Micro-aerobic digestion of high-solid anaerobically digested sludge: further stabilization, microbial dynamics and phytotoxicity reduction†
Xiaowei Liab,
Zonghan Lia,
Xiaohu Dai*a,
Bin Dong*a and
Yanfei Tanga
aState Key Laboratory of Pollution Control and Resources Reuse, National Engineering Research Center for Urban Pollution Control, College of Environmental Science and Engineering, Tongji University, Shanghai 200092, PR China. E-mail: daixiaohu@tongji.edu.cn; tj_dongbin@vip.163.com
bSchool of Environmental and Chemical Engineering, Shanghai University, Shanghai 200444, PR China
First published on 8th August 2016
Abstract
Micro-aerobic digestion was firstly applied for further stabilization and phytotoxicity reduction of high-solid anaerobically digested sludge (ADS) in room temperature, mesophilic and thermophilic conditions. Organic matter degradation and microbial community succession were determined by fluorescent and X-ray photoelectron spectrometers, and Illumina MiSeq sequencing analysis during the process. Results showed that specific oxygen uptake rate, volatile solid and ammonia nitrogen contents of the ADS reduced by 36.1–86.4%, 8.4–16.2% and 70.2–85.4%, respectively after micro-aerobic digestion, and these changes had an increasing tendency with the temperature. They implied that micro-aerobic digestion promoted in-depth stabilization of the ADS, which temperature increase had a positive effect on. Protein-like and carbohydrate-like groups decreased, and humic acid-like and carboxyl materials enriched, while microbial community succession shifted from unassigned bacteria and Tepidimicrobium to Pseudomonas and Desulfuromonadales during the micro-aerobic process. Phytotoxicity tests revealed that micro-aerobic digestion reduced the inhibition of the ADS to germination and root growth of three plant seeds, but temperature had an adverse impact on the phytotoxicity reduction. Overall, the findings indicated that mesophilic micro-aerobic digestion was an alternative technique for the post-treatment of high-solid ADS.
1. Introduction
Anaerobic digestion is one of the most widely used processes to stabilize sludge by converting a part of its biodegradable organic matter into biogas, a renewable energy source.1 Especially the development of high-solid anaerobic digestion makes it more economical and efficient.1 Meanwhile, large amount of anaerobically digested sludge (ADS) is generated during the process. The ADS has a great agronomic or land-utilization potential value, due to its high proportion of mineral N and nutrients.2 However, the ADS in its basic form may cause poor plant growth and damage crops because insufficiently-biodegraded organic matter and small-molecule substances (e.g. ammonia and volatile organic acids) in the ADS will compete for oxygen or cause phytotoxicity to plants.3,4 In addition, unstable ADS would continue to decompose even after application to soil, in which case, soil microbes scavenge for the nutrients that should have been made available to plants,4 causing the immobilization of the nutrients (e.g. nitrogen) instead of its release for plant growth. Thus, post-treatment of the ADS was required before it was used for soil organic amendments or agronomic fertilizer.Recently, micro-aerobic digestion was commended to treat sewage sludge5,6 because of its excellent treatment efficiency and low energy consumption. Researches showed that the efficiency of H2S removal even reached up to 99% in the micro-aerobic condition,5 and sufficient micro-aeration could improve the hydrolysis of carbohydrates and protein.6 Meanwhile, both of strictly aerobic bacteria and anaerobes could simultaneously grow in the micro-aerobic system,5 causing higher VS degradation, compared with a purely anaerobic system. Micro-aerobic system was only supplied limited oxygen, and thus needed low energy consumption for aeration. In fact, the micro-aerobic system had many cases. In one case, it described an anaerobic system into which a trace amount of oxygen is supplied, while in another it represented an aerobic system with low oxygen supply.5 Previous reports focused on an anaerobic system with small amount of oxygen, but an aerobic system with limited oxygen was paid little attention, because the latter was liable to cause the accumulation of volatile fatty acids (VFA).7 Compared with raw sludge, the ADS had less biodegradable organic matter, which would relieve the VFA accumulation. Thus, the micro-aerobic digestion might be a potential and economic process for post-treatment of the ADS.
Previous study about the post-aerobic digestion of the ADS focused on the treatment performance, e.g. VS reduction and nitrogen removal, and paid little attention to chemical changes of unstable organic matter in the ADS. Fluorescence excitation–emission matrix (EEM) spectroscopy was a useful method to investigate the changing characteristics of fluorescent organic matter in different samples1,8 and X-ray photoelectron spectroscopy (XPS) analysis was applied to characterize change in chemical speciation of organic matter of the samples.9–11 In the present study, the two techniques were used to investigate the degradation and transformation characteristics of organic matters during micro-aerobic digestion of the ADS.
Microorganisms in sludge have a close relation to the degradation of unstable organics and small-molecule phytotoxins. Microbial community succession would occur when the anaerobic environment transfers to the micro-aerobic one. So, it's significant to explore the changes of microbial population before and after ADS micro-aerobic digestion. Compared to traditional analytical methods (e.g. PCR-DGGE technique), next-generation sequencing technology can obtain more comprehensive and acute data in a shorter analytical time.12 In the present study, Illumina MiSeq sequencing technology was applied to characterize the microbial community succession during the micro-aerobic digestion of the ADS.
