Influence of high-mannose glycan whose glucose moiety is substituted with 5-thioglucose on calnexin/calreticulin cycle†
Masafumi Sakono*ab,
Akira Sekoa,
Yoichi Takedaac,
Masakazu Hachisuad,
Akihiko Koizumiae,
Kohki Fujikawaaf,
Hideharu Setog and
Yukishige Ito*ag
aJapan Science and Technology Agency (JST), ERATO Ito Glycotrilogy Project, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan. E-mail: msakono@eng.u-toyama.ac.jp; yukito@riken.jp
bDepartment of Applied Chemistry, University of Toyama, 3190 Gofuku, Toyama, Toyama 930-855, Japan
cDepartment of Biotechnology, Ritsumeikan University, 1-1-1 Noji-higashi, Kusatsu, Shiga 525-8577, Japan
dDepartment of Biological Science and Technology, Tokyo University of Science, 6-3-1 Niijuku, Katsushika-ku, Tokyo 125-8585, Japan
eFaculty of Pharmaceutical Sciences, Josai University, 1-1 Keyakidai, Sakado, Saitama 350-0295, Japan
fSUNTORY Foundation for Life Sciences, Bioorganic Research Institute, 8-1-1 Seikadai Seika-cho, Soraku-gun, Kyoto 619-0284, Japan
gSynthetic Cellular Chemistry Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan
First published on 9th August 2016
Abstract
Our study first revealed that UDP-5-thioglucose functions as a glycosyl donor of UDP-glucose: glycoprotein glucosyltransferase to produce 5-thio-glucosylated Man9 (5S-G1M9). Subsequently, we observed that only calreticulin can interact with 5S-G1M9. Finally, the 5-thioglucose residue was resistant to hydrolysis by glucosidase II.
The calnexin (CNX)/calreticulin (CRT) cycle in the endoplasmic reticulum (ER) plays an important role in glycoprotein quality control (GQC).1–3 Nascent proteins that are modified co-translationally by high-mannose type glycan acquire mature structures through assistance of the CNX/CRT cycle, a glycoprotein specific folding machinery composed of molecular chaperones, glycosyltransferase and glycosidases (Fig. 1(A)). This process is initiated by attachment of a Glc3Man9GlcNAc2 glycan which is catalyzed by oligosaccharyltransferase, which is followed by glycan trimming by glucosidase I/II. Resultant glycoform Glc1Man9GlcNAc2 (G1M9) is recognized by lectin chaperones CNX and CRT. As strength of their affinity to the glycan is modest, glycoproteins are transiently liberated from chaperones and trimmed further by the second action of glucosidase II (G-II), which ultimately gives non-glucosylated glycoform Man9GlcNAc2 (M9). At this stage, glycoproteins are bifurcated depending on their folding status. Namely, when correctly folded, they are transported to Golgi apparatus for further processing, whereas rests of them are recognized as substrates of a folding sensor enzyme, UDP-glucose: glycoprotein glucosyltransferase (UGGT).4 This enzyme is able to regenerates the G1M9 glycoform, allowing folding defective glycoprotein can re-enter the CNX/CRT cycle.
 | ||
| Fig. 1 (A) Schematic illustration of CNX/CRT cycle. (B) Chemical structure of acceptor and donor substrates. | ||
Our group has conducted analysis of the glycoprotein folding machinery by using chemically synthesized high-mannose type glycan derivatives (Fig. 1(B)).5,6 Especially, fluorescently labeled glycans were shown to provide highly versatile platform for the analysis of CNX/CRT cycle. Most notably, M9 derivatives were excellent substrates of UGGT,7 suggesting that the substituents are suitable as surrogates of unfolded polypeptides. Importantly, kinetic constants of glucose transfer reaction to synthetic N-glycans by UGGT were found to be quite similar to using glycoproteins,8 underscoring relevance of our approach.
