Sigma-spacer regulated thiophenyl triazine conjugates: synthesis and crystal, electronic and luminescent properties†
Zhuangli Zhu,
Ersha Shao,
Shan Xu,
Huaming Sun,
Guofang Zhang,
Zunyuan Xie,
Weiqiang Zhang* and
Ziwei Gao*
Key Laboratory of Applied Surface and Colloid Chemistry, Ministry of Education, School of Chemistry & Chemical Engineering, Shaanxi Normal University, 199 South Chang'an Road, Xi'an, China. E-mail: zwq@snnu.edu.cn; zwgao@snnu.edu.cn
First published on 8th August 2016
Abstract
A water-accelerated Pd catalyzed Suzuki–Miyaura cross-coupling reaction was developed for the synthesis of thiophenyl triazine conjugates (TTCs). The compounds (2a–2e and 3a–3e) with five σ-spacers were obtained and fully characterized. The X-ray analysis of 2a, 2d, and 3d found that the length of the intermolecular H bond was 3.465(3) Å and the π–π interactions ranged from 3.6538(2)–3.9519(1) Å. Five σ-spacers of TTCs had an effect on their emissions from blue (363 nm) to yellow (530 nm) whilst 2- and 3-thiophenyl chromophores finely tune the emission from 530 to 504 nm. The DFT computation showed the TTCs had low HOMO (−6.8 eV) and LUMO (−2.5 eV) energy and high triplet energy (ET, 2.4 eV). The energy level and gap of TTCs were regularly modulated by the σ-spacer.
Introduction
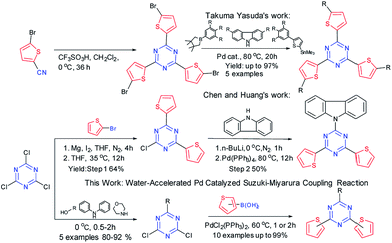
1,3,5-Triazine is a fascinating π-electron-deficient aromatic building block, which has been widely employed as a C2V electron acceptor (A) for designing D–A luminescent organic molecular devices. Introducing a 1,3,5-triazine unit into the backbone of the conjugated molecules not only improved the abilities of electron-injection and electron-transportation of the molecules,1 but also increased the heat resistance of the molecules.2 Accordingly, to explore new triazine conjugates with a modulated electron donating spacer is essential for developing photophysical organic devices. Thiophenes as electron donor derivatives have drawn great interest due to their low electron affinity (EA = −1.17 eV) and high ionization potential (IP = 8.87 eV).3 The thiophenyl derivatives were widely used in liquid-crystalline materials,4–6 OLEDs,7 photosensitizers8 and more9–11 in the past few decades. Strohriegl et al. reported a series of asymmetric triazine derivatives with a flexible link-age (C–O bond) between the triazine core and the donor moiety for blue PHOLEDs with high glass transition temperature (up to 170 °C) and triplet energy (up to 2.96 eV).12 In addition, asymmetric triazines with donors connected directly to the triazine core (acceptor) can benefit both from the D–A architectures and from the fine turning of the energy levels of the materials.10 What’s more, the introduction of σ-spacer not only have a benefit from the asymmetric aspect, but also can have a fine modulation of singlet and triplet excited states.13Palladium catalyzed C–C and C–N cross-coupling reactions played a vital role for the rapid construction of TTCs (Scheme 1). From the pre-prepared 5-bromothio-phene-2-carbonitrile, Takuma Yasuda et al.3 constructed the propeller-shaped TTCs bearing different π-conjugated groups via Pd catalyzed Suzuki–Miyaura cross-coupling reaction,14 Stille cross-coupling reaction15,16 and Buchwald–Hartwig amination17,18 respectively. Chen and Huang et al.7 prepared a kind of conjugated asymmetric donor-substituted TTC via the Pd catalyzed nucleophilic substitution of the cyanuric chloride with magnesium thiophenyl bromide and lithium carbazole. At present, few synthetic efforts were devoted to the new-type moderate synthesis of TTCs with sophisticated structures. Herein, we reported water-accelerated Pd catalyzed Suzuki–Miyaura cross-coupling reactions for the preparation of TTCs bearing σ-spacers such as methoxyl (CH3O), phenoxyl (PhO), 1-naphthoxyl (1-NaphthO), diphenylaminyl (N-Ph2) and morpholinyl (N-Morph), by which 10 examples of 2-TTCs (2a–2e) and 3-TTCs (3a–3e) were obtained up to 99% yield. The crystal structures and luminescent properties of thiophen-2-yl triazine conjugates (2-TTCs) and thiophen-3-yl triazine conjugates (3-TTCs) were investigated, respectively. The experiment results indicated that five σ-spacers of CH3O, PhO, N-Morph, 1-NaphthO, N-Ph2 finely tuned the HOMO, LUMO, Eg and ET of TTCs, demonstrating a regular fluctuation. It was also found that the 2- and 3-position of thiophenyl group had a significant impact on luminescence property of TTCs.
