen atmosphere. Thermal gravity analysis was performed on a TGA Q500 V6.4 Build 193 thermal analysis instrument under nitrogen at a heating rate of 10 °C from 50 °C to 800 °C. Dynamic Mechanical Analysis (DMA) was performed on TA-Q800 apparatus operating in tensile mode at a frequency of 1 Hz in the temperature range from 30 to 300 °C, with a heating rate of 5 °C min−1. The stress–strain behavior was measured on an Instron Corporation 4302 instrument at room temperature. Parallel plate rheometer (Bohlin Gemini 200, Britain) was fitted with 2.5 cm diameter stainless steel parallel plates. At a frequency of 1 Hz, a temperature sweep test was performed under nitrogen atmosphere (350–290 °C).
Results and discussion
Synthesis of monomers
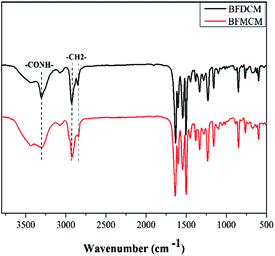
The semiaromatic amides monomers were synthesized by an interfacial reaction through a one-step procedure from 4-fluorobenzoyl chloride and 4,4′-diamino dicyclohexyl methane or 3,3′-dimethyl-4,4′-diamino dicyclohexyl methane. The synthesis route was presented in Scheme 1. The reaction temperature should be controlled below 30 °C to avoid side reaction. The chemical structure of monomers BFDCM and BFMCM was studied by FT-IR and 1H-NMR. Fig. 1 is the 1H-NMR spectra of BFDCM and BFMCM. In the spectra, the proton signals of aromatic rings were in the range of 7.25–7.92 ppm. The proton signals ranged from 8.08–8.22 ppm were attributed to the amide units. However it is obvious that –CONH– of BFDCM have two proton signals. This phenomenon is attributed to the trans- and cis-isomerism of the dicyclohexyl methane.28 Because the dicyclohexyl methane has three different conformations, so the methylenes in the cyclohexane units have different proton signals. The proton signals of methylene are in the range of 1.18–1.86 ppm. Compared with BFDCM, the proton signals of BFMCM did not change a lot. The proton signals of three methylenes were in the range of 1.31–1.75 ppm. The sharp peak at 0.85 ppm was attributed to the pendent methyl groups. Fig. 2 is the FT-IR spectra of the monomers. Amide absorptions can be found near 3300, 1635 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1, methylene absorptions were near 2927 and 2850 (C–H) cm−1. The absorptions of benzene ring and P-substituted benzene ring were near 1602, 1502, 1450 (C
O) cm−1, methylene absorptions were near 2927 and 2850 (C–H) cm−1. The absorptions of benzene ring and P-substituted benzene ring were near 1602, 1502, 1450 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) and 850 (C–H) cm−1. The results of FT-IR and 1H-NMR indicate that the monomers containing cyclohexane units were successfully synthesized through the interfacial reaction.
C) and 850 (C–H) cm−1. The results of FT-IR and 1H-NMR indicate that the monomers containing cyclohexane units were successfully synthesized through the interfacial reaction.
Synthesis of polymer
Two kinds of novel semiaromatic polyamides were synthesized through the reaction of bisphenol (BHPPE) and difluorobenzamide monomers with K2CO3 as the base. The molecular weights of the resultant polyamides were characterized by the intrinsic viscosity (ηint). As shown in Table 1, the intrinsic viscosity of Ph-DCM and Ph-MCM was 0.48 dl g−1, 0.49 dl g−1, respectively. These values indicate that the synthesized polyamides exhibited moderate molecular weights. The chemical structures of polyamides were characterized by FT-IR and 1H NMR.| Samples | Intrinsic viscosity (dl g−1) | Tg (°C) | T5% (°C) | Char yield (%) | Tensile strength (MPa) | Elongation at break (%) | Storage modulus (GPa) | Storage modulus at 200 °C (GPa) |
|---|---|---|---|---|---|---|---|---|
| Ph-DCM | 0.48 | 217.2 | 442.3 | 6.09 | 75.6 | 23.91 | 1.3 | 0.67 |
| Ph-MCM | 0.49 | 236.9 | 429.8 | 7.63 | 77.6 | 21.64 | 2.1 | 1.5 |
As shown in Fig. 3 amide absorptions can be found near 3435 (–NH–), 1635 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) cm−1. Compared with monomers the absorption of C–F (1330 cm−1) disappeared and a new absorption peak near 1100 cm−1 (–O–) appeared. It indicates the reaction of difluorobenzamide monomers and bisphenol. Fig. 4 and 5 show the 1H NMR of the resultant semiaromatic polyamides Ph-DCM and Ph-MCM. Because of different conformations, the protons of amide unit and methylenes in the cyclohexane unit have different proton signals. As show in Fig. 4, the signals at 8.01 and 8.13 ppm were attributed to the protons of amide. The proton signals in the range of 0.96–3.91 ppm belong to aliphatic structure. Compared with Ph-DCM, the proton signals of Ph-MCM did not change a lot. The proton signals at 0.71–3.47 ppm are attributed to the aliphatic moiety.