To extend analysis of carbohydrate active enzymes and lectins, utilization of 5-thio-sugars has been shown to be effective.9 For instance, some 5-thio-sugar derivatives have been reported to function as inhibitors of various glycosidases.10–12 Further, 5-thio-sugar nucleotides were reported to be active as either glycosyl donors,13–15 or inhibitors of glycosyltransferases.16 For instance, Adlercreutz et al. reported that α-galactosyltransferase was able to smoothly transfer 5-thiogalactose to lactose from UDP-5-thiogalactose, and resultant trisaccharide was shown to be resistant to hydrolysis by human α-galactosidase.15 On the contrary, transfer of 5-thiogalactose to βGlcNAc by β-galactosyltransferase was more sluggish.13 In the latter case, the produced 5-thio-LacNAc derivative was found to be susceptible to hydrolysis by β-galactosidase, although the velocity was 200 times lower than LacNAc. Moreover, recent study showed that O-GlcNAc transferase was inhibited efficiently by 5-thio-GlcNAc.16 The usage 5-thio-sugar for the analysis of carbohydrate-active proteins is promising in various contexts.
Based on these, our interest was attracted to introducing 5-thio-sugar to the CNX/CRT cycle, which consists of glycosyltransferase, glycosidase and lectin chaperones. Specifically, we planned to examine the influence of 5-thioglucose on CNX/CRT cycle, as it consists of bidirectional interconversion between mono- and non-glucosylated glycoforms, former of which is captured by lectin chaperones.
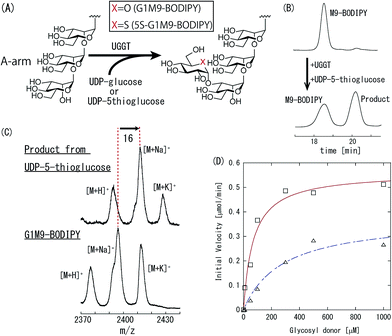
UGGT is a well-documented enzyme that transfers a glucose residue from UDP-glucose to the A-arm of M9 glycan (Fig. 2(A)). We examined the ability of UGGT to transfer thioglucose by using UDP-5-thioglucose and M9-BODIPY as donor and acceptor substrate, respectively (Fig. 1(B)). Monitoring by HPLC revealed a new peak with longer retention time (Fig. 2(B)), the identity of which was confirmed by MALDI-TOF-MS analysis. Namely, the aliquot of the fraction corresponding to the new peak was separated, which exhibited 16 higher m/z value than G1M9-BODIPY, indicating that the 5-thioglucose was transferred indeed (Fig. 2(C)). To evaluate the influence of ring-oxygen substitution with sulfur in detail, analysis of reaction kinetics was performed. Correlation of initial velocity with concentration of the donor substrate is depicted in Fig. 2(D). The Km and kcat value of UGGT estimated from fitting curve of Michaelis–Menten kinetics are given in Table 1. Intriguingly, reduction of the activity associated with the O to S substitution was not significant. Specifically, the Km value was increased only by a factor of 4, and reduction of the kcat value was marginal (66% of UDP-Glc). This result was somewhat surprising, UGGT is known to have narrow donor specificity. For instance, TDP- and ADP-glucoses were reported to be completely inactive.17 More recently, we compared reactivity of various UDP-glucose analogues.18 Although some UDP-deoxyglucose derivatives as well as UDP-galactose exhibited detectable activity, other derivatives were totally inactive. Consequently, the finding that replacement of C-5 oxygen with sulfur caused only marginal reduction of the activity is noteworthy. Several studies examined nucleotide 5-thio-sugar derivatives as donor substrates of glycosyltransferases.13,14 However, their reactivity, with few exceptions,19 was by far lower than the natural substrate. In this context, our results indicate that the oxygen atom at C-5 position is not of primary importance in UGGT–donor interaction.