Results and discussion
Synthetic strategy and characterization
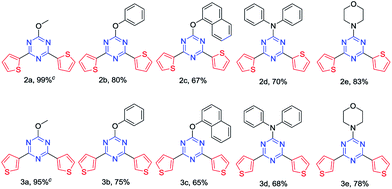
Ten σ-spacer regulated luminescent TTCs were synthesized via a sequent two-step strategy (Scheme 2). In the first step, the five σ-spacers of CH3O, PhO, 1-NaphthO, N-Ph2 and N-Morph were introduced into triazine core by a mild nucleophilic substitution reactions with high yield (80–92%). In the second step, the water-accelerated Pd catalyzed Suzuki reaction were employed for the synthesis of 2- and 3-TTCs up to 99% yield. The reaction condition of the water-accelerated Pd catalyzed Suzuki–Miyaura cross-coupling reaction were optimized in Table 1.| Entry | Catalyst | Solvent | Co- | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a 0.5 mmol, thiophen-2-yl boronic acid 1 mmol, Na2CO3 3 equiv., catalyst (2% mmol Pd), 60 °C, 1 h, under air.b Yield was determined by 1H NMR.c K2CO3 as base. | ||||
| 1 | Pd(PPh3)4 | CH3CN (5 mL) | — | 20 |
| 2 | Pd(PPh3)4 | CH3CN (3 mL) | H2O (2 mL) | 96 |
| 3 | Pd(PPh3)4 | THF (3 mL) | H2O (2 mL) | 95 |
| 4 | Pd(PPh3)4 | Toluene (3 mL) | H2O (2 mL) | 10 |
| 5c | Pd(PPh3)4 | CH3CN (3 mL) | H2O (2 mL) | 85 |
| 6 | Pd(PPh3)4 | CH3CN (2 mL) | H2O (3 mL) | 5 |
| 7 | Pd(PPh3)4 | CH3CN (1 mL) | H2O (4 mL) | — |
| 8 | Pd(PPh3)4 | — | H2O (5 mL) | — |
| 9 | Pd2(dba)3 | CH3CN (3 mL) | H2O (2 mL) | — |
| 10 | PdCl2(PPh3)2 | CH3CN (3 mL) | H2O (2 mL) | 99 |
| 11 | PdCl2(PPh3)2 | CH3CN (1.5 mL) | H2O (1 mL) | 97 |
In the preliminary experiment, the Suzuki–Miyaura coupling reaction of thiophen-2-yl boronic acid and 1a was in very low reactivity and efficiency under standard reaction condition (Table 1, entry 1, 20%). Fortunately, we found adding small amount of water significantly improved efficiency of the cross-coupling reaction (Table 1, entry 2, 96%). In the participation of co-solvent water, the CH3CN performed best as the solvent (Table 1, entries 2–4). And the Na2CO3 was found to be optimum base in the coupling reaction (Table 1, entries 2 and 5). It was noted that the most efficient and accelerating effect of the water proportion was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (V
3 (V![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VOrg) (Table 1, entries 6–8). The P-ligand-free Pd2(dba)3 failed in the reaction (Table 1, entry 9) while the PdCl2(PPh3)2 performed better in the reaction even in the half volume of solvent (Table 1, entries 10 and 11).
VOrg) (Table 1, entries 6–8). The P-ligand-free Pd2(dba)3 failed in the reaction (Table 1, entry 9) while the PdCl2(PPh3)2 performed better in the reaction even in the half volume of solvent (Table 1, entries 10 and 11).
To our knowledge, the water-accelerated effect was mainly attributed to the water-promoted activation of palladium catalysis.19 It was well known that the formation of an active Pd(0) complex is most chiefly and commonly accomplished in the Pd catalyzed Suzuki catalytic cycle. The activation could be monitored visually by color change as shown in Fig. 1. In the participation of water, the PdIICl2(PPh3)2 was easily and rapidly converted to the Pd(0) catalysis in five minutes. However, in the absence of water the reduction did not proceed. This showed that water played a direct role in the formation of the active Pd(0) catalyst to accelerate the Suzuki cross-coupling reaction.
 | ||
| Fig. 1 Visualization of water-promoted activation of palladium catalysis (PdCl2(PPh3)2 0.02 mmol, Na2CO3 1.5 mmol, H2O 2 mL, CH3CN 3 mL, 60 °C). | ||
With the optimized condition in hand, we finally install the thiophene group to the 1,3,5-triazine backbone bearing the different σ spacers (Table 2). With the decrease of the electron donating ability of σ spacers, the yields of 2-TTCs decreased (2a > 2b > 2c). When the steric hindrances of them decreased, the yields of 2-TTCs increased (3d < 3e). Meanwhile, water-accelerated catalytic system was also employed in the synthesis of 3-TTCs. The yields of 3-TTCs were slightly lower than those of 2-TTCs and were seriously influenced by the electron donating ability and steric hindrances of σ spacers in the same way as well. These compounds were readily soluble in common organic solvents by virtue of the attached flexible σ-spacers. All the compounds were fully characterized by 1H and 13C NMR spectroscopy, HRMS (ESI) and DSC (ESI†). We also employed the method to synthesize the propeller-shaped TTCs (Scheme 3).
Crystal structure
The crystal structure of 2a (coplanar CH3O σ-spacer/2-TTC), 2d (non-coplanar N-Ph2 σ-spacer/2-TTC) and 3d (non-coplanar N-Ph2 σ-spacer/3-TTC) were obtained by X-ray single crystal analysis (Fig. 2). Suitable single crystals for X-ray diffraction were obtained by slow evaporation of CDCl3 solution. All the three compounds crystallized as colourless cubes in the monoclinic system, in space group C2/c (2a) and P21/c (2d and 3d). In the molecular structures of them, one donor thiophene (Th1) and acceptor triazinal (T) units were almost coplanar (1.7–6.7) whilst the other one of those (Th2⋯T) were non-coplanar (5.4–24.1). These crystallographic observation suggest that the degree of conjugation between Th1⋯T and Th2⋯T were different. With the decrease of the dihedral angle for Th⋯T (Th2⋯T > Th1⋯T), the degree of conjugation between them was increased and the conjugated group showed shorter bond length (L1 > L2) (Fig. 2e).It is noteworthy that each two molecules of 2a were connected by weak H-bond (Fig. 2a). There are intermolecular π–π interactions between two neighboring molecules in every three layers (Fig. 2b). When the non-plane diphenylamino group was introduced, the H-bond and the π–π interactions were destroyed to form the structure of 2d (Fig. 2c). These crystallographic observation suggest that the conjugated degree of it was decreased (the dihedral angle for Th⋯T: 2a < 2d). The π–π interactions in every three layers were destroyed and became into an intermolecular force in every two layers (Fig. S2†). In the following, the thiophen-3-yl group took the place of the thiophen-2-yl group. Its conjugated degree was decreased further more (the dihedral angle for Th⋯T: 2a < 2d < 3d) which made the unit cell more loose (Table S1†) to form the structure of 3d (Fig. 2d).
Electronic structure
To modulating singlet and triplet excited states through σ spacers, theoretical calculation was executed in fact. From the HOMOs and LUMOs of TTCs shown in Fig. 3, the LUMOs have high distribution density on thiophen-yl triazine core, whereas the HOMOs are tuned by the different σ-spacers. This D–A architecture of TTCs can lead to separated electron density distribution between the HOMOs and LUMOs. This separation provides the material with a charge-transfer (C-T) state, efficient hole- and electron-transporting properties and the prevention of reverse energy-transfer, which enables the related TTCs to have excellent potential as host materials.20,21 Since the LUMOs of TTCs are dominated by thiophen-yl triazine core, their energy levels of LUMOs are similar with little fluctuation, whereas for HOMOs, a larger fluctuation was observed. This independent modification of HOMOs and LUMOs of TTCs with various σ-spacers can greatly facilitate the molecular design of the desired materials. Due to the different σ-spacers, the energy of HOMO increased in the following order: CH3O-TTCs ≈ PhO-TTCs < N-Morpth-TTCs < 1-NaphthO-TTCs ≈ N-Ph2-TTCs while the Eg (LUMO–HOMO) and ET performed in the opposite way. Comparing the different position isomerism, the HOMOs of the 2-TTCs are higher while the LUMOs and Eg of the 3-TTCs are higher. The low LUMOs of all TTCs suggest properties such as good electron ejection and transportation of the material, which is very crucial for organic optoelectronic devices.Optical properties
The normalized UV/vis absorption in CH2Cl2 solution (3 × 10−5 M) of TTCs were shown in Fig. 4 and summarized in Table 3 where the thermal properties of TTCs were also summarized. | ||
| Fig. 4 Normalized UV/vis absorption in CH2Cl2 solution (top) and photoluminescent spectra in CH2Cl2 solution (right lower) and solid powder (left lower). | ||
| Product | UVλmax [nm] | PLλmax [nm] | Tm | Td | ||
|---|---|---|---|---|---|---|
| CH2Cl2 | CH2Cl2 | Powder | [°C] | [°C] | ||
| 2a | 274 | 315 | 363 | 451 | 102.2 | 222.8 |
| 2b | 275 | 314 | 367 | 398 | 103.1 | 227.9 |
| 2c | 277 | 313 | 503 | 406 | 161.5 | 275.8 |
| 2d | 268 | 305 | 530 | 476 | 215.2 | 255.2 |
| 2e | 266 | 300 | 437 | 410 | 166.8 | 240.1 |
| 3a | 271 | 363, 443 | 426 | 95.4 | 244.8 | |
| 3b | 273 | 381 | 427 | 120.7 | 222.1 | |
| 3c | 278 | 481 | 421 | 150.2 | 275.1 | |
| 3d | 272 | 504 | 451 | 212.2 | 297.7 | |
| 3e | 265 | 407 | 400 | 183.8 | 224.5 | |
In the absorbance spectra, the absorption bands around 265 nm were observed for all the TTCs which can be associated with n–π* transition, while the bands around 300 nm just observed for the 2-TTCs which should be assigned to π–π* transition of the conjugated backbone.22,23 The photoluminescent (PL) emission spectra in CH2Cl2 solution and solid powder of TTCs were also shown in Fig. 4 and summarized in Table 3. The TTCs exhibit emissions from blue to yellow in the CH2Cl2 solution according to the different σ-spacers of the 1,3,5-triazine. With the increase of the electron-donating ability of the σ-spacers (CH3O < PhO < N-Morph < 1-NaphthO < N-Ph2), a blue shift was observed (363–530 nm and 363–504 nm). In the solid powder, the emissions of TTCs are blue shifted up to 97 nm to form stable blue emissions in comparison with that in the solution state, probably due to the better stabilizing effects of the solvent on the base state (S0) than the excited state (S1) of the D–A structure of TTCs.24 The abnormal red shifted emissions were probably caused by the hydrogen bonds or π–π stacking interactions which can enhance the conjugate such as the molecular structure of 2a (Fig. 2).
Experimental
General methods and materials
All manipulations were performed in an atmosphere of air. Tetrahydrofuran and acetonitrile were dried and purified by routine procedures while other solvents for reactions were used without distilled. All reagents were purchased from the commercial approaches and used without further purification unless specified otherwise. Flash chromatography was performed using 200–300 mesh silica gel with the indicated solvent system according to standard techniques and analytical thin layer chromatography was carried out using 250 μm commercial silica gel plates. Visualization of the developed chromatogram was performed by UV/vis absorbance. 1H and 13C NMR spectra were recorded on a Bruker EQUINX55 (400 MHz for 1H; 101 MHz for 13C) spectrometer in CDCl3. For 1H NMR, tetramethylsilane (TMS) served as internal standard (δ = 0) and 1H NMR chemical shifts are reported in ppm downfield of tetramethylsilane and referenced to residual solvent peak (CDCl3 at 7.26 ppm) unless otherwise noted. The data are reported as follows: chemical shift, integration, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet and m = multiplet), and coupling constant in Hz. For 13C NMR, CDCl3 was used as internal standard (δ = 77.16) and spectra were obtained with complete proton decoupling. HRMS (ESI) analysis was performed and (HRMS) data were reported with sodium mass/charge (m/z) ratios as values in atomic mass units. Differential scanning calorimetry (DSC) was run on a Pyris 1 DSC (Perkin Elmer Co.) thermal analyst system under a heating rate of 20 °C min−1 and an argon flow rate of 50 cm3 min−1. Ultraviolet/visible (UV/vis) spectra were recorded on an UV-3600 SHIMADZU UV/vis-NIR spectrophotometer and fluorescence spectra were obtained using a RF-5301PC spectrofluorophotometer with a xenon lamp as a light source. The concentrations of the compound solutions (in CH2Cl2) were adjusted to 3 × 10−5 M.Computational methods
Theoretical calculations were performed on Gaussian 09 program with the Becke's three-parameter exchange functional along with the Lee Yang Parr's correlation functional (B3LYP) using 6-31G (d) basis sets. The ground and lowest triplet-state geometries were fully optimized and these optimized stationary points were further characterized by harmonic vibration frequency analysis to ensure that real local minima had been found. The properties of the designed model compounds, such as highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy, energy gap (Eg), and triplet-state energy (ET) were derived from the computed results.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as eluent to afford 1e as a white solid (2.89 g, 12.3 mmol, 82%). The NMR data can be found in the literature.
3) as eluent to afford 1e as a white solid (2.89 g, 12.3 mmol, 82%). The NMR data can be found in the literature.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent to afford 2a as a white solid (0.1363 g, 0.495 mmol, 99%). 1H NMR (400 MHz, CDCl3) δ (ppm): 8.24 (d, J = 3.7 Hz, 2H), 7.62 (d, J = 6.0 Hz, 2H), 7.20 (t, 2H), 4.16 (s, 3H); 13C NMR (101 MHz, CDCl3) δ (ppm): 170.11, 168.40, 140.01, 131.45, 130.82, 127.36, 53.99. HRMS (ESI) m/z: [M + Na]+ calcd for C12H9N3NaOS2: 298.0079. Found: 298.0090.
1) as eluent to afford 2a as a white solid (0.1363 g, 0.495 mmol, 99%). 1H NMR (400 MHz, CDCl3) δ (ppm): 8.24 (d, J = 3.7 Hz, 2H), 7.62 (d, J = 6.0 Hz, 2H), 7.20 (t, 2H), 4.16 (s, 3H); 13C NMR (101 MHz, CDCl3) δ (ppm): 170.11, 168.40, 140.01, 131.45, 130.82, 127.36, 53.99. HRMS (ESI) m/z: [M + Na]+ calcd for C12H9N3NaOS2: 298.0079. Found: 298.0090.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent to afford 4a as a white solid (0.1005 g, 0.307 mmol, 61%). 1H NMR (400 MHz, CDCl3) δ (ppm): 8.28 (d, J = 3.7 Hz, 2H), 7.63 (d, J = 4.9 Hz, 2H), 7.22 (t, J = 8.7 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ (ppm): 167.87, 141.58, 132.47, 131.84, 128.57.
1) as eluent to afford 4a as a white solid (0.1005 g, 0.307 mmol, 61%). 1H NMR (400 MHz, CDCl3) δ (ppm): 8.28 (d, J = 3.7 Hz, 2H), 7.63 (d, J = 4.9 Hz, 2H), 7.22 (t, J = 8.7 Hz, 1H); 13C NMR (101 MHz, CDCl3) δ (ppm): 167.87, 141.58, 132.47, 131.84, 128.57.Conclusions
In summary, ten examples of σ-spacers regulated luminescent TTCs have been synthesized via a water-accelerated Suzuki cross-coupling method which provided it appeared to be a facile synthesis of more complex D–A molecules and bearing multiple triazinal groups. This will greatly promote the research of the triazine-based materials. The prepared TTCs exhibit good solubility and high thermostability for device fabrications. The D–A compounds with separated electron density distribution show low LUMO as revealed by the theoretical investigations. The DFT computations and optical property demonstrated that the modulation of σ-spacers is a pretty rational strategy for building promising optoelectronic materials and the 3-TTCs can be applied to new optoelectronic materials. Given these promising properties of these compounds, development of new photophysical materials with structurally complexity as well as the excellent photoproperty is underway.Acknowledgements
This work was supported by the 111 Project (B14041), the grant from National Natural Science Foundation of China (21271124, 21272186, 21371112, 21446014), the Fundamental Funds Research for the Central Universities (No. GK201501005, GK201503029, 2016CSY002), the grant from Fundamental Doctoral Fund of Ministry of Education of China (20120202120005), the Program for Changjiang Scholars and Innovative Research Team in University (IRT_14R33).References
- H. Zhong, H. Lai and Q. Fang, J. Phys. Chem. C, 2011, 115, 2423–2427 CAS.
- T. Fang and D. A. Shimp, Prog. Polym. Sci., 1995, 20, 61–118 CrossRef CAS.
- T. Yasuda, T. Shimizu, F. Liu, G. Ungar and T. Kato, J. Am. Chem. Soc., 2011, 133, 13437–13444 CrossRef CAS PubMed.
- T. Yasuda, H. Ooi, J. Morita, Y. Akama, K. Minoura, M. Funahashi, T. Shimomura and T. Kato, Adv. Funct. Mater., 2009, 19, 411–419 CrossRef CAS.
- K. Minoura, Y. Akama, J. Morita, T. Yasuda, T. Kato and T. Shimomura, J. Appl. Phys., 2009, 105, 113513–113516 CrossRef.
- T. Yasuda, K. Kishimoto and T. Kato, Chem. Commun., 2006, 3399–3401 RSC.
- Z. F. An, R. F. Chen, J. Yin, G. H. Xie, H. F. Shi, T. Tsuboi and W. Huang, Chem.–Eur. J., 2011, 17, 10871–10878 CrossRef CAS PubMed.