O) cm−1. Compared with monomers the absorption of C–F (1330 cm−1) disappeared and a new absorption peak near 1100 cm−1 (–O–) appeared. It indicates the reaction of difluorobenzamide monomers and bisphenol. Fig. 4 and 5 show the 1H NMR of the resultant semiaromatic polyamides Ph-DCM and Ph-MCM. Because of different conformations, the protons of amide unit and methylenes in the cyclohexane unit have different proton signals. As show in Fig. 4, the signals at 8.01 and 8.13 ppm were attributed to the protons of amide. The proton signals in the range of 0.96–3.91 ppm belong to aliphatic structure. Compared with Ph-DCM, the proton signals of Ph-MCM did not change a lot. The proton signals at 0.71–3.47 ppm are attributed to the aliphatic moiety.
Thermal properties of the resultant semiaromatic polyamides (Ph-DCM and Ph-MCM)
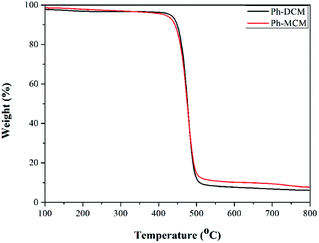
The thermal properties of these resultant semiaromatic polyamides were evaluated by DSC and TGA under nitrogen atmosphere. The DSC and TGA curves of these polyamides based on BFDCM and BFMCM are displayed in Fig. 6 and 7, respectively. The Tg of Ph-DCM and Ph-MCM was 217.2 °C and 236.9 °C, respectively. It is about 80–100 °C higher than that of commercial product such as PA6T (Tg is about 130 °C). However, compared with semiaromatic polyamides containing cyclohexane unit, the resultant semiaromatic polyamides containing dicyclohexane units have slightly lower Tg.28 This is because that the resultant semiaromatic polyamides have lower proportion of rigidly benzene rings. Beyond our expectation, the glass transition temperature (Tg) of the semiaromatic polyamide was found to increase by introducing methyl groups into the polyamide backbones. The main reason was that the methyl groups increased the polymer interaction, then the movement of polymer chain was hindered. Compared with traditional semiaromatic polyamides (such as PA9T, Tm = 305 °C), the melting peaks can't be found from the DSC curves. This was attributed to the introduction of large pendent phenyl groups and methyl groups which decreased the close packing of molecular chains, so the chains can't keep regular arrangement. As shown in the Fig. 7, under nitrogen atmosphere, the 5% weight-loss temperature (T5%) of Ph-DCM and Ph-MCM was 442.3 °C and 429.8 °C, respectively. The char yield of Ph-DCM and Ph-MCM at 800 °C was 6.09% and 7.63%, respectively. The values of corresponding TGA were listed in Table 1. The thermostability of polyamide decreased with the introduction of methyl groups. This can be attributed to the relatively lower stability of aliphatic methyl at the high temperature. However, the thermal properties of polyamides were maintained with the introduction of aliphatic segment (cyclohexane and methyl units).Dynamic mechanical analysis of the resultant semiaromatic polyamides (Ph-DCM and Ph-MCM)
DMA was used to get the information of the thermal mechanical properties of these resultant semiaromatic polyamides. As shown in Fig. 8, one apparent transition was found, which corresponded to the α-relaxation of resin. The glass transition temperature (Tg) is related to the segment movements in the noncrystalline area, so Tg is determined by α-relaxation. These Tg values were close to those obtained by the DSC method. The differences could be mainly ascribed to the different responses to both the measurements. The Tg values increased with introducing methyl groups into the semiaromatic polyamide backbones that was similar with the results measured by DSC. The curves of storage modulus versus temperature of Ph-DCM and Ph-MCM are shown in Fig. 9. The semiaromatic polyamides were found to have good storage modulus of 1.3 GPa and 2.1 GPa, respectively. The storage modulus of Ph-DCM remained 0.67 GPa and 1.5 GPa at about 200 °C. It indicates the resultant semiaromatic polyamides have good thermal mechanical performance.Tensile properties of the resultant semiaromatic polyamides