| Glycosyl donor | Km [μM] | kcat [min−1] | kcat/Km [μM−1 min−1] |
|---|---|---|---|
| UDP-glucose | 69.3 | 1.86 × 105 | 2.68 × 103 |
| UDP-5-thioglucose | 279 | 1.23 × 105 | 4.40 × 102 |
Having the 5-thioglucose analogue of G1M9, we subsequently examined if it can interact with ER lectin chaperones. For this purpose, human CNX (hCNX), human CRT (hCRT) and human testis chaperone calmegin (hCMG), a homologue of hCNX,20,21 was employed. The binding assay was conducted based on the ultrafiltration method as reported previously.22 Correlation between protein concentration and degree of complex formation is recapitulated in Fig. S1.† Their dissociation constants with G1M9 and 5S-G1M9 calculated from curve fitting of the plots are provided in Table 2. As affinities to G1M9 were similar to previously reported values, it was assumed that all lectin chaperones were expressed in active forms.
Although the dissociation constant was 4 times higher, 5S-G1M9 retained binding affinity to hCRT. In sharp contrast, no meaningful value was obtained for hCNX under our assay conditions, as its affinity to 5S-G1M9 was quite low. Similar observation was made for hCMG, suggesting that glucose recognition manner is different between CRT and CNX/calmegin.
Hydroxyl groups of the terminal glucose residue of G1M9 are proposed to interact with Lys111, Gly124, Asp125, Tyr128 and Asn154 of mouse CRT (mCRT).23 hCRT shows significant amino acid sequence homology with mCRT,24 and these glucose-bound amino acids are fully conserved in hCRT (Fig. 3(A) inset). Further, superimposed structure of mCRT and hCRT indicates that spatial location of glucose-bound amino acids are almost identical although slight structural gap is found around D125 because it is located on the loop region (Fig. 3(A)). Therefore, amino acids responsible for glucose binding is presumed to be same between two CRTs. Bond angles of C1–S–C5 and S–C1–C2 of 5-thioglucose show more acute than normal glucose.9,10 This means that the ring conformation of glucose is changed partially by sulfur substitution. Thus, the change of the ring structure is predicted to be one of the reasons causing difference of complex formation between G1M9 and 5S-G1M9 with hCRT. hCRT mutants altered at D125 and N154 were prepared to reveal the interaction manner between hCRT and thioglucose. Previous study indicates that D125 recognizes oxygen at C-1 and C-2 position of glucose, and N154 interacts with that at C-3 and C-4.23 Fig. 3(B) indicates influence of mutation of amino acids on interaction between hCRT and high-mannose type glycan bound to glucose or thioglucose. Ratio of the complexation yields of G1M9 substrate interacted with hCRT to that of 5S-G1M9 substrate are shown in Table S1.† Only marginal differences were observed for the complexation yields of both G1M9 and 5S-G1M9 to hCRTs mutated in D125. Therefore, D125 seems to exhibit little influence on interaction with glucose moiety. In contrast, the complexation yield to 5S-G1M9 substrate was reduced significantly in N154-mutated hCRT. Ratio G1M9 vs. 5S-G1M9 were clearly higher than D125 mutants, indicating that the residue of N154 plays an important role in the molecular recognition that discriminates glucose and 5-thioglucose. Fig. S2† shows glucose recognition site of hCRT which is superimposed on predicted structure of hCNX and hCMG based on canine CNX.25 The superimposed structure indicates that glutamic acid residues of hCNX and hCMG are positioned proximal spatially to N154 of hCRT, while other amino acids that constitute canonical glucose binding sites are oriented in similar manner. Thus, the lack of asparagine in hCNX and hCMG is speculated to be a cause of reduced interaction with 5S-G1M9.
As illustrated in Fig. 4(A), G-II catalyzes hydrolysis reaction of non-reducing terminal glucose of N-type glycan.26 In general, reactions catalyzed by glycosyl hydrolases and transferases both proceed through oxocarbenium ion like intermediates.27 Since our analysis revealed that UGGT transfers 5-thioglucose to N-glycan, it may be expected that 5S-G1M9 is susceptible to hydrolysis by G-II. Accordingly, we examined the hydrolysis of 5S-G1M9 in comparison with G1M9. Fig. 4(B) shows initial velocity of hydrolysis reaction in each substrate. Hydrolysis of G1M9-BODIPY proceeded smoothly, in accordance with the previous report.28 In contrast, 5S-G1M9-BODIPY was highly resistant to the hydrolytic activity of G-II. To additional note, hydrolysis of G1M9-BODIPY was not influenced in the presence of 5S-G1M9-BODIPY (data not shown), indicating that G-II activity is not inhibited by addition of 5S-G1M9. This result suggests that interaction of G-II with 5S-G1M9 glycan is rather weak. Hence, we speculate that recognition of glucose by G-II requires the presence of ring oxygen. As our previous study revealed that G-II has promiscuous activity,18 which is able to cleave deoxyglucoses, galactose and glucuronic acid residues from the A-arm of high-mannose glycans, presence of ring oxygen is a critical element in order to be recognized by this enzyme.
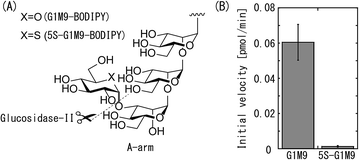 | ||
| Fig. 4 Glucose hydrolysis reaction by glucosidase II. (A) Illustration of hydrolysis reaction by glucosidase II. (B) Initial velocity of hydrolysis reaction toward G1M9-BODIPY or 5S-G1M9-BODIPY. | ||
In conclusion, we found that 5-thioglucose exhibited intriguing behavior toward GQC-related proteins. It was first shown that UGGT possessed ability to transfer 5-thioglucose to M9 glycan from UDP-5-thioglucose. The generated 5S-G1M9 was able to interact with CRT, whereas its affinity to CNX was low. From interaction assay between glycan and mutated CRT, D154 in CRT was suggested to play an important role in molecular recognition to 5S-G1M9 derivatives, and the lack of corresponding Asn in CNX might lead a cause of decreased affinity. It was also shown that 5S-G1M9-BODIPY was resistant to GII, leaving the thioglucose moiety unhydrolysed. In the ER, reason of existing seemingly redundant chaperones, CNX and CRT, has been obscure. It is also difficult to narrow down which glycoproteins enter the CNX/CRT cycle. To answer these questions, introduction of UDP-5-thioglucose to living cells would be a promising strategy.
Acknowledgements
We thank Ms Akemi Takahashi and Ms Satoko Shirahata for their technical assistance. We also thank the Support Unit for Bio-material Analysis, RIKEN BSI Research Resources Center for DNA sequencing analysis.Notes and references
- A. Helenius and M. Aebi, Annu. Rev. Biochem., 2004, 73, 1019 CrossRef CAS PubMed.
- J. L. Brodsky and W. R. Skach, Curr. Opin. Cell Biol., 2011, 23, 464 CrossRef CAS PubMed.
- Y. Ito, Y. Takeda, A. Seko, M. Izumi and Y. Kajihara, Semin. Cell Dev. Biol., 2015, 41, 90 CrossRef CAS PubMed.
- Y. Takeda, A. Seko, M. Hachisu, S. Daikoku, M. Izumi, A. Koizumi, K. Fujikawa, Y. Kajihara and Y. Ito, Glycobiology, 2014, 24, 344 CrossRef CAS PubMed.
- Y. Takeda, K. Totani, I. Matsuo and Y. Ito, Curr. Opin. Chem. Biol., 2009, 13, 582 CrossRef CAS PubMed.
- Y. Ito and Y. Takeda, Proc. Jpn. Acad., Ser. B, 2012, 88, 31 CrossRef CAS PubMed.
- K. Totani, Y. Ihara, I. Matsuo, H. Koshino and Y. Ito, Angew. Chem., Int. Ed., 2005, 44, 7950 CrossRef CAS PubMed.
- M. Sakono, A. Seko, Y. Takeda, M. Hachisu and Y. Ito, Biochem. Biophys. Res. Commun., 2012, 426, 504 CrossRef CAS PubMed.