In spite of undisputable potential resulting from the application of the ADS in agriculture or landscape, it also involves some threats due to the presence of pathogens and organic pollutants.13 The identification of potential threats was needful to control and reduce the risk involved in the application of the ADS. Phytotoxicity test could not only evaluate the applicability of the ADS for agricultural or soil reclamation purposes, but also identify potential threats for the environment and for human health.13
The main objectives of this paper were to: (1) investigate treatment performance of micro-aerobic digestion for the high-solid ADS further stabilization by chemical parameters and phytotoxicity test; (2) explore possible mechanisms about the micro-aerobic digestion treating ADS by fluorescence and XPS spectra, and Illumina MiSeq sequencing techniques; (3) study the effect of temperature on the micro-aerobic process of the ADS. The study contributes toward development of a feasible and low-cost process for the ADS post-treatment.
2. Materials and methods
2.1. High-solid anaerobically digested sludge
The high-solid ADS were collected from a 12 L mesophilic anaerobic digestion reactor with the sludge retention time (SRT) of 20 days, which had been operated for 60 days and reached the stable state with VS removal rate of 47.0% ± 2.3%. The anaerobic digestion reactor was operated semi-continuously (once-a-day draw-off and feeding) and fed with dewatered sludge collecting from Anting WWTP in Shanghai, China. The characteristics of the ADS were shown as followings: pH, 7.62 ± 0.05; total alkalinity (TA), 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 822 ± 754 mg CaCO3 per L; total solid content (TS), 145.2 ± 2.1 g kg−1 (wet weight); volatile solid content (VS), 455.2 ± 9.3 g kg−1 (dry basis); VFA contents, 3563 ± 906 mg L−1; total kjeldahl nitrogen (TKN), 8.60 ± 0.95 mg N per g; total ammonium nitrogen (TAN), 3.93 ± 0.26 mg N per g.
822 ± 754 mg CaCO3 per L; total solid content (TS), 145.2 ± 2.1 g kg−1 (wet weight); volatile solid content (VS), 455.2 ± 9.3 g kg−1 (dry basis); VFA contents, 3563 ± 906 mg L−1; total kjeldahl nitrogen (TKN), 8.60 ± 0.95 mg N per g; total ammonium nitrogen (TAN), 3.93 ± 0.26 mg N per g.
2.2. Micro-aerobic digestion experiment
Micro-aerobic digestion experiments were carried out in three lab-scale polyvinyl chloride cylinders, which were designed according to the ref. 14, and 100 mm in inner-diameter, 385 mm in inner-height and 5 mm of thickness, with an effective volume of about 3 L. Two perforated pipes with 2 mm mesh were installed at the bottom of each reactor to facilitate aeration. The aeration rate was about 2.4 L min−1 to provide a micro-aerobic condition (dissolved oxygen below 0.2 mg L−1) during the entire experiment.5,6 An air inlet was installed at the bottom and the outlet on the top. A vertical stirrer (20 rpm) was fixed in the middle of each reactor for mixing.The reactors were also operated semi-continuously (once-a-day draw-off and feeding), and fed with the above ADS. The SRT of the micro-aerobic reactor was 8 d. At the beginning of experiment, about 800 g of the ADS was fed into each reactor. The reactors were incubated in room temperature (25 °C), mesophilic (37 °C) and thermophilic (55 °C) conditions, respectively. Moisture loss was replenished by adding distilled water to the reactor daily to keep the original volume (subtracting the volume of sample).15
2.3. Sampling and chemical analysis
The influent and effluent sludge (IS and ES) of the micro-aerobic reactors were periodically sampled for the chemical analysis after the reactors reached the stable state, in order to evaluate their treatment performances. The experiment ended after the reactors were operated for 32 days, and the IS and ES samples were collected for fluorescence EEM, XPS, and microbial community analyses, and phytotoxicity test.pH values were determined by a Mettler Toledo pH meter (Switzerland). Electric conductivity (EC) values were determined by a Mettler Toledo EC meter (Switzerland). Specific oxygen uptake rates (SOUR) were determined using about 20 g (wet basis) sludge sample according to the ref. 12. TS contents were estimated through drying at 105 °C for 24 h, while VS contents were measured through maintaining the drying sludge at 600 °C for 1 h in a muffle furnace. Dissolved organic matter (DOC), VFA and TAN contents were determined using the filtrate of the samples. The filtrate were gained as followings: about 5 g (wet basis) sludge samples were added 25 ml distilled water, and then mixed on a horizontal shaker at 350 rpm for 15 min; the mixture was centrifuged at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 min, and then the supernatant was passed through a 0.45 μm microfiber filter. DOC contents were determined by a TOC VCPN analyzer (Shimadzu, Japan), and VFA contents were analyzed by a GC (GC-2010plus, Shimadzu, Japan) with flame ionization detector, and TAN content were estimated according to the standard methods.16
000 rpm for 20 min, and then the supernatant was passed through a 0.45 μm microfiber filter. DOC contents were determined by a TOC VCPN analyzer (Shimadzu, Japan), and VFA contents were analyzed by a GC (GC-2010plus, Shimadzu, Japan) with flame ionization detector, and TAN content were estimated according to the standard methods.16
2.4. Organic matter analysis
Fluorescence EEM spectra of the samples were analyzed according to the ref. 17 by a Hitachi F-7000 fluorescence spectrometer (Hitachi High Technologies, Tokyo, Japan). The filtrate of each sample was gained using the above method and normalized to a DOC concentration of 10 mg L−1. The emission spectra were scanned from 290 to 550 nm at 5 nm increments by varying the excitation wavelength from 250 to 450 nm at 5 nm increments. Surfer 8.0 software was used to analyze fluorescence spectral data.The freeze-dried and 0.149 mm sieved samples were used for XPS analysis, which was carried out on a RBD upgraded PHI-5000C ESCA system (Perkin Elmer). The sample was directly pressed to a self-supported disk (10 × 10 mm) and mounted on a sample holder, and then transferred into the analyzer chamber. Binding energies were calibrated by using the containment carbon (C 1s = 284.6 eV). RBD AugerScan 3.21 software was used for the analysis of the XPS data.