In order to study the tensile properties, the resultant semiaromatic polyamides were made into films by hot press molding at 315 °C. The tensile properties of Ph-DCM and Ph-MCM films were studied at room temperature and the results of tensile strength were listed in Table 1. The average tensile strength of the semiaromatic polyamides was 75.6 and 77.6 MPa, respectively. The average tensile strength of films which prepared from PA6T-Dupont was 50.9 MPa. The results indicate that the resultant semiaromatic polyamides have better tensile strength than that of commercial product PA6T.Rheological properties of the resultant semiaromatic polyamides
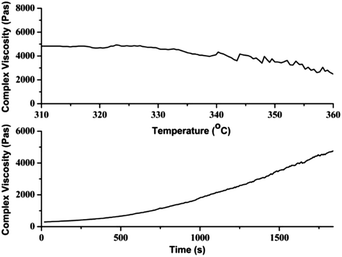
We studied the effects of chemical structure on the rheological properties of these polyamides by parallel plate rheometer. The effects of temperature on complex viscosities of Ph-DCM and Ph-MCM were studied. As shown in Fig. 10, the complex viscosities had a closed relationship versus temperature: with the increase of temperature (from 290 °C to 350 °C), the complex viscosities decreased dramatically. With the increase of temperature, the complex viscosities of Ph-MCM decreased significantly quickly than the complex viscosities of Ph-DCM. This result suggested that the introduction of side methyl groups made the resin be more sensible to temperature. Also we can observe that when the methyl groups were introduced into the polyamide backbone, the complex viscosities of polyamides increased obviously. This reason may be that the introduction of methyl groups increased the interaction between the molecular chains and hindered the movement of polyamide chains. Then the glass transition temperature increased with the incorporation of side methyl group. So the zero-shear complex viscosity of Ph-MCM is much higher than the zero-shear complex viscosity of Ph-MCM. In addition, compared with the commercial product PA6T-Dupont, the complex viscosities of the resultant semiaromatic polyamides were smaller at the temperature of 320–340 °C. These results indicate the synthesized polyamides have good processability. The effects of sheared frequency (0.01–100 Hz) at 300 °C on the complex viscosities of synthesizing polyamides were also been studied. The complex viscosities of these polyamides at different shear rates were in the range of 300–13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 Pa s and 800–30
500 Pa s and 800–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 Pa s, respectively. As shown in Fig. 11, the complex viscosities of semiaromatic polyamides decreased with increasing shear rates, suggesting a shear-thinning behavior of the samples. In addition, the semiaromatic polyamides had excellent stability under high shear rates. The effects of testing time (300 °C) on the complex viscosities of these semiaromatic polyamides were also been studied (Fig. 12). The viscosities values of the resultant semiaromatic polyamides changed slightly during the whole testing period, the complex viscosities of Ph-MCM decreased slowly, however an increase in the complex viscosities of Ph-DCM could be observed. When the testing time was more than 500 s the complex viscosities remained stable, whereas the complex viscosities of PA6T-Dupont increased during the testing time (Fig. 13). These evidences suggest the resultant semiaromatic polyamides have better melt stability than PA6T-Dupont. The results of rheological testing indicate that these semiaromatic polyamides are suitable for melting processing.
200 Pa s, respectively. As shown in Fig. 11, the complex viscosities of semiaromatic polyamides decreased with increasing shear rates, suggesting a shear-thinning behavior of the samples. In addition, the semiaromatic polyamides had excellent stability under high shear rates. The effects of testing time (300 °C) on the complex viscosities of these semiaromatic polyamides were also been studied (Fig. 12). The viscosities values of the resultant semiaromatic polyamides changed slightly during the whole testing period, the complex viscosities of Ph-MCM decreased slowly, however an increase in the complex viscosities of Ph-DCM could be observed. When the testing time was more than 500 s the complex viscosities remained stable, whereas the complex viscosities of PA6T-Dupont increased during the testing time (Fig. 13). These evidences suggest the resultant semiaromatic polyamides have better melt stability than PA6T-Dupont. The results of rheological testing indicate that these semiaromatic polyamides are suitable for melting processing.