- J. G. Fernandez-Bolaños, N. A. AL-Masoudi and I. Maya, Adv. Carbohydr. Chem. Biochem., 2001, 57, 21 CrossRef.
- T. Kajimoto, K. K. C. Liu, R. L. Pederson, Z. Zhong, Y. Ichikawa, J. A. Porco Jr and C. H. Wong, J. Am. Chem. Soc., 1991, 113, 6187 CrossRef CAS.
- H. Hashimoto, M. Kawanishi and H. Yuasa, Carbohydr. Res., 1996, 282, 207 CrossRef CAS.
- S. Mehta, J. S. Andrews, B. Svensson and B. M. Pinto, J. Am. Chem. Soc., 1995, 117, 9783 CrossRef CAS.
- H. Yuasa, O. Hindsgaul and M. M. Palcic, J. Am. Chem. Soc., 1992, 114, 5891 CrossRef CAS.
- O. Tsuruta, H. Yuasa, H. Hashimoto, K. Sujino, A. Otter, H. Li and M. M. Palcic, J. Org. Chem., 2003, 68, 6400 CrossRef CAS PubMed.
- D. Adlercreutz, Y. Yoshimura, K. Mannerstedt, W. W. Wakarchuk, E. P. Bennett, N. J. Dovichi, O. Hindsgaul and M. M. Palcic, ChemBioChem, 2012, 13, 1673 CrossRef CAS PubMed.
- T. M. Gloster, W. F. Zandberg, J. E. Heinonen, D. L. Shen, L. Deng and D. J. Vocadlo, Nat. Chem. Biol., 2011, 7, 174 CrossRef CAS PubMed.
- S. E. Trombetta and A. J. Parodi, J. Biol. Chem., 1992, 267, 9236 CAS.
- A. Miyagawa, K. Totani, I. Matsuo and Y. Ito, Biochem. Biophys. Res. Commun., 2010, 403, 322 CrossRef CAS PubMed.
- M. Yang, M. R. Proctor, D. N. Bolam, J. C. Errey, R. A. Field, H. J. Gilbert and B. G. Davis, J. Am. Chem. Soc., 2005, 127, 9336 CrossRef CAS PubMed.
- D. Watanabe, M. Okabe, N. Hamajima, T. Morita, Y. Nishina and Y. Nishimune, FEBS Lett., 1995, 368, 509 CrossRef CAS PubMed.
- D. Watanabe, K. Yamada, Y. Nishina, Y. Tajima, U. Koshimizu, A. Nagata and Y. Nishimune, J. Biol. Chem., 1994, 269, 7744 CAS.
- Y. Takeda, A. Seko, M. Sakono, M. Hachisu, A. Koizumi, K. Fujikawa and Y. Ito, Carbohydr. Res., 2013, 375, 112 CrossRef CAS PubMed.
- G. Kozlov, C. L. Pocanschi, A. Rosenauer, S. Bastos-Aristizabal, A. Gorelik, D. B. Williams and K. Gehring, J. Biol. Chem., 2010, 285, 38612 CrossRef CAS PubMed.
- D. P. McCauliffe, E. Zappi, T. S. Lieu, M. Michalak, R. D. Sontheimer and J. D. Capra, J. Clin. Invest., 1990, 86, 332 CrossRef CAS PubMed.
- M. Sakono, A. Seko, Y. Takeda, J. Aikawa, M. Hachisu, A. Koizumi, K. Fujikawa and Y. Ito, Biochim. Biophys. Acta, 2014, 1840, 2904 CrossRef CAS PubMed.
- J. Roth, M. Ziak and C. Zuber, Biochimie, 2003, 85, 287 CrossRef CAS PubMed.
- L. L. Lairson and S. G. Withers, Chem. Commun., 2004, 2243 RSC.
- K. Totani, Y. Ihara, I. Matsuo and Y. Ito, J. Biol. Chem., 2006, 281, 31502 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra16476e |
| This journal is © The Royal Society of Chemistry 2016 |