- J. Liu, K. Wang, X. Zhang, C. Li and X. You, Tetrahedron, 2013, 69, 190–200 CrossRef CAS.
- V. Lukeš, P. Rapta, K. R. Idzik, R. Beckert and L. Dunsch, J. Phys. Chem. B, 2011, 115, 3344–3353 CrossRef PubMed.
- A. J. Heeger, Chem. Soc. Rev., 2010, 39, 2354–2371 RSC.
- P. Leriche and F. Piron, Tetrahedron Lett., 2009, 50, 5673–5676 CrossRef CAS.
- M. M. Rothmann, S. Haneder, C. E. Da, C. Lennartz, C. Schildknecht and P. Strohriegl, Chem. Mater., 2010, 22, 2403–2410 CrossRef CAS.
- Z. An, Q. Wu, J. Xiao, R. Chen, J. Yin, H. Shi, Y. Tao, Y. Wang, Z. Wang, H. Li, H. Zhang, X. Zhou, Y. Zhao and W. Huang, RSC Adv., 2013, 3, 13782–13788 RSC.
- J. J. Shie and J. M. Fang, J. Org. Chem., 2003, 68, 1158–1160 CrossRef CAS PubMed.
- N. Miyaura and A. Suzuki, Chem. Rev., 1995, 95, 2457–2483 CrossRef CAS.
- J. K. Stille, Angew. Chem., Int. Ed. Engl., 1986, 25, 508–524 CrossRef.
- M. Kosugi, K. Sasazawa, Y. Shimizu and T. Migita, Chem. Lett., 1977, 301–302 CrossRef CAS.
- J. F. Hartwig, Angew. Chem., Int. Ed., 1998, 37, 2046–2067 CrossRef CAS.
- B. P. Fros, P. Krattiger, E. Strieter and S. L. Buchwald, Org. Lett., 2008, 10(16), 3505–3508 CrossRef PubMed.
- J. L. Brédas, D. Beljonne, V. Coropceanu and J. Cornil, Chem. Rev., 2004, 104, 4971–5003 CrossRef PubMed.
- G. Qian, B. Dai, M. Luo, D. B. Yu, J. Zhan, Z. Q. Zhang, D. G. Ma and Z. Y. Wang, Chem. Mater., 2008, 20, 6208–6216 CrossRef CAS.
- Z. Q. Gao, M. Luo, X. H. Sun, H. L. Tam, M. S. Wong, B. X. Mi, P. F. Xia, K. W. Cheah and C. H. Chen, Adv. Mater., 2009, 21, 688–692 CrossRef CAS.
- S. L. Zhang, R. F. Chen, J. Yin, F. Liu, H. J. Jiang, N. E. Shi, Z. F. An, C. Ma, B. Liu and W. Huang, Org. Lett., 2010, 12, 3438–3441 CrossRef CAS PubMed.
- J. Yin, R. F. Chen, S. L. Zhang, Q. D. Ling and W. Huang, J. Phys. Chem. A, 2010, 114, 3655–3667 CrossRef CAS PubMed.
- T. Tanaka, M. Noguchi, K. Watanabe, T. Misawa, M. Ishihara, A. Kobayashi and S. Shoda, Org. Biomol. Chem., 2010, 8, 5126–5132 CAS.
- R. J. Goetz, A. Robertazzi, I. Mutikainen, U. Turpeinen, P. Gamez and J. Reedijk, Chem. Commun., 2008, 3384–3386 RSC.
- X. Su, Y. Yi, J. Tao, H. Qi and D. Li, Polym. Degrad. Stab., 2014, 105, 12–20 CrossRef CAS.
- W. Chen, J. F. Chu and Y. Q. Wang, J. Mol. Struct., 2014, 1068, 237–244 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1430747, 1430748 and 1435751. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra13795d |
| This journal is © The Royal Society of Chemistry 2016 |