 | ||
| Fig. 10 The curves of complex viscosity versus temperature for the resultant semiaromatic polyamides. | ||
 | ||
| Fig. 11 The curves of complex viscosity versus sheared frequency for the resultant semiaromatic polyamides. | ||
 | ||
| Fig. 12 The curves of complex viscosity versus times (300 °C) for the resultant semiaromatic polyamides. | ||
Conclusions
Difluoro-monomers containing cyclohexane unit were synthesized with a facile interface reaction at room temperature. It was then used to react with bisphenol BHPPE with bulky group through nucleophilic polycondensation to synthesize two kinds of semiaromatic polyamides. We found that the pendent methyl group was beneficial for the polymer glass transition temperature, tensile strength and storage modulus. While the melt flowability of the prepared resins decreased with the incorporation of methyl group. Additionally, it was found that with the introduction of dicyclohexane units, the thermal properties (the glass transition temperature was about 80–100 °C higher than that of commercial product PA6T), mechanical and melt flowability was better than that of semiaromatic polyamides which containing linear aliphatic chain such as the commercial product PA6T. Therefore, these semiaromatic polyamides with well processability and high glass transition temperature could be potentially used as heat resistant thermoplastic materials especially in the field of thin-walled products.Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (Grant No. 21304060) and Outstanding Young Scholars Fund of Sichuan University (Grant No. 2015SCU04A25) for the financial support.References
- J. K. Fink, High performance polymers, William Andrew, 2014 Search PubMed.
- J. M. García, F. C. García, F. Serna, L. Jose and D. L. Pena, High-performance aromatic polyamides, Prog. Polym. Sci., 2010, 35, 623–686 CrossRef.
- D. J. Liaw, W. H. Chen, C. K. Hu, K. R. Lee and J. Y. Lai, High optical transparency, low dielectric constant and light color of novel organosoluble polyamides with bulky alicyclic pendent group, Polymer, 2007, 48, 6571–6580 CrossRef CAS.
- W. Z. Wang and Y. H. Zhang, Environment-friendly synthesis of long chain semiaromatic polyamides, eXPRESS Polym. Lett., 2009, 3, 470–476 CrossRef CAS.
- W. Z. Wang, X. W. Wang, R. X. Li, B. Y. Liu, E. G. Wang and Y. H. Zhang, Environment-friendly synthesis of long chain semiaromatic polyamides with high heat resistance, J. Appl. Polym. Sci., 2009, 114, 2036–2042 CrossRef CAS.
- C. H. Zhang, X. B. Huang, X. B. Zeng, M. Cao, T. M. Cai, S. J. Jiang and Q. F. Yi, Fluidity improvement of semiaromatic polyamides: Modification with oligomers, J. Appl. Polym. Sci., 2014, 13 Search PubMed.
- D. J. Liaw, F. C. Chang, M. Leung, M. Y. Chou and K. Muellen, High thermal stability and rigid rod of novel organosoluble polyimides and polyamides based on bulky and noncoplanar naphthalene–biphenyldiamine, Macromolecules, 2005, 38, 4024–4029 CrossRef CAS.
- D. J. Liaw, B. Y. Liaw, C. M. Yang, P. N. Hsu and C. Y. Hwang, Synthesis and characterization of new organosoluble polyamides derived from bis[4-(4-carboxyphenoxy)phenyl]diphenylmethane, J. Polym. Sci., Part A: Polym. Chem., 2001, 39, 1156–1161 CrossRef CAS.
- D. J. Liaw, C. C. Huang and W. H. Chen, Color lightness and highly organosoluble fluorinated polyamides, polyimides and poly(amide–imide)s based on noncoplanar 2,2′-dimethyl-4,4′-biphenylene units, Polymer, 2006, 47, 2337–2348 CrossRef CAS.
- S. H. Hsiao, S. C. Peng, Y. R. Kung, C. M. Leu and T. M. Lee, Synthesis and electro-optical properties of aromatic polyamides and polyimides bearing pendent 3,6-dimethoxycarbazole units, Eur. Polym. J., 2015, 73, 50–64 CrossRef CAS.
- G. S. Liou, S. H. Hsiao, N. K. Huang and Y. L. Yang, Synthesis, photophysical, and electrochromic characterization of wholly aromatic polyamide blue-light-emitting materials, Macromolecules, 2006, 39, 5337–5346 CrossRef CAS.
- S. H. Hsiao, H. M. Wang, J. W. Lin, W. J. Guo, Y. R. Kung, C. M. Leu and T. M. Lee, Synthesis and electrochromic properties of polyamides having pendent carbazole groups, Mater. Chem. Phys., 2013, 141, 665–673 CrossRef CAS.
- V. L. Sergei, D. W. Edward and L. Menachem, Thermal Decomposition of Aliphatic Nylons, Polym. Int., 1999, 48, 532–557 CrossRef.
