A simple ‘in situ’ co-precipitation method for the preparation of multifunctional CoFe2O4–reduced graphene oxide nanocomposites: excellent microwave absorber and highly efficient magnetically separable recyclable photocatalyst for dye degradation†
Debabrata Moitraa,
Madhurya Chandela,
Barun Kumar Ghosha,
Raj Kumar Janib,
Manoj Kumar Patrab,
Sampat Raj Vaderab and
Narendra Nath Ghosh*a
aNanomaterials Lab, Department of Chemistry, Birla Institute of Technology and Science, Pilani K.K. Birla Goa Campus, Zuarinagar, Goa 403726, India. E-mail: naren70@yahoo.com; Fax: +91 832 2557033; Tel: +91 832 2580318
bDefence Lab, Jodhpur 342011, India
First published on 9th August 2016
Abstract
Here, an ‘in situ’ co-precipitation reaction method has been reported for the preparation of CoFe2O4–RGO (CF–RGO) nanocomposites. To the best of our knowledge, this is the first time a simple synthetic method is reported for the preparation of CoFe2O4–RGO nanocomposites where a hydrothermal technique was not used. The novelty of this technique lies in its simplicity, cost-effectiveness, and the capability of large scale production of CoFe2O4–RGO nanocomposites. The synthesized CoFe2O4–RGO nanocomposites possess excellent microwave absorbing properties as well as high photocatalytic activity towards the degradation of various dyes under visible light irradiation. 85CF–15RGO (85 wt% CF and 15 wt% RGO) showed excellent microwave absorption properties with a Reflection Loss (RL) of −31.31 dB (∼99.94% absorption) at 9.05 GHz with an 8.2–10.92 GHz effective band width range. To the best of our knowledge 85CF–15RGO nanocomposite exhibited comparable and even superior microwave absorption properties in the X-band region than most of the ferrite based composites. 75CF–25RGO (75 wt% CF and 25 wt% RGO) acted as a very good magnetically separable photocatalyst for the degradation of various synthetic dyes (such as methyl orange, methylene blue, rhodamine B and a mixture of these dyes) under visible light irradiation emitted from a 100 W reading lamp. Moreover, CoFe2O4–RGO catalyst also showed easy magnetic separation with high reusability. The photocatalytic activity of 75CF–25RGO was found to be comparable and in some cases better than the various reported RGO–ferrite composites. The simple method of preparation and multifunctional character make CF–RGO nanocomposites attractive materials for application in the area of photocatalysis as well as microwave absorption.
1. Introduction
Performance, functionality and durability are the keywords in today's scientific and technological research in the area of materials science. Keeping these points in mind, scientists are developing new multifunctional materials, which can be used in different areas of life science, energy, environment, etc.1–3 It has already been recognized that graphene and graphene based materials have the potential to be employed in plethora of applications due to their two-dimensional sheet structure where sp2 hybridized carbon atoms form a hexagonal network.4 Graphene possesses a large surface area (∼2600 m2 g−1),5 high Young's modulus (1 TPa), high electron mobility (2.5 × 105 cm2 V−1 s−1), high thermal conductivity (5000 W m−1 K−1) and excellent chemical durability.4,6 Hence, by exploiting these interesting properties of graphene opportunities exist to design multifunctional materials which can be utilized in more than one application. We have targeted to synthesize graphene-based nanocomposites which can be used as microwave absorber as well as a photocatalyst to degrade various synthetic dyes, present in industrial waste water. Recently different RGO based composites have demonstrated their capability to act as photocatalyst.7–10We have fixed the objective of our current research in this direction because of the following reasons: (i) extensive usage of mobile phones, personal computers, local area network, radar, etc. in modern life style causes over exposure of electromagnetic waves to human bodies as well as living beings. As a result several harmful effects to biological systems, such as possibility of cancer, damage of DNA strands in brain cells, weakening of immune system, fluctuation of heart rate, etc. are increasing day by day.11 Moreover, problems associated with electromagnetic interference (EMI) are increasing in electronic and military communication systems due to increase of usage of electronic devices and their component sensitivity. Light weight Radar Absorbing Materials (RAM) are in great demand in both commercial and military applications because of their promising applications in stealth technology of aircraft, television image interference of high rise buildings, near field absorber, anechoic chamber, etc.12,13 Hence, development of a microwave absorber which is capable of absorbing microwave in X-band region is very important. (ii) Water pollution caused by the colored effluents, discharged from various dye based industries (such as textile, printing, leather, cosmetic, etc.) is increasing day by day and poses major threat to human life and water ecology. Most of the synthetic dyes are not only carcinogenic and mutagenic in nature but also their strong color creates serious aesthetic and ecological problems to the receiving aquatic ecosystem, such as inhibition of benthic photosynthesis.14 Hence, decolorization/degradation of these colored effluents have immerged as one of the important areas of research. For the last one decade several attempts have been made to address this issue and as a result various types of catalysts have been reported which had shown their capabilities to reduce/decolorize these dyes to their colorless forms. Various metal nanoparticles (e.g. Pt, Au, Ag, Ru, Cu, etc.) have been reported as catalyst for reduction of dyes.15–20 However, due to their nanosize separation of these catalysts from the reaction mixture by easy filtration is not possible and this factor limits their large scale application. Another popular approach is photo degradation of these dyes under UV or visible light in presence of a photocatalyst (such as TiO2, ZnO, etc.).21–26 Here also, separation of TiO2 or ZnO nanoparticles remains as a challenging issue. Moreover, in most of the cases usage of UV light also limits their applications. In case of photo-Fenton based advanced oxidation process generation of iron sludge, which is a secondary pollutant, is a major limitation. As photo degradation of dyes is a relatively clean process, there is a demand to develop suitable photocatalyst which not only will exhibit high photocatalysis efficiency towards degradation of dyes but also will be able to show its activity under visible light along with easy separation from the reaction mixture. Therefore, to address the above mentioned issues, we have synthesized nanocomposites composed of CoFe2O4 (CF) nanoparticles and reduced graphene oxide (RGO). These CoFe2O4–reduced graphene oxide (CF–RGO) nanocomposites are expected to exhibit good microwave absorption property in X-band region as well as photocatalytic efficiency towards degradation of synthetic dyes under visible light irradiation.
Microwave absorption properties of various types of magnetic materials, particularly ferrites are well reported in the literature, where the working mechanism is magnetic loss and depends upon the magnetic properties of the materials.11,27–35 However, magnetic materials are incapable of producing very high dielectric loss and magnetic loss simultaneously, which limits their applications as microwave absorber. Moreover, the requirements of high weight ratios of ferrite powders (∼60–80%) in the microwave absorbing coating and higher thickness are major limitations of usage of pure ferrites as light weight microwave absorber.30 Recently, reduced graphene oxide–ferrite nanocomposites have shown their promising prospect in this application. The presence of residual defects and functional groups on the surface of RGO sheets, its large aspect ratio and high conductivity in combination with excellent magnetic properties of ferrite nanoparticles make them promising candidates for fabrication of light weight microwave absorbers.36,37
Though different ferrite–RGO composites have been reported in the literature as microwave absorber and catalyst, but to the best of our knowledge most of these composites have been prepared by employing hydrothermal or solvothermal technique.24,25,38–57 Fu et al.50 have reported a one step hydrothermal process to prepare CoFe2O4–graphene composites using NaHSO3 as reducing agent and 1-propyl-3-hexadecylimidazolium bromide as structure growth-directing agent. The reaction was carried in an autoclave at 150 °C for 15 h. This composite showed minimum RL of −25.8 dB at 16.7 GHz. A vapor diffusion method48 was reported by the same group for preparation of CoFe2O4 hollow sphere–graphene composites. Here, the composite was prepared by hydrothermal treatment of a mixture of CoCl2, FeCl3, PVP and GO at 160 °C for 24 h in presence of urea, followed by the annealing the precipitate at 550 °C in argon atmosphere. This composite showed minimum RL of −18.5 dB at 12.9 GHz. Recently Jian et al.58 have reported Fe3O4–graphene capsules composites as microwave absorber which exhibited minimum reflection loss (RL) of −32 dB at 8.76 GHz. To prepare Fe3O4–graphene composites, graphene capsules were first prepared by CCVD (catalytic chemical vapor deposition) method, followed by hydrothermal treatment of a mixture of graphene, FeCl3, sodium acetate and ethylene glycol in a steel autoclave at 200 °C for 24 h. Gan et al.38 have reported the synthesis of CoFe2O4–graphene nanocomposites under hydrothermal condition at 180 °C for 12 h. This catalyst exhibited its efficiency to degrade methylene blue completely within 3 h when irradiated by a tungsten halogen lamp with 500 W. Fu et al.41 have prepared CoFe2O4–graphene photocatalyst using hydrothermal technique. Though hydrothermal technique is a popular method to prepare CoFe2O4–graphene composites, the requirement of a high pressure autoclave and operation temperature above ∼150 °C limit its application for the large scale synthesis of these composites. Hence, development of a simple synthetic route to prepare ferrite–RGO nanocomposites is in high demand.
In this paper, we have reported a simple ‘in situ’ co-precipitation reduction based synthetic methodology for preparation of CF–RGO nanocomposites. The synthesized materials were characterized by X-ray diffraction (XRD), High Resolution Transmission Electron Microscope (HRTEM), energy dispersive X-ray analysis (EDAX), Fourier transform-infrared (FT-IR), Raman spectroscopy, UV-vis diffuse reflectance spectra (DRS), thermogravimetric analysis (TGA). Magnetic properties of the synthesized CF–RGO nanocomposites were determined by using Vibrating Sample Magnetometer (VSM). Microwave absorption of the synthesized nanocomposites was measured by using a Vector Network Analyzer (VNA). Photocatalytic activity of CF–RGO nanocomposites was investigated towards degradation of various synthetic dyes (such as methyl orange (MO), Methylene Blue (MB), Rhodamine B (RhB) as well as mixture of dyes), under visible light irradiation, generated from a 100 W reading lamp and the reactions were monitored using a UV-vis spectrophotometer.
2. Experimental
2.1. Materials
The chemicals used for synthesis of CF–RGO nanocomposites and photocatalysis tests are cobalt nitrate Co(NO3)2·6H2O (Merck, India), iron nitrate Fe(NO3)3·9H2O (Merck, India), sodium hydroxide (NaOH) (Merck, India), sodium nitrate (NaNO3) (Merck, India), sulphuric acid (H2SO4) (Merck, India), potassium permanganate (KMnO4) (Merck, India), 30% H2O2 (Merck, India), polyethylene glycol 400 (PEG 400) (s d fine-chem limited). Methyl orange (Fisher scientific), rhodamine-B (Fluka analytical), methylene blue (Fluka analytical), graphite powder (Sigma Aldrich with mean particle size of <20 μm), acetylene black (Sigma Aldrich), 1-methyl-2-pyrrolidinone (Sigma Aldrich) and poly(vinylidene fluoride) (Sigma Aldrich, Mw-180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000). All chemicals were used as received and without further purification. De-ionized water was used throughout the experiment.
000). All chemicals were used as received and without further purification. De-ionized water was used throughout the experiment.
2.2. Synthesis of CoFe2O4–Reduced graphene oxide (CF–RGO) nanocomposites
CoFe2O4–RGO nanocomposites were synthesized by employing an ‘in situ’ co-precipitation reduction method using following steps: (i) in a round bottomed flask stoichiometric amount of Co(NO3)2·6H2O and Fe(NO3)3·9H2O were mixed with a PEG and H2O mixture (PEG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O weight ratio = 1
H2O weight ratio = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). (ii) In a separate beaker calculated amount of graphene oxide (prepared by modified Hummer's method)59 was well dispersed in de-ionized water using an ultrasonicator. (iii) This graphene oxide dispersion was then added to the mixture, which was prepared in step (i). (iv) In this mixture an aqueous NaOH (2 M) solution was added drop wise till pH was reached to ∼11. (v) Then the temperature of the reaction mixture was raised to 160 °C and refluxed for 16 h. (vi) After reflux the reaction mixture was allowed to cool down at room temperature. The black colour solid thus formed was separated from mixture by applying a bar magnet externally. This solid was then washed with de-ionized water for several times, till pH of the washing became ∼7. This solid was then dried at 60 °C for 10 h. Using this protocol several CF–RGO nanocomposites, having different weight ratios of CF and RGO, were prepared. Now onwards, nanocomposites having 5, 10, 15, 20, 25 and 50 wt% RGO content will be referred as 95CF–5RGO, 90CF–10RGO, 85CF–15RGO, 80CF–20RGO, 75CF–25RGO and 50CF–50RGO respectively. Using this method pure CoFe2O4 (CF) nanoparticles were also synthesized where GO was not mixed. Except this all parameters were kept same. CF nanoparticles thus obtained were used as reference for magnetic, microwave absorption and photocatalytic activity studies.
5). (ii) In a separate beaker calculated amount of graphene oxide (prepared by modified Hummer's method)59 was well dispersed in de-ionized water using an ultrasonicator. (iii) This graphene oxide dispersion was then added to the mixture, which was prepared in step (i). (iv) In this mixture an aqueous NaOH (2 M) solution was added drop wise till pH was reached to ∼11. (v) Then the temperature of the reaction mixture was raised to 160 °C and refluxed for 16 h. (vi) After reflux the reaction mixture was allowed to cool down at room temperature. The black colour solid thus formed was separated from mixture by applying a bar magnet externally. This solid was then washed with de-ionized water for several times, till pH of the washing became ∼7. This solid was then dried at 60 °C for 10 h. Using this protocol several CF–RGO nanocomposites, having different weight ratios of CF and RGO, were prepared. Now onwards, nanocomposites having 5, 10, 15, 20, 25 and 50 wt% RGO content will be referred as 95CF–5RGO, 90CF–10RGO, 85CF–15RGO, 80CF–20RGO, 75CF–25RGO and 50CF–50RGO respectively. Using this method pure CoFe2O4 (CF) nanoparticles were also synthesized where GO was not mixed. Except this all parameters were kept same. CF nanoparticles thus obtained were used as reference for magnetic, microwave absorption and photocatalytic activity studies.
2.3. Characterization
Room temperature powder X-ray diffraction (XRD) pattern of the synthesized nanopowder was recorded using a powder X-ray diffractometer (Mini Flex II, Rigaku, Japan) with Cu Kα (λ = 0.15405 nm) radiation at a scanning speed of 2° min−1. Thermogravimetric analysis (TGA) analysis were carried out using DTA-60 (Shimaduzu, Japan). High Resolution Transmission Electron Microscopy (HRTEM) images of samples were obtained using JEOL JEM 1400, Japan. Energy Dispersive X-ray (EDX) spectra of the synthesized material was recorded using Carl-Zeiss EVOMA15 (Carl-Zeiss, Germany) electron microscope. Fourier transform-infrared (FT-IR) spectra were recorded in KBr by using FT-IR spectrophotometer (Model Shimadzu DR-8031). Raman spectra were taken on a Renishawin Via Raman microscope with a 633 nm laser excitation. UV-vis diffuse reflectance spectra (DRS) analysis was recorded using JASCO V-770 spectrophotometer and energy band gap was calculated from the plot of Kubelka–Munk function versus photon energy. IVIUMSTAT (10 V/5 A/8 MHz) workstation was used to perform Electrochemical Impedance Spectroscopy (EIS) measurements of pure CoFe2O4 nanoparticles, GO and CF–RGO nanocomposites. The test electrode was fabricated by mixing 80 wt% of active materials, 10 wt% acetylene black and 10 wt% polyvinylidine difluoride in N-methyl-2-pyrrolidene. Then the slurry was coated on a Cu wire by dip coating using a dip coater Xdip-SV1 (Apex Eqipments) and vacuum dried at 60 °C for 24 h. A three-electrode cell was constructed using Ag/AgCl electrode as the reference electrode, the as prepared electrode as working electrode and platinum wire electrode as counter electrode. EIS measurements were carried out in 3 M KOH solution. Room temperature magnetization with respect to external field was measured by using Vibrating Sample Magnetometer (VSM) (EV5, ADE technology, USA).2.4. Microwave absorption measurement
For the measurement of microwave absorption of the synthesized nanocomposites in X-band (8.2–12.4 GHz) range, HP 8510 Vector Network Analyzer (USA) was used and reflection loss (RL) was calculated using the measured values of complex permittivity and permeability. To prepare the samples for this purpose, nanocomposite powders were first mixed with aqueous solution of 10 wt% polyvinyl alcohol (PVA), which acted as a binder and the mixture was dried. This mixture was further ground to powders and then compressed under a pressure of 10 tons and shaped into rectangular pellet with size of 10.16 mm × 22.86 mm × 2 mm, so as to fit exactly into rectangular waveguide of X-band.2.5. Photocatalytic activity test
To evaluate the photocatalytic activity of the synthesized CF–RGO nanocomposites towards photo degradation of methyl orange (MO), methylene blue (MB), rhodamine B (RhB) and dye mixture, photocatalyst reactions were conducted under visible light in presence of H2O2. A 100 W reading lamp was used as visible light source. In a typical photocatalysis reaction, 50 mL aqueous solution of 20 mg L−1 MO (in case of MB and RhB the concentrations were 30 mg L−1 and 25 mg L−1 respectively) was mixed with 25 mg of catalyst in a 100 mL beaker. The mixture was then stirred mechanically in dark for 1 h to reach adsorption desorption equilibrium between the dye and catalyst. Then, 2 mL of 30% H2O2 was added to the reaction mixture and the lamp (placed at a distance of 10 cm from the reaction mixture) was turned on. This point was consider as the starting point (t = 0) of the photochemical reaction. The change of concentration of dye in the reaction mixture with time was monitored spectrophotometrically (UV-vis spectrophotometer, V-570 Jasco, Japan) by following the decrease of absorbance at λmax (λmax (MO) = 464 nm, λmax (RhB) = 554 nm and λmax (MB) = 663 nm). As the absorbance of the dye is proportional to its concentration, the ratio of absorbance of the dye At (measured at time t) to A0 (at t = 0) is equal to Ct/C0 (where Ct is the concentration of dye at time t and C0 is the initial concentration of the dye). After completion of the reaction the catalyst was separated magnetically from the reaction mixture, washed several times with water and dried for recycling. Each catalysis reaction was performed three times and reproducible results were obtained.3. Results and discussion
3.1. Structure and morphology of CF–RGO nanocomposites
XRD patterns of CF, pure GO and CF–RGO nanocomposites are shown in Fig. 1. XRD of CF sample showed the diffraction peaks at 2θ = 18.23°, 30.35°, 35.72°, 37.83°, 43.32°, 53.94°, 57.20° and 62.89° corresponding to (111), (220), (311), (222), (400), (422), (511) and (440) planes of CoFe2O4 (JCPDS card no. 22-1086). XRD patterns of GO exhibited a strong peaks at 2θ = 9.76° and a small peak at 2θ = 42.14° for (001) and (101) planes of GO.60,61 In the XRD patterns of CF–RGO nanocomposites following important points were observed: | ||
| Fig. 1 Room temperature wide angle powder XRD patterns of (a) pure CF (b) 95CF–5RGO (c) 90CF–10RGO (d) 85CF–15RGO (e) 80CF–20RGO (f) 75CF–25RGO (g) 50CF–50RGO and (h) GO. | ||
(i) Presence of all the peaks corresponding to CF indicated the presence of CF nanoparticles in the composites, (ii) absence of peaks for GO (i.e. 2θ = 9.76° and 42.14°) indicated that during preparation of CF–RGO composites, GO flakes were converted to RGO and RGO sheets were exfoliated.42,47,55,61 This transformation of GO to RGO was also detected from the results obtained from TGA, FT-IR and Raman spectroscopy, which will be discussed later, (iii) no impurity peaks were detected, indicating the purity of CF–RGO nanocomposites. This is very important as presence of impurity phases significantly influences the magnetic, microwave absorption as well as photocatalytic activities of the composites, (iv) crystallite size of CF nanoparticles, calculated by Scherrer's equation using (311) diffraction plane, was ∼15 nm, (v) intensities of the peaks for CF were increased with increasing CF content in the composites.
Fig. 2 displays TEM micrographs of pure RGO (Fig. 2A and B), pure CF (Fig. 2C and D) and 85CF–15RGO nanocomposite (Fig. 2E and F). Fig. 2A shows a general view of RGO and a higher magnified image is shown in Fig. 2B. These micrographs exhibit the stacking of nanometer thin RGO flakes. Fig. 2C and D reveal that the average particle size of pure CF is ∼15–20 nm. TEM images in Fig. 2E and F depict the general view and higher magnified image of 85CF–15RGO sample as representative. These images show that CF nanoparticles are homogeneously embedded on the surface of nanometer thin RGO flakes. In HRTEM micrographs (Fig. 2G) well resolved lattice fringes corresponding to (311) plane of CF were observed. SAED patterns (Fig. 2H) also show Debye–Scherrer diffraction rings for pure CF. EDAX analysis of the synthesized nanocomposites also confirmed the compositions of these composites (Fig. S1 (ESI†)).
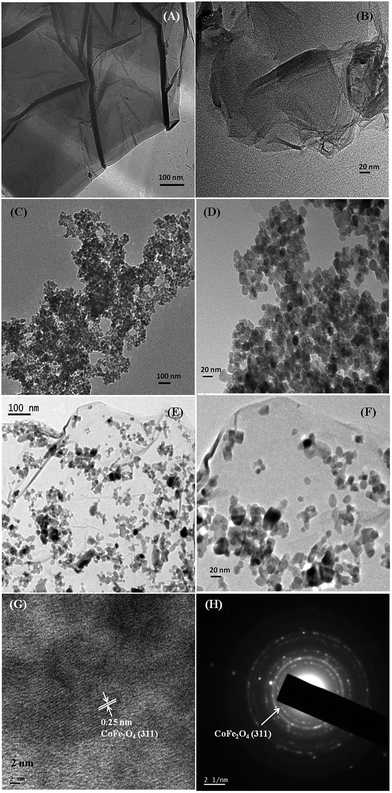 | ||
| Fig. 2 TEM micrographs of synthesized RGO (A) and (B), pure CoFe2O4 (C) and (D), 85CF–15RGO nanocomposites (E) and (F), HRTEM micrograph of 85CF–15RGO (G) and SAED pattern of 85CF–15RGO (H). | ||
TGA analysis of GO and CF–RGO was performed in air atmosphere in the temperature range of 30–850 °C with a heat rate of 10 °C min−1. Fig. 3 shows TGA thermograms of pure GO, pure CF and CF–RGO nanocomposites having various compositions. In TGA thermograms following points were observed: (i) pure CF is quite stable in the temperature range of 30–850 °C. (ii) In the temperature range of 30–100 °C, GO exhibited ∼17% wt loss, which might be due to evaporation of H2O.61 In this temperature range ∼9% wt loss occurred for 50CF–50RGO. (iii) In 100–200 °C temperature range, GO showed ∼3% wt loss and a sharp weight loss occurred in the range of 200–250 °C with 15% weight loss. This was due to the removal of oxygen containing groups from GO. However, only 9% wt loss was observed for 50CF–50RGO. This fact clearly indicated that during synthesis of CF–RGO, GO was converted to RGO via reduction of oxygen containing groups (e.g. carbonyl, carboxyl, epoxy groups, etc.)61 during reflux with NaOH (iv) in 325–600 °C temperature range, the oxidative decomposition of carbon atoms of GO was observed whereas, in case of 50CF–50RGO, this decomposition of carbon occurred in the range of 275–375 °C. It was also observed that, ∼50 wt% CF remained undecomposed as residue. Similarly for 85CF–15RGO and 75CF–25RGO nanocomposites 85 wt% and 75 wt% CF was remained as residue after thermal decomposition of the RGO component of the composites. So, we can also conclude that, this ‘in situ’ co-precipitation-reduction method for CF–RGO composite preparation is capable of producing composites with desired CF and RGO composition.
 | ||
| Fig. 3 TGA curves of (a) pure CF, (b) 85CF–15RGO (c) 75CF–25RGO (d) 50CF–50RGO nanocomposite and (e) GO. | ||
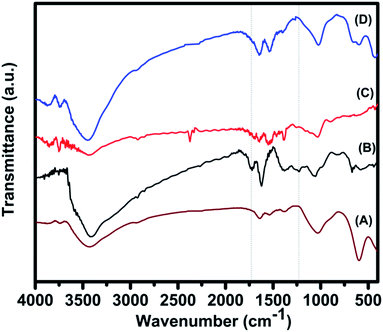
Fig. 4 shows FT-IR spectra of pure GO, pure CF pure RGO (prepared by refluxing GO at 160 °C for 16 h) and 85CF–15RGO. It was observed that, in case of GO peaks appeared at (i) 1384 cm−1 corresponding to the stretching vibration of C–O of carboxylic group, (ii) 1720 cm−1 for carbonyl group, (iii) 1226 cm−1 for C–O stretching vibration of epoxy group (iv) 1054 cm−1 for C–O stretching vibration.56,61 This fact indicated the presence of oxygen containing functional groups (e.g. epoxy, carbonyl, carboxyl and hydroxyl) on the surface of GO. Moreover, the peak at 1621 cm−1 can be assigned to the contribution from the skeletal vibration of the graphitic domains.39,48,61,62 In case of pure RGO the peak at 1720 cm−1 (for carbonyl group) was found to be disappeared and intensities of the peaks at 1226 and 1054 cm−1 (corresponding to C–O) have decreased. Appearance of a peak at 1544 cm−1 was also observed. The band at 1621 cm−1 (in GO sample), which can be assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C skeletal vibration of graphitic domains of GO, has been red shifted to 1544 cm−1 for RGO and indicated the partial restoration of π–π conjugation of graphene sheet in RGO.39 Absorption bands at the same positions were observed in FT-IR spectra of CF–RGO nanocomposite samples. Here also carboxylic group vibration band (νC
C skeletal vibration of graphitic domains of GO, has been red shifted to 1544 cm−1 for RGO and indicated the partial restoration of π–π conjugation of graphene sheet in RGO.39 Absorption bands at the same positions were observed in FT-IR spectra of CF–RGO nanocomposite samples. Here also carboxylic group vibration band (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1720 cm−1) was found to be disappeared and absorption intensities corresponding to C–O at 1226 and 1054 cm−1 were decreased. These results implied that, most of the oxygen containing groups of GO, particularly carboxyl groups, had been removed and some of the hydroxyl and epoxy groups were remained on the surface of RGO in CF–RGO nanocomposites. In the FTIR spectra of pure CF appearance of a peak at 591 cm−1, could be ascribed to lattice absorption of M–O (M = Fe3+, Co2+), confirmed the formation of CoFe2O4.49 Presence of this peak in CF–RGO samples indicated the existence of CoFe2O4 in CF–RGO nanocomposite.48,61
O at 1720 cm−1) was found to be disappeared and absorption intensities corresponding to C–O at 1226 and 1054 cm−1 were decreased. These results implied that, most of the oxygen containing groups of GO, particularly carboxyl groups, had been removed and some of the hydroxyl and epoxy groups were remained on the surface of RGO in CF–RGO nanocomposites. In the FTIR spectra of pure CF appearance of a peak at 591 cm−1, could be ascribed to lattice absorption of M–O (M = Fe3+, Co2+), confirmed the formation of CoFe2O4.49 Presence of this peak in CF–RGO samples indicated the existence of CoFe2O4 in CF–RGO nanocomposite.48,61
Raman spectra of CF–RGO composites (Fig. 5A and B) also confirmed the presence of RGO in the composites. In Raman spectra of pure GO, characteristic peaks for D and G band were found at 1355 and 1599 cm−1 respectively. In case of 85CF–15RGO samples these peaks were appeared at 1349 and 1580 cm−1. It has been reported that Raman shift of D and G bands shifts to lower values when GO is reduced to RGO.42 ID/IG ratio of 85CF–15RGO was found to be ∼1.06 where as this ratio was ∼0.9 for pure GO.48 This increase of ID/IG value for 85CF–15RGO can be attributed to the decrease in the average size of sp2 domains upon reduction of GO during formation of CF–RGO composite.36,48
The results obtained from XRD, TGA, HRTEM, FT-IR and Raman spectroscopic measurement clearly indicated that when the mixture of GO, metal nitrates and NaOH were refluxed for 16 h at 160 °C, two reactions proceeded simultaneously (i) reduction of GO to RGO and (ii) formation of CoFe2O4 nanoparticles.
Formation of CoFe2O4 nanoparticles can be presented by the following reactions:
| Fe3+ + 3OH− → FeOOH + H2O | (1) |
 | (2) |
The growth mechanism of CoFe2O4 nanoparticles in co-precipitation technique has been described in our previous paper.63
Formation of CF–RGO nanocomposites has been illustrated in Scheme 1.
As the formation of CF and RGO occurred simultaneously their interfacial interaction was expected to be superior to the composites where RGO and CoFe2O4 were prepared separately. As a consequence CF nanoparticles were well dispersed on the surface of RGO. Presence of PEG in the reaction mixture during CF formation restricted the formation of large CF particles or agglomeration of CF nanoparticles.
Room temperature magnetic property measurement of synthesized CF and CF–RGO nanocomposites (Fig. 6) by vibrating sample magnetometer (VSM) showed that, saturation magnetization (Ms) and coercivity (Hc) values for pure CF were decreased. In these composites presence of nonmagnetic RGO along with several other factors (e.g., attachment of CF nanoparticles on the surface of RGO sheets which might influence particle surface spin, disordered surface spin structure, dipolar inter particle interactions, etc. of CF nanoparticle)48,64–66 play important roles in determining their Ms values. This also indicated that, the magnetic properties of these composites materials could also be tuned by changing the ratio of metal nitrate salts to GO.67
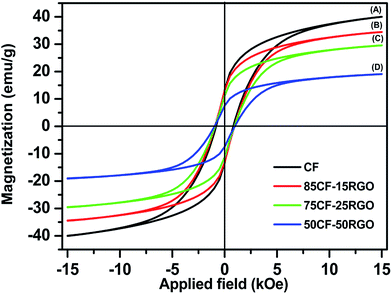 | ||
| Fig. 6 Room temperature magnetic hysteresis loops for (A) pure CF, (B) 85CF–15RGO, (C) 75CF–25RGO and (D) 50CF–50RGO. | ||
3.2. Microwaves absorption property of CF–RGO nanocomposites
The complex permittivity and permeability are usually used to analyze the dielectric and magnetic properties of absorber materials. Generally, real parts (ε′ and μ′) signify the storage capability of dielectric and magnetic energy, whereas the imaginary parts (ε′′ and μ′′) stand for the loss of dielectric and magnetic energy.28 From the measurement of ε′, ε′′, μ′ and μ′′ of pure CF and CF–RGO nanocomposites over the frequency range of 8.2 to 12.4 GHz (X-band) (Fig. 7) it was observed that (i) the values of ε′ and ε′′ were increased with increasing RGO content in the composite. This might be due to the fact that larger number of RGO sheets might enhance the electrical polarization and electrical conductivity of the samples because ε′ is an expression of the polarizability of materials and consists of dipolar polarization and electric polarization at microwave frequency.36,61,68 (ii) Over the frequency range ε′ and ε′′ remained almost constant. (iii) the values of μ′ and μ′′ were found to be increased with increasing CF content in the composite and the values were decreased with increasing frequency form 8.2–12.4 GHz. Therefore, it is suggested that both dielectric loss and magnetic loss play important roles in microwave absorption of CF–RGO nanocomposite.The reflection loss (RL) was calculated from the complex relative permeability and permittivity at a given frequency and specimen thickness using a model of single-layered plane wave absorber, proposed by Naito and Suetake.69
 | (3) |
 | (4) |
For CoFe2O4 nanocrystals and CF–RGO nanocomposites, having various compositions, reflection loss (RL) values were calculated (Fig. 8) and following important points were observed: (i) pure CF exhibited minimum RL value of −7.5 dB at 9.98 GHz when thickness was 3.2 mm (ii) with increasing RGO content in the composites minimum RL value was increased up to 15 wt% of RGO. Beyond 15 wt% of RGO content RL values were started to decrease. This might be due to the high permittivity value of composite having 20 wt% RGO (80CF–20RGO), which is harmful to the impedance match and leads to strong reflection resulting in weak absorption.48 (iii) Effective band width (i.e. RL < −10 dB and >90% absorption) was found to be increased with increasing RGO content in the composite. (iv) With increasing thickness of the absorber minimum RL values were increased up to certain value of thickness depending upon the composition of the composite. (v) With increasing thickness of the absorber the frequency, at which minimum RL was observed, was decreased. (vi) Composite having 85 wt% CF and 15 wt% RGO (85CF–15RGO) exhibited highest value of minimum RL of −31.31 dB (i.e. 99.94% absorption) at 9.05 GHz when thickness was 2.15 mm with effective band width in 8.2–10.92 GHz range. This composite also exhibited minimum 99.5% microwave absorption (>−26.6 dB) for all the thickness (1.9–2.4 mm) at various frequencies in X-band region. The frequencies, at which minimum RL values were observed, were decreased with increasing thickness. The optimum reflection loss can be achieved by impedance matching, when Zin = Z0, where Z0 and Zin are free space impedance and absorber impedance respectively63 and corresponding frequency and absorber thickness are known as matching frequency (fm) and matching thickness (tm). In the present case, Zin/Z0 was ∼0.98 (Z0 = 376.7 ohm (ref. 63) and Zin = 368.58 ohm (calculated value)), when fm was 9.05 GHz and tm was 2.15 mm.
Several factors play important roles in the enhancement of microwave absorption of CF–RGO nanocomposites such as (i) the existence of residual defects and functional groups in RGO, which favours the electromagnetic energy absorption,61,68 (ii) the high aspect ratio and high conductivity of RGO sheets provide its better absorbing ability,61 (iii) the interfaces between RGO sheets and CF nanoparticle, which cause the interfacial polarization (known as Maxwell–Wagner polarization)68 and associated relaxation contribute to the dielectric loss of the CF–RGO nanocomposites.
These important intrinsic physical properties of the hierarchical structure of the CF–RGO nanocomposites are responsible for their stronger microwave absorption ability than pure CF and pristine RGO. To the best of our knowledge 85CF–15RGO nanocomposite exhibited comparable and even superior microwave absorption property than most of the ferrite based composite in X-band region.12,28,29,31,33,47–54,61,68,70–85 Table S1 (ESI†) summarizes microwave absorption properties of some of the ferrite and ferrite based composites.
3.3. Photocatalytic activity of CF–RGO nanocomposites
The photocatalytic activities of CoFe2O4 nanocrystals and CF–RGO nanocomposites were evaluated by the degradation of dyes (MO, RhB, MB and mixture of dyes) in presence of H2O2 under visible light irradiation. The UV-vis spectra of these photocatalysis reactions are shown in Fig. 9. It has been observed that almost no photocatalysis reaction occurred in absence of catalyst. Pure CoFe2O4 nanocrystals and RGO also exhibited their inertness as catalyst towards these photocatalysis reactions. Photocatalysis reaction did not occur in presence of (i) only visible light irradiation but absence of any catalyst and H2O2, (ii) visible light irradiation and H2O2 but without CF or catalyst, (iii) visible light irradiation, H2O2 and CF (iv) visible light irradiation and 75CF–25RGO but without H2O2 (Fig. S2, ESI†), whereas the nanocomposites (CF–RGO) showed dramatically enhancement of photocatalytic activity in presence of visible light and H2O2 (Fig. 10A). The photocatalytic activity of CF–RGO was increased with increasing RGO content in composites till 25 wt% of RGO. The times required to degrade MO completely for the catalyst having 5, 10, 15 and 25 wt% RGO were 270, 150, 120 and 60 min respectively. Further increase of RGO content caused to increase the completion time of the photocatalysis reaction. Fig. 10A shows the effect of different catalysts on photocatalytic degradation of MO. It has also been observed that the time required to complete photo-degradation of MO has decreased with increasing catalyst dose up to 500 mg L−1 and then no significant decrease of time was observed when more catalyst was used (Fig. 10B). Similarly use of 2 mL of H2O2 was found to be optimum amount for the photocatalysis reaction (Fig. 10C).Hence to conduct photocatalysis reactions 500 mg L−1 of catalyst dose and 2 mL of H2O2 were used for all the catalysis reactions. The photo degradation rates of MO using different CF–RGO nanocomposites under visible light irradiation decreased in the following order 75CF–25RGO > 85CF–15RGO > 90CF–10RGO > 50CF–50RGO > 95CF–5RGO. When 75CF–25RGO was used as catalyst then times required to complete photo degradation of MO, MB, RhB and mixture of dyes by 75CF–25RGO were 60 min, 75 min, 45 min and 120 min respectively (Fig. 9).
As mentioned above CF–RGO nanocomposites exhibit higher photocatalytic activity than pure CF towards degradation of various dyes under visible-light irradiation. Fig. 11A shows the UV-vis absorption spectra, also named as diffuse reflectance spectra (DRS), of CF and 75CF–25RGO nanocomposite.
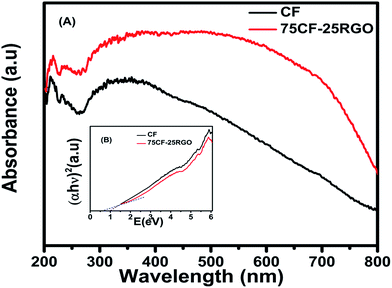 | ||
| Fig. 11 (A) UV-vis absorption spectra of CF and 75CF–25RGO nanocomposite and (B) the inset is the plot of transformed Kubelka–Munk function versus the energy of light. | ||
From absorption spectra it was clearly observed that, pure CF absorbs light in the entire range ∼200 to 800 nm. However, the nanocomposites composed of RGO and CF (75CF–25RGO), absorbs more light energy in both near UV (300–400 nm) region and visible light region (400–800 nm). These features clearly suggest that incorporation of RGO into the system plays an important role in the optical absorption behavior of these nanocomposites in the near UV region and visible light region. The optical absorption coefficient (α) near the band edge follows the Kubelka–Munk function86 as:
| αhν = A(hν − Eg)n/2 | (5) |
Upon irradiation with visible light CoFe2O4 nanoparticles yield electrons (e) and holes (h) by charge separation (reaction (6)).
| CoFe2O4 + hν → CoFe2O4(h + e) | (6) |
It was observed that CoFe2O4 is showing inertness as catalyst towards photo degradation reaction. Recombination of holes and electrons which are photo generated in CoFe2O4 might be the main reason for this. Presence of highly conductive RGO in CF–RGO prevents the recombination of holes and electrons because electrons are quickly transferred to RGO sheets via a percolation mechanism (reaction (7)).
| CoFe2O4(e) + RGO → CoFe2O4 + RGO(e) | (7) |
These electrons produce free radicals (˙OH), super oxides (O2˙−) by reacting with H2O2, H2O, dissolved O2 (reaction (8)–(12)). Holes (generated in reaction (6)) also produce ˙OH when react with OH−.87
| RGO(e) + O2 → O2˙− + RGO | (8) |
| O2˙− + H2O → HO2˙ + OH− | (9) |
| HO2˙ + H2O → H2O2 + ˙OH | (10) |
| H2O2 → 2˙OH | (11) |
| CoFe2O4(h) + OH− → CoFe2O4 + ˙OH | (12) |
These free radicals and superoxide anions oxidize the dye molecules, which are adsorbed on the surface of the catalyst (reaction (13)).
| Dye + ˙OH (and/or O2˙−) → intermediates → CO2 + H2O | (13) |
As shown in these reactions the RGO in RGO–CF catalysts prevents the hole–electron recombination, which in turns helps the formation of ˙OH, O2˙−. This explains the enhanced photocatalytic activity of CF–RGO nanocomposites.
To understand the synergistic effect between CF and RGO on the electrical conductivity of CF–RGO nanocomposites the electrochemical impedance measurements of pure CF, GO and CF–RGO nanocomposites were performed. The impedance plots of the synthesized materials are shown in Fig. 12. It was observed that, the impedance plot of 75CF–25RGO nanocomposite has a smaller radius than those of GO and pure CF. This fact indicates that, charge transfer resistance of 75CF–25RGO has significantly reduced and GO has been reduced to RGO during the synthesis of CF–RGO nanocomposites.38 This is because of graphene is a zero band gap semiconductor and a two dimensional π conjugated system in which charge carriers behave as mass less fermions, resulting in its unique electron transport properties.38,40,42 Thus, the photogenerated electrons of CF could transfer easily from the conduction band to RGO and rapidly transport the instant that they formed. As a result of the great inhibition for the recombination of photogenerated electrons and holes, the photocatalytic activity of CF–RGO was significantly enhanced.
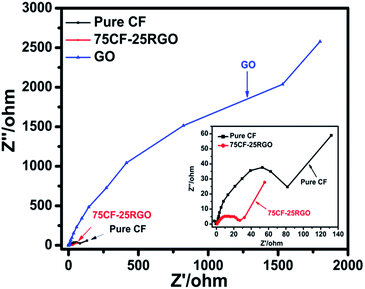 | ||
| Fig. 12 The electrochemical impedance spectra (EIS) of 75CF–25RGO, pure CF and GO. The spectrum in the inset is enlarged to show the impedance of 75CF–25RGO and pure CF. | ||
The lowering of band gap energy of CoFe2O4 in presence of RGO in CF–RGO nanocomposites and the photo degradation of dye molecules catalyzed by CF–RGO composite are illustrated in Scheme 2.
 | ||
| Scheme 2 Schematic illustration of the photocatalysis reaction mechanism of CF–RGO nanocomposites towards degradation of synthetic dye. | ||
75CF–25RGO exhibited high catalytic efficiency towards degradation of mixture of dyes. The dye mixture (MO, RhB and MB) was decolorized (Fig. 9D) within 120 min when treated with 75CF–25RGO ([catalyst] = 500 mg L−1, [MO] = 20 mg L−1, [MB] = 32 mg L−1 and [RhB] = 24 mg L−1 and [H2O2] = 40 mL L−1). After separating the catalyst from reaction mixture after reaction, catalyst was washed with alcohol and it was observed that no unreacted dye molecule was remained adsorbed in the catalyst indicating complete photo degradation of dyes. As CF is magnetic in nature, 75CF–25RGO nanocatalyst offers an additional advantage along with high catalytic activity.
The catalyst can be easily separable from the reaction mixture after completion of the reaction by using an external magnet. This easy magnetic separation of this catalyst also helps to overcome the limitation of separation problem associated with nanoparticle catalyst. Fig. 13 illustrates the compete decomposition of the dyes, resulting in decolorization of dye solution due to photocatalysis reaction and magnetic separation of the catalyst by employing a bar magnet externally.
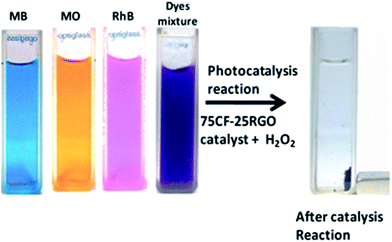 | ||
| Fig. 13 Decolorization of dye solutions due to photocatalysis reaction and magnetic separation of the catalyst by applying a magnet after completion of reaction. | ||
3.4. Reusability of CF–RGO nanocomposites
After completion of the reaction, the catalyst was separated by applying an external magnetic. Then the catalyst was washed thoroughly by de-ionized water several times and dried at 90 °C for 4 h and reused. The experiment was repeated for several times. The activity of the catalyst was found almost same up to 5 consecutive cycles (Fig. 14). XRD patterns and TEM micrograph of the reused catalyst also showed no significant change in their crystal structure as well as morphology compared to the fresh catalyst (Fig. 15A and B). | ||
| Fig. 14 Reusability of magnetically separable catalyst (75CF–25RGO) for the photo degradation of MO, MB and RhB. | ||
4. Conclusions
Here, we have described an ‘in situ’ co-precipitation reduction method for preparation of CF–RGO nanocomposites with various compositions. In this method reduction of GO to RGO and formation of CF nanoparticles from metal nitrates occurred simultaneously, which helped to anchor CF nanoparticles on the sheets of RGO homogenously.To the best of our knowledge, this is the first time a simple method is reported for preparation of CoFe2O4–RGO nanocomposites where hydrothermal technique was not used. Moreover, the methodology employed to prepare these nanocomposites is very simple and does not require any elaborate setup. It is important to note that; here NaOH acted the role of a precipitating agent during the formation of CoFe2O4 nanoparticles from metal ions, as well as a reducing agent to convert GO to RGO. In this synthetic methodology no other reducing agent was used. The novelty of this technique lies in its simplicity, cost-effectiveness and capability of large scale production of CoFe2O4–RGO nanocomposites. By employing this synthetic method a good light weight microwave absorber as well as a good magnetically separable catalyst can be prepared by simply tailoring the compositions of CF–RGO nanocomposite.
It has been demonstrated that, the synthesized CoFe2O4–RGO nanocomposites possess excellent microwave absorbing property as well as high photocatalytic activity towards degradation of various dyes under visible light irradiation generated from a 100 W reading lamp.
The heteroarchitectural structure of RGO–CF composites caused to enhance its catalytic property as well as microwave absorption property. Moreover, CoFe2O4–RGO catalyst also showed easy magnetic separation with high reusability.
The synthesized CF–RGO nanocomposites having 25 wt% RGO content (75CF–25RGO) exhibited high photocatalytic efficiency towards the decolorization of individual dyes as well as mixture of dyes under visible light generated from a 100 W bulb. The significant enhancement in photocatalytic activity of 75CF–25RGO than pure CF can be ascribed to the efficient separation of photogenerated carriers in the CF and RGO coupling system and synergistic effect of CF and RGO.41 The photocatalytic activity of 75CF–25RGO under visible light was found to be comparable and in some cases better than the various reported RGO–ferrite composites24–26,38–46,88 (Table S2 (ESI†)). Magnetic nature of 75CF–25RGO makes it a magnetically separable catalyst, which solves the separation related problems associated with the nanosized catalysts. It is believed that, these materials will find promising applications in the field of catalysis particularly waste water treatment.
85CF–15RGO also exhibited ∼99.94% minimum RL (−31.31 dB) at 9.05 GHz in X-band region when thickness was 2.15 mm with effective bandwidth in 8.2–10.92 GHz. To the best of our knowledge, the minimum RL value of 85CF–15RGO is comparable and even superior than most of the pure ferrites and ferrite–RGO composites.11,12,28–31,33,36,47–53,55,56,61,63,68,70–85 This nanocomposite not only shows higher minimum RL in comparison with pure CF but also its light weight, due to presence of RGO, offers an added advantage. The heteroarchitectural structure of RGO–CF composites caused to enhance its photocatalytic property as well as microwave absorption property. CF–RGO nanocomposites have demonstrated its capability to act as a multifunctional material as heterogeneous photocatalyst as well as microwave absorber.
Acknowledgements
Dr N. N. Ghosh gratefully acknowledges financial support from DRDO, New Delhi, India (ERIP/ER/1305004/M/01/1523). We also express our thanks to Prof. Paul A. Millner and Martin Fuller, University of Leeds, UK, for recording TEM micrographs.Notes and references
- X. An and C. Y. Jimmy, RSC Adv., 2011, 1, 1426 RSC.
- V. Sugathan, E. John and K. Sudhakar, Renewable Sustainable Energy Rev., 2015, 52, 54 CrossRef CAS.
- M. A. Oturan and J. J. Aaron, Crit. Rev. Environ. Sci. Technol., 2014, 44, 2577 CrossRef CAS.
- O. C. Compton and S. T. Nguyen, Small, 2010, 6, 711–723 CrossRef CAS PubMed.
- M. D. Stoller, S. Park, Y. Zhu, J. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498 CrossRef CAS PubMed.
- A. A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. N. Lau, Nano Lett., 2008, 8, 902 CrossRef CAS PubMed.
- L. Hu, S. Dong, Q. Li, J. Feng, Y. Pi, M. Liu, J. Sun and J. Sun, J. Alloys Compd., 2015, 633, 256 CrossRef CAS.
- S. Dong, J. Sun, Y. Li, C. Yu, Y. Li and J. Sun, Appl. Catal., B, 2014, 144, 386 CrossRef CAS.
- S. Dong, L. Hu, J. Feng, Y. Pi, Q. Li, Y. Li, M. Liu, J. Sun and J. Sun, RSC Adv., 2014, 4, 64994 RSC.
- S. Dong, Y. Pi, Q. Li, L. Hu, Y. Li, X. Han, J. Wang and J. Sun, J. Alloys Compd., 2016, 663, 1 CrossRef CAS.
- W. Zhu, L. Wang, R. Zhao, J. Ren, G. Lu and Y. Wang, Nanoscale, 2011, 3, 2862 RSC.
- J. Cao, W. Fu, H. Yang, Q. Yu, Y. Zhang, S. Liu, P. Sun, X. Zhou, Y. Leng and S. Wang, J. Phys. Chem. B, 2009, 113, 4642 CrossRef CAS PubMed.
- P. Xu, X. Han, J. Jiang, X. Wang, X. Li and A. Wen, J. Phys. Chem. C, 2007, 111, 12603 CAS.
- H. Lee, S. H. Park, Y.-K. Park, B. H. Kim, S. J. Kim and S. C. Jung, Chem. Cent. J., 2013, 7, 156 CrossRef PubMed.
- B. Naik, S. Hazra, V. S. Prasad and N. N. Ghosh, Catal. Commun., 2011, 12, 1104 CrossRef CAS.
- B. Naik, S. Hazra, D. Desagani, B. K. Ghosh, M. K. Patra, S. R. Vadera and N. N. Ghosh, RSC Adv., 2015, 5, 40193 RSC.
- Y.-C. Chang and D.-H. Chen, J. Hazard. Mater., 2009, 165, 664 CrossRef CAS PubMed.
- S. El-Sheikh, A. A. Ismail and J. F. Al-Sharab, New J. Chem., 2013, 37, 2399 RSC.
- B. K. Ghosh, S. Hazra, B. Naik and N. N. Ghosh, Powder Technol., 2015, 269, 371 CrossRef CAS.
- B. K. Ghosh, S. Hazra, B. Naik and N. N. Ghosh, J. Nanosci. Nanotechnol., 2015, 15, 6516 CrossRef PubMed.
- B. Li and H. Cao, J. Mater. Chem., 2011, 21, 3346 RSC.
- Y. Liang, X. He, L. Chen and Y. Zhang, RSC Adv., 2014, 4, 18132 RSC.
- Y. Liu, RSC Adv., 2014, 4, 36040 RSC.
- Z. Q. Li, H. L. Wang, L. Y. Zi, J. J. Zhang and Y. S. Zhang, Ceram. Int., 2015, 41, 10634 CrossRef CAS.
- X. Yang, W. Chen, J. Huang, Y. Zhou, Y. Zhu and C. Li, Sci. Rep., 2015, 5, 10632 CrossRef PubMed.
- C. Haw, W. Chiu, S. Abdul Rahman, P. Khiew, S. Radiman, R. Abdul Shukor, M. A. A. Hamid and N. Ghazali, New J. Chem., 2016, 40, 1124 RSC.
- P. Fannin, C. Marin, I. Malaescu, N. Stefu, P. Vlazan, S. Novaconi, P. Sfirloaga, S. Popescu and C. Couper, Mater. Des., 2011, 32, 1600 CrossRef CAS.
- S. Hazra, B. K. Ghosh, H. R. Joshi, M. K. Patra, R. K. Jani, S. R. Vadera and N. N. Ghosh, RSC Adv., 2014, 4, 45715 RSC.
- S. Hazra, B. K. Ghosh, M. K. Patra, R. K. Jani, S. R. Vadera and N. N. Ghosh, J. Nanosci. Nanotechnol., 2015, 15, 6559 CrossRef CAS PubMed.
- X. Huang, J. Zhang, S. Xiao and G. Chen, J. Am. Ceram. Soc., 2014, 97, 1363 CrossRef CAS.
- W. Fu, S. Liu, W. Fan, H. Yang, X. Pang, J. Xu and G. Zou, J. Magn. Magn. Mater., 2007, 316, 54 CrossRef CAS.
- L. Xi, Z. Wang, Y. Zuo and X. Shi, Nanotechnology, 2010, 22, 045707 CrossRef PubMed