2.5. Microbial community analysis
The DNA of the samples were extracted using a Mo Bio Power Soil® DNA Isolation Kit (Mo Bio laboratories, Inc. Carsbad, CA, USA). The DNA samples were submitted to Shanghai Majorbio Bio-pharm Technology Co., Ltd (Shanghai, China) for Illumina MiSeq sequencing analysis. Gene amplicons (16S rRNA) were conducted using PCR with primers 515F 5′-GTGCCAGCMGCCGCGG-3′ and 907R 5′-CCGTCAATTCMTTTRAGTTT-3′. Each primer was pre-pended with a 8 base barcode sequence and a unique barcode was applied for each sample.18 The amplicons were extracted from 2% agarose gels and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, U.S.). Purified amplicons were pooled in equimolar and paired-end sequenced (2 × 250) on an Illumina MiSeq platform according to the standard protocols. The raw reads were deposited into the NCBI Sequence Read Archive (SRA) database (Accession number: SRP067951). Raw fastq files were demultiplexed, quality-filtered using QIIME (version 1.17) software and reads which could not be assembled were discarded. Operational taxonomic units (OTUs) were clustered with 97% similarity cutoff using UPARSE (version 7.1 http://drive5.com/uparse/) and chimeric sequences were identified and removed using UCHIME. The taxonomy of each 16S rRNA gene sequence was analyzed by RDP Classifier (http://rdp.cme.msu.edu/) against the silva (SSU115) 16S rRNA database using confidence threshold of 70%.2.6. Phytotoxicity assays
Three kinds of ornamental plant seeds, including sunflower (Helianthus annuus), cornflower (Centaurea cyanus) and purple morning glory (Ipomea hederacea), which were commended by the OECD for ecotoxicological testing,19 were used for the acute and subchronic phytotoxicity assay of the samples.Acute phytotoxicity test (APT) was conducted according to the National Standard of the People's Republic of China for disposal of sludge from municipal wastewater treatment (GB/T 23486-2009). In brief, about 10 g of each sample was dissolved in 30 ml distilled water, and then the mixture was filtrated after shaking at 160 rpm for 60 min. A Petri dish with filter paper was added 5 ml filtrate and 20 seeds, and then incubated in the dark at 25 °C for 48 h. At the end, the seed germinate rate and root length of the germinated seed were determined. Each test was repeated five times. The distilled water was used as control under other similar conditions.
Subchronic phytotoxicity test (SPT) was carried out according to OECD Guidelines.19 Shortly, growth substrate consisting of inorganic and organic matrices (volumetric ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was mixed well before adding to the polystyrol seed tray with 20 holes. The inorganic growth substrate consisted of quartz sand, while organic growth substrate were composed of peat and sludge sample with the ratio of 1
3) was mixed well before adding to the polystyrol seed tray with 20 holes. The inorganic growth substrate consisted of quartz sand, while organic growth substrate were composed of peat and sludge sample with the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w, dry basis), and 100% peat was served as control. The prepared polystyrol seed trays were kept in the incubation chamber at 21 °C with light/dark regime 16/8 h for 14 d. Seed germination rate were recorded every days, and root length and fresh weight of the germinated seeds were determined at the end. The inhibition of the seed germination for APT and SPT test were estimated with respect to the control according to the ref. 4 and 13.
1 (w/w, dry basis), and 100% peat was served as control. The prepared polystyrol seed trays were kept in the incubation chamber at 21 °C with light/dark regime 16/8 h for 14 d. Seed germination rate were recorded every days, and root length and fresh weight of the germinated seeds were determined at the end. The inhibition of the seed germination for APT and SPT test were estimated with respect to the control according to the ref. 4 and 13.
3. Results and discussion
3.1. Treatment performance of the micro-aerobic digesters for high-solid ADS
Fig. 1 showed the chemical changes in the IS and ES samples of the three micro-aerobic digesters. Compared with the IS, the pH of ES increased to 7.80 ± 0.06 at 25 °C, but decreased to 7.55 ± 0.10 and 7.08 ± 0.15 at 37 and 55 °C, respectively (Fig. 1a). The pH value decrease probably resulted from mineralization of ammonia nitrogen (Fig. 1g), while the pH value increase might be attributed to the loss of volatile aids (Fig. 1f).20 EC values of the ES were significantly lower than that of IS (Fig. 1b), which might be attributed to loss of soluble salts by leaching and/or microbial immobilization, and/or to formation of insoluble salts.21The ES of the micro-aerobic reactor had lower SOUR and VS content, compared with the IS. After micro-aerobic digestion, the removal rates of SOUR and VS were 36.1–86.4% and 8.4–16.2%, respectively, corresponding to the previous results from aerobic composting.12 The results showed that the organic matter of the ADS was further biodegraded during the process, implying that the micro-aerobic digestion promoted the bio-stabilization of the ADS. Compared with the results of raw sewage sludge reported in the previous ref. 5, the VS reduction rates of the ADS in this study was lower, which might be attributed to the presence of more biostable organic matter in the ADS.20
Kumar proposed that there are 4 kinds of VS fractions in sludges: a fraction degradable only under aerobic conditions, a fraction degradable only under anaerobic conditions, a fraction degradable under both anaerobic and aerobic conditions, and a non degradable fraction.22 The micro-aerobic digestion might promote the biodegradation of the fraction degradable only under aerobic conditions. Additionally, the removal rates of SOUR and VS gradually increased with temperature, implying that temperature increase could promote the biodegradation of the ADS organic matter.
Compared with the IS, the ES of micro-aerobic reactor had lower DOC and VFA contents, which were similar to the changes of the SOUR and VS content, indicating the degradation of C-containing organic matter and the enhancement of the ADS stability. Compared with ES of the reactors at 25 and 37 °C (ES-25 and ES-37), the ES at 55 °C (ES-55) had higher DOC and VFA contents, which was possibly attributed to higher organic matter hydrolysis rate and limited oxygen supply in the micro-aerobic condition.5 Additionally, the TAN removal rates of the ADS were 70.2–85.4% after the micro-aerobic digestion, which similar to the results of the ref. 22, which might be resulted from two pathways, i.e. nitrification/denitritation and stripping.22 Fig. S1 of the ESI† showed that TAN removal went more through stripping (47–75%) than through nitrification/denitritation (25–53%).
In general, after micro-aerobic digestion, the EC, SOUR, VS, DOC, VFA and TAN contents of high-solid ADS significantly reduced, and the changes had an increasing tendency as the temperature. These results implied that the micro-aerobic digestion promoted further stabilization of organic matter in the ADS, which temperature had a positive effect on.
3.2. Degradation and transformation of organic matters
| Samples | Peak 1 | Peak 2 | Peak 3 | Peak 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Ex/Ema (nm) | SFIb | Ex/Em (nm) | SFI | Ex/Em (nm) | SFI | Ex/Em (nm) | SFI | |
| a Excitation/emission wavelength pairs.b Specific fluorescence intensity.c Influent sludge.d No data. | ||||||||
| ISc | 275/305 | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 526 526 |
—d | — | 250/460 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 767 767 |
— | — |
| ES-25 | 275/310 | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 915 915 |
275/340 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 261 261 |
250/465 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 788 788 |
330/430 | 6307 |
| ES-37 | 275/310 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 503 503 |
270/410 | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 638 638 |
250/460 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 305 305 |
345/420 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 186 186 |
| ES-55 | 275/305 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 747 747 |
— | — | 250/460 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 277 277 |
330/410 | 8238 |
Compared with the IS, the EEM spectra of the ES samples had lower intensity of peak 1, and higher intensity of peak 3, implying that micro-aerobic digestion promoted the degradation of protein-like group and the formation of humic-like substance. Additionally, the spectra of the ES samples had two additional peaks (peak 2 and peak 4), which had longer emission than peak 1 or peak 3 (Fig. S2 of the ESI†). It was reported that the peak position emission shifted to longer wavelength with the increasing content of aromaticity and polycondensation of humic materials.8 The results showed that the aromaticity and polycondensation of organic matter in the ADS increased after the micro-aerobic digestion, implying the formation of more biostable groups.
As the temperature increased, the intensities of the ES samples had a decreasing tendency in peak 1 and peak 2, and an increasing trend in peak 4. The results showed that the temperature increase promoted the degradation of protein-like materials, and the formation of humic acid-like groups. They complemented and confirmed the above findings that temperature could promote further stabilization of the ADS organic matter.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in carbonyl and carboxylate. The O peaks were decomposed into three bonds: O
O) in carbonyl and carboxylate. The O peaks were decomposed into three bonds: O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C band in carboxylic acid, carboxylate, ester, carbonyl and amide; O–C bond, including hydroxide (C–OH); and acetal and hemiacetal (C–O–C) in polysaccharides. The N peaks were attributed to three bonds: nitrogen in amine groups (
C band in carboxylic acid, carboxylate, ester, carbonyl and amide; O–C bond, including hydroxide (C–OH); and acetal and hemiacetal (C–O–C) in polysaccharides. The N peaks were attributed to three bonds: nitrogen in amine groups (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–); N–O/C–N bonds in amide or amine; and N–H bond in ammonia or protonated amine.
N–); N–O/C–N bonds in amide or amine; and N–H bond in ammonia or protonated amine.
Table 2 outlined the chemical composition and percentages, in terms of atomic concentration, of C, O, N, Si, P and S. Compared with the IS, the ES of the micro-aerobic reactors had lower carbon, C/O and C/N ratios, and higher nitrogen, oxygen and sulphur. The results showed that the proportion of C-containing materials decreased, and that of N-, O-, S-containing groups increased after micro-aerobic digestion, which was possibly resulted from stronger degradation of C-containing compounds, implying an increase in maturity degree of the ADS.12 The changes of the elements had an increasing tendency with temperature, indicating that temperature increase was beneficial to the degradation and transformation of unstable organic matter in the ADS.
| Elements | Peak (eV) | IS | ES-25 | ES-37 | ES-55 | Assignments |
|---|---|---|---|---|---|---|
| Atomic ratios (%) | ||||||
| a No data. | ||||||
| Total C | 284.6 | 53.53 | 50.77 | 49.47 | 47.96 | —a |
| C 1s | 283.96 ± 0.25 | 34 | 39.9 | 34.3 | 48.9 | C–C |
| C 1s | 284.63 ± 0.09 | 31.5 | 15.7 | 25.2 | 16.4 | C–H |
| C 1s | 285.69 ± 0.10 | 20.4 | 15.7 | 13 | 18.7 | C–(O, N) |
| C 1s | 287.25 ± 0.60 | 14.1 | 28.8 | 27.5 | 16 | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH C–OH |
| Total O | 532.65 ± 0.10 | 40.63 | 42.38 | 44.11 | 45.17 | — |
| O 1s | 531.60 ± 0.20 | 25.7 | 45.7 | 29.4 | 28.9 | O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C |
| O 1s | 532.67 ± 0.19 | 37.1 | 17.3 | 34.3 | 35.7 | O–C |
| O 1s | 533.79 ± 0.05 | 37.2 | 36.9 | 36.3 | 35.5 | C–O–C |
| Total N | 400 ± 0.16 | 2.29 | 3.03 | 2.66 | 3.42 | — |
| N 1s | 398.82 ± 0.26 | 23.1 | 25.7 | 43.8 | 34.3 | ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– N– |
| N 1s | 400.10 ± 0.16 | 30.1 | 30.2 | 32.6 | 35.7 | N–O/C–N |
| N 1s | 401.37 ± 0.41 | 46.9 | 44.1 | 23.6 | 30.1 | N–H |
| Total Si | — | 2.41 | 2.39 | 2.50 | 2.09 | — |
| Total P | — | 0.76 | 0.87 | 0.64 | 0.69 | — |
| Total S | — | 0.38 | 0.56 | 0.62 | 0.67 | — |
| C/O | 1.76 | 1.60 | 1.50 | 1.41 | ||
| C/N | 27.27 | 19.54 | 21.70 | 16.36 | ||
After the micro-aerobic digestion, the percentages of C–H, C–(O, N), O–C, C–O–C and N–H bonds decreased, and that of C–C, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–OH, O
C–OH, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C,
C, ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– and N–O/C–N bonds increased in the ADS, showing that micro-aerobic digestion promoted the degradation of carbohydrates and amines, and the removal of ammonia, and led to the enrichment of graphitic carbon, carboxylic acid and nitrocompound. However, the changes of the groups had no obvious trend with the temperature, which was possibly resulted from the presence of different functional microbe at different temperature. The results indicated that the degradation and transformation of these organic matters were complex and distinctive during the micro-aerobic digestion, and still needed further study.
N– and N–O/C–N bonds increased in the ADS, showing that micro-aerobic digestion promoted the degradation of carbohydrates and amines, and the removal of ammonia, and led to the enrichment of graphitic carbon, carboxylic acid and nitrocompound. However, the changes of the groups had no obvious trend with the temperature, which was possibly resulted from the presence of different functional microbe at different temperature. The results indicated that the degradation and transformation of these organic matters were complex and distinctive during the micro-aerobic digestion, and still needed further study.
In sum, XPS analysis showed that the micro-aerobic digestion promoted the degradation of C-containing materials (e.g. carbohydrates) and amines, and the enrichment of carboxylic acid and nitrocompound.
3.3. Microbial community succession during the aerobic digestion process
Illumina MiSeq sequencing was used to investigate the change of microbial community composition before and after micro-aerobic digestion. Each sample possessed about 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 027–15
027–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 356 quality-filtered reads with the mean length of the sequences of 428–438 bp (Table S1 of the ESI†). Fig. S4a of the ESI† showed the rarefaction curves at distance cutoff levels of 3%, which indicated reasonable numbers of sample sequences in this study. Chao and ACE values showed that compared with the IS, the phylotype richness of the ES-55 increased, and that of the ES-25 and ES-37 decreased (Table S1 of the ESI†). Shannon diversity index revealed that compared with the IS, bacterial diversities of ES-25 and ES-37 increased (Fig. S4b of the ESI†). The results showed that the micro-aerobic digestion caused a decrease in bacterial richness of the ADS, and an increase in the diversity, indicating that microbial community succession occurred during the micro-aerobic digestion process of the ADS.
356 quality-filtered reads with the mean length of the sequences of 428–438 bp (Table S1 of the ESI†). Fig. S4a of the ESI† showed the rarefaction curves at distance cutoff levels of 3%, which indicated reasonable numbers of sample sequences in this study. Chao and ACE values showed that compared with the IS, the phylotype richness of the ES-55 increased, and that of the ES-25 and ES-37 decreased (Table S1 of the ESI†). Shannon diversity index revealed that compared with the IS, bacterial diversities of ES-25 and ES-37 increased (Fig. S4b of the ESI†). The results showed that the micro-aerobic digestion caused a decrease in bacterial richness of the ADS, and an increase in the diversity, indicating that microbial community succession occurred during the micro-aerobic digestion process of the ADS.
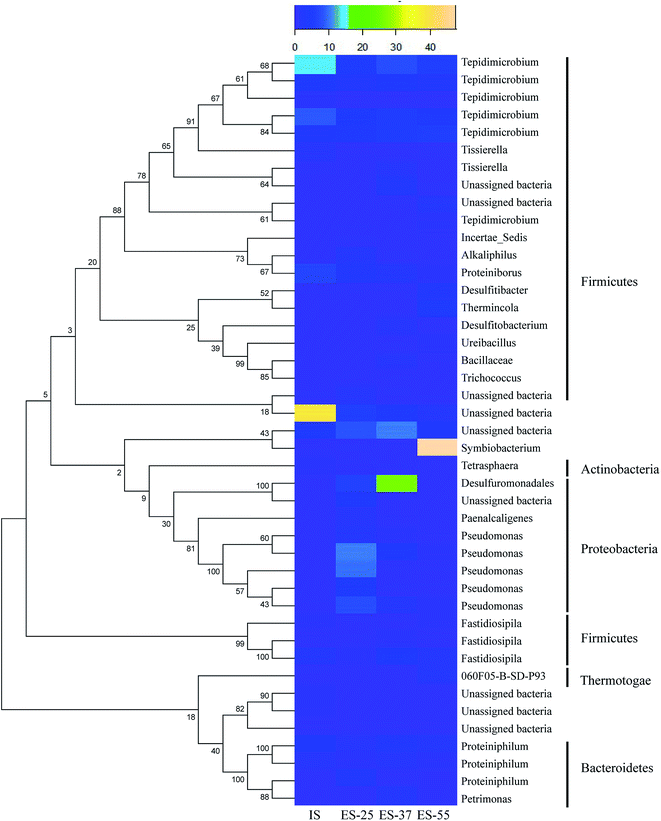
The sequence tags were assigned into different phylogenetic bacterial taxa using the RDP classifier and the relative bacterial abundances of the samples on the phylum level were shown in Fig. 4. The results showed that the samples were dominated by Firmicutes (38.55–88.14%), Proteobacteria (2.04–47.89%), Bacteroidetes (2.67–6.09%), Actinobacteria (0.80–1.59%) and unclassified bacteria (2.49–41.36%), with the total abundances of 97.95–99.74%. Other phylum with little abundance were also found, including Thermotogae (0.00–1.64%), Synergistetes (0.07–0.31%), Chloroflexi (0.04–0.21%), Acidobacteria (0.02–0.06%) and Tenericutes (0.00–0.04%). After aerobic digestion, the percentage of Firmicutes in the ADS decreased, and that of Bacteroidetes and Proteobacteria increased. Previous study showed that Proteobacteria were usually found to dominate in activated sludge of WWTP, followed by Bacteroidetes and Firmicutes,23 while Firmicutes was the most dominated phylum in the anaerobic digestion system.24 Therefore, the results indicated the microbial community of the ADS had a distinctive succession from the anaerobic bacteria to aerobic bacteria during the micro-aerobic digestion. As the treatment temperature increased, the percentage of Bacteroidetes and Proteobacteria had a decreasing tendency, and Firmicutes tended to increase. The results showed that temperature increase had an adverse on the succession from the anaerobic bacteria to aerobic bacteria.
The top 20 abundant OTU in each sample (a total of 43 OTUs from the four samples) at 3% cutoff level were selected and compared with the abundances in other samples. The phylogenetic tree of the 43 OTUs using neighbor-joining analysis was shown in Fig. 5. For the IS, unassigned bacteria (38.86%) was the most abundant OTU, followed by two OTUs of Tepidimicrobium (14.26% and 9.01%) and Proteiniborus (7.16%). The OTUs were mostly affiliated within Clostridiales, Firmicutes, which were well-known obligate anaerobes. Then, three OTUs of Pseudomonas (28.39% in total) were the most abundant OTUs in the ES-25, followed by unassigned bacteria (8.54%) and Desulfuromonadales (6.44%). For the ES-37, the most abundant OTUs were Desulfuromonadales (26.51%), unassigned bacteria (10.77%) and two OTUs of Tepidimicrobium (7.87% and 5.64%). For the ES-55, the OTUs of Symbiobacterium (47.83%) and Tepidimicrobium (11.92%) were the most abundant. Fig. 5 showed that there were considerable changes in the dominate OTUs composition of the samples after aerobic digestion and with the temperature, which complemented and confirmed the results at the phylum level.
Pseudomonas species were universal in the environment and found to be the dominant bacteria in the mature compost.25 The Lalucat et al.26 reported that Pseudomonas strains were capable of denitrification and the degradation of pollutants. Ueda et al.27 found that Symbiobacterium could consume various forms of organic matter, such as carbohydrate and amino acid, and produce low molecular weight organic matter, which were well associated with high VS removal rate of the micro-aerobic reactor at 55 °C. Desulfuromonadales was also described as a genera of sulfate-reducing long-chain fatty acids (LCFA) oxidizers.28 Tepidimicrobium was described as a thermophilic, peptolytic and strictly nonsaccharolytic bacterium related to the Clostridia and grows organotrophically on a number of proteinaceous substrates,29 which might be responsible for the degradation of protein-like materials during the micro-aerobic digestion of ADS. The results indicated that the microbes such as Pseudomonas, Desulfuromonadales and Symbiobacterium, played an important part in the degradation and transformation of organic matter during the micro-aerobic digestion of the ADS.
3.4. Phytotoxicity test
In the preset study, three kinds of ornamental plant seeds, i.e. Helianthus annuus, Centaurea cyanus L. and Pharbitis nil Choisy, were picked for the phytotoxicity assay of the sludge samples, which was significant to investigate the effect of the micro-aerobic digestion on the ADS landscape application.Fig. 6 and S5 of the ESI† outlined the results of the acute and subchronic phytotoxicity tests of the IS and ES samples. Both of the acute and subchronic tests showed that the IS had high inhibition on the germination rate and average root length of the seeds, corresponding to the previous results that the ADS in basic form induced the poor plant growth.3,4 The inhibition of the ES had a more or less decrease after the micro-aerobic digestion at three temperatures, indicating that the micro-aerobic digestion reduced the phytotoxicity of the ADS. Additionally, the inhibition of the ES samples had an increasing tendency as the treatment temperature, indicating that the temperature had an adverse effect on the reduction of the inhibition. Therefore, the results showed that micro-aerobic digestion caused a decrease in the phytotoxicity of the ADS, but treatment temperature increase was not beneficial for improvement of the ADS land-utilization quality.
Previous study showed that the phytotoxicity of sewage sludge was always attributed to the presence of ammonia, volatile organic acids (VOA), phenolic compounds, salts and heavy metals.30,31 Brinton reported that VOA in plant growth media as low as 300–500 ppm could make a phytotoxic influence on plant seedling, mainly through the ways of nutrient-ion leakage and root suppression.32 McLachlan et al. showed that high soluble salts, which could be reflected by EC value, in the extracts of digestates may be an important component of phytotoxicity.33 During composting process, short-chain volatile fatty acids, primarily acetic acid, was found to cause the phytotoxic effects of immature compost and the inhibitory effect of acetic acid on seed germination and root growth was a metabolic phenomenon.34 In the present study, Fig. 1 showed that the EC, TAN and VFA contents of the ADS decreased after micro-aerobic digestion, which might be main factors to cause the improvement of the ES phytotoxicity. Meanwhile, the ES at 55 °C (ES-55) had higher VFA contents than the ES at other temperatures, which might be an important reason for its higher inhibition on the germination of the seeds (Fig. S6 of the ESI†). Additionally, Himanen et al.3 found that the phytotoxicity had an increasing trend with the increase of VFA molecule chain length. Relatively high iso-valeric acid concentration was also observed in ES-55 (Fig. S6 of the ESI†), which may be another reason for the ES-55 possessing the high inhibition.
4. Conclusions
Micro-aerobic digestion promoted further stabilization of the high-solid ADS organic matter, and significant improvement of the seed germination and seedling growth. The protein-like and polysaccharides-like groups in the ADS reduced, and the humic acid-like and carboxyl materials had an increasing trend after micro-aerobic digestion. The microbial community had a distinctive succession from anaerobic bacteria to aerobic bacteria. However, temperature had a positive effect on the further stabilization of the ADS and an adverse impact on the microbial succession and phytotoxicity improvement. In consideration of sludge stabilization, phytotoxicity reduction and energy consumption, mesophilic micro-aerobic digestion appears more feasible process for the high-solid ADS post-treatment, compared with the room-temperature and thermophilic processes.Acknowledgements
The work was financially supported by the National Natural Scientific Foundation of China (51408423), National Water Pollution Control and Management Technology Major Projects (2013ZX07315003), National Key Technology Support Program (2014BAC29B01) and Postdoctoral Science Foundation of China (2015T80452).References
- X. Li, X. Dai, J. Takahashi, N. Li, J. Jin, L. Dai and B. Dong, Bioresour. Technol., 2014, 159, 412–420 CrossRef CAS PubMed.
- M. Bustamante, J. Alburquerque, A. Restrepo, C. De la Fuente, C. Paredes, R. Moral and M. Bernal, Biomass Bioenergy, 2012, 43, 26–35 CrossRef CAS.
- M. Himanen, P. Prochazka, K. Hänninen and A. Oikari, Chemosphere, 2012, 88, 426–431 CrossRef CAS PubMed.
- G. Ofosu-Budu, J. Hogarh, J. Fobil, A. Quaye, S. Danso and D. Carboo, Resour., Conserv. Recycl., 2010, 54, 205–209 CrossRef.
- P. Jenicek, J. Koubova, J. Bindzar and J. Zabranska, Water Sci. Technol., 2010, 62, 427–434 CrossRef CAS PubMed.
- M. Zhu, F. Lü, L.-P. Hao, P.-J. He and L.-M. Shao, Waste Manage., 2009, 29, 2042–2050 CrossRef CAS PubMed.
- N. Jin, B. Jin, N. Zhu, H. Yuan and J. Ruan, Bioresour. Technol., 2015, 175, 120–127 CrossRef CAS PubMed.
- X. Li, M. Xing, J. Yang, L. Zhao and X. Dai, J. Hazard. Mater., 2013, 261, 491–499 CrossRef CAS PubMed.
- H. Liu, G.-Q. Luo, H.-Y. Hu, Q. Zhang, J.-K. Yang and H. Yao, J. Hazard. Mater., 2012, 235, 298–306 CrossRef PubMed.
- B. Liao, H. Lin, S. Langevin, W. Gao and G. Leppard, Water Res., 2011, 45, 509–520 CrossRef CAS PubMed.
- L. Hao, S. Liss and B. Liao, Water Res., 2016, 89, 132–141 CrossRef CAS PubMed.
- X. Li, X. Dai, S. Yuan, N. Li, Z. Liu and J. Jin, Bioresour. Technol., 2015, 175, 245–253 CrossRef CAS PubMed.
- P. Oleszczuk, A. Malara, I. Jośko and A. Lesiuk, Water, Air, Soil Pollut., 2012, 223, 4937–4948 CrossRef CAS PubMed.
- H. M. Jang, S. K. Park, J. H. Ha and J. M. Park, Bioresour. Technol., 2013, 145, 80–89 CrossRef CAS PubMed.
- L. Shao, T. Wang, T. Li, F. Lü and P. He, Bioresour. Technol., 2013, 140, 131–137 CrossRef CAS PubMed.
- APHA, Standard methods for the examination of water and wastewater, American Public Health Association (APHA), Washington, DC, USA, 21st edn, 2005 Search PubMed.
- M. Xing, X. Li, J. Yang, Z. Huang and Y. Lu, J. Hazard. Mater., 2012, 205, 24–31 CrossRef PubMed.
- L. Ye, M. F. Shao, T. Zhang, A. H. Y. Tong and S. Lok, Water Res., 2011, 45, 4390–4398 CrossRef CAS PubMed.
- OECD, in OECD Guidelines for the Testing of Chemicals, 2006 Search PubMed.
- X. Li, X. Dai, L. Dai and Z. Liu, RSC Adv., 2015, 5, 82087–82096 RSC.
- E. Romero, C. Plaza, N. Senesi, R. Nogales and A. Polo, Geoderma, 2007, 139, 397–406 CrossRef CAS.
- N. Kumar, Master Science, the Virginia Polytechnic Institute and State University, 2006.
- T. Zhang, M.-F. Shao and L. Ye, ISME J., 2012, 6, 1137–1147 CrossRef CAS PubMed.
- C. Sundberg, W. A. Al-Soud, M. Larsson, E. Alm, S. S. Yekta, B. H. Svensson, S. J. Sørensen and A. Karlsson, FEMS Microbiol. Ecol., 2013, 85, 612–626 CrossRef CAS PubMed.
- M. Danon, I. H. Franke-Whittle, H. Insam, Y. Chen and Y. Hadar, FEMS Microbiol. Ecol., 2008, 65, 133–144 CrossRef CAS PubMed.
- J. Lalucat, A. Bennasar, R. Bosch, E. García-Valdés and N. J. Palleroni, Microbiol. Mol. Biol. Rev., 2006, 70, 510–547 CrossRef CAS PubMed.
- K. Ueda, A. Yamashita, J. Ishikawa, M. Shimada, T.-o. Watsuji, K. Morimura, H. Ikeda, M. Hattori and T. Beppu, Nucleic Acids Res., 2004, 32, 4937–4944 CrossRef CAS PubMed.
- D. Sousa, M. Pereira, J. Alves, H. Smidt, A. Stams and M. Alves, Water Sci. Technol., 2008, 57, 439–444 CrossRef CAS PubMed.
- A. Slobodkin, T. Tourova, N. Kostrikina, A. Lysenko, K. German, E. Bonch-Osmolovskaya and N.-K. Birkeland, Int. J. Syst. Evol. Microbiol., 2006, 56, 369–372 CrossRef CAS PubMed.
- M. Garcia-Sanchez, I. Garrido, J. Casimiro Ide, P. J. Casero, F. Espinosa, I. Garcia-Romera and E. Aranda, Chemosphere, 2012, 89, 708–716 CrossRef CAS PubMed.
- M. F. Drennan and T. D. DiStefano, Bioresour. Technol., 2010, 101, 537–544 CrossRef CAS PubMed.
- W. F. Brinton, Compost Sci. Util, 1998, 6, 75–82 CrossRef.
- K. L. McLachlan, C. Chong, R. P. Voroney, H. W. Liu and B. E. Holbein, in Sustainability of Horticultural Systems in the 21st Century, ed. L. Bertschinger and J. D. Anderson, 2004, pp. 225–230 Search PubMed.
- A. Shiralipour, D. B. McConnell and W. H. Smith, Compost Sci. Util., 1997, 5, 47–52 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: One table showing the summary of high-throughput sequencing data of the samples; one figure showing the contribution of nitrification/denitritation and stripping ways to TAN reduction; one figure showing the distribution of excitation–emission maxima; one figure showing the XPS full spectra; one figure showing the rarefaction and Shannon diversity curves; one figure showing the inhibition of germination index in APT and fresh weight in SPT; one figure showing the changes in the VFA contents. See DOI: 10.1039/c6ra11964f |
| This journal is © The Royal Society of Chemistry 2016 |