- G. Zhang, D. T. Bai, D. S. Li, S. R. Long, X. J. Wang and J. Yang, Synthesis and properties of polyamides derived from 4,6-bis(4-chloroformylphenylthio)pyrimidine and 3,6-bis(4-chloroformylphenylthio)pyridazine, Polym. Int., 2013, 62, 1358–1367 CrossRef CAS.
- O. Persyn, V. Miri, J. M. Lefebvre, V. Ferreiro, T. Brink and A. Stroeks, Mechanical behavior of films of miscible polyamide 6/polyamide 6I–6T blends, J. Polym. Sci., Part B: Polym. Phys., 2006, 44, 1690–1701 CrossRef CAS.
- S. H. Hsiao, C. W. Chen and G. S. Liou, Novel aromatic polyamides bearing pendent diphenylamino or carbazolyl groups, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 3302–3313 CrossRef CAS.
- A. Ballistreri, D. Garozzo, M. Giuffrida, P. Maravigna and G. Montaudo, Thermal decomposition processes in aliphatic–aromatic polyamides investigated by mass spectrometry, Macromolecules, 1986, 19, 2693–2699 CrossRef CAS.
- A. J. Uddin, Y. Ohkoshi, Y. Gotoh, M. Nagura and T. Hara, Influence of moisture on the viscoelastic relaxations in long aliphatic chain contained semiaromatic polyamide, (PA9-T) fiber, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 2878–2891 CrossRef CAS.
- M. Y. Liu, K. F. Li, S. H. Yang, P. Fu, Y. D. Wang and Q. X. Zhao, Synthesis and thermal decomposition of poly(dodecamethylene terephthalamide), J. Appl. Polym. Sci., 2011, 122, 3369–3376 CrossRef CAS.
- G. Zhang, G. S. Huang, D. S. Li, X. J. Wang, S. R. Long and J. Yang, Facile synthesis of processable semiaromatic polyamides containing thioether Units, Ind. Eng. Chem. Res., 2011, 50, 7056–7064 CrossRef CAS.
- P. H. Li, C. Y. Wang, G. Li and J. M. Jiang, Highly organosoluble and transparent polyamides containing cyclohexane and trifluoromethyl moieties: synthesis and characterization, eXPRESS Polym. Lett., 2009, 3, 703–712 CrossRef CAS.
- S. H. Yang, P. Fu and M. Y. Liu, et al., Synthesis, characterization of polytridecamethylene 2,6-naphthalamide as semiaromatic polyamide containing naphthalene-ring, eXPRESS Polym. Lett., 2010, 4, 442–449 CrossRef CAS.
- S. H. Yang, P. Fu, M. Y. Liu, Y. D. Wang, Y. C. Zhang and Q. X. Zhao, Synthesis of polyundecamethylene 2,6-naphthalamide as semiaromatic polyamide-containing naphthalene ring, J. Appl. Polym. Sci., 2010, 118, 1094–1099 CAS.
- S. H. Hsiao and Y. H. Chang, New soluble aromatic polyamides containing ether linkages and laterally attached p-terphenyls, Eur. Polym. J., 2004, 40, 1749–1757 CrossRef CAS.
- J. M. Garcia, J. G. Campa, G. Schwarz and J. Abajo, Thermotropic aromatic poly(amide-ether)s, Macromol. Chem. Phys., 2001, 202, 1298–1305 CrossRef CAS.
- G. Zhang, Y. X. Zhou, Y. Kong, Z. M. Li, S. R. Long and J. Yang, Semiaromatic polyamides containing ether and different numbers of methylene (2–10) units: synthesis and properties, RSC Adv., 2014, 4, 63006–63015 RSC.
- G. Zhang, Y. X. Zhou, Y. Li, X. J. Wang, S. R. Long and J. Yang, Investigation of the synthesis and properties of isophorone and ether units based semi-aromatic polyamides, RSC Adv., 2015, 5, 49958–49967 RSC.
- G. Zhang, G. M. Yan, H. H. Ren, Y. Li, X. J. Wang and J. Yang, Effects of a trans- or cis-cyclohexane unit on the thermal and rheological properties of semi-aromatic polyamides, Polym. Chem., 2016, 7, 44–53 RSC.
- G. Zhang, X. J. Xing and D. S. Li, et al., Effects of thioether content on the solubility and thermal properties of aromatic polyesters, Ind. Eng. Chem. Res., 2013, 52, 16577–16584 CrossRef CAS.
- G. Zhang, Y. Zhang, D. S. Li, G. S. Huang, X. J. Wang and J. Yang, Polyamide derived from 4,4′-thiobis(methylene)dibenzoyl chloride and aliphatic diamine: interfacial synthesis and characterization, Polym. Bull., 2013, 70, 789–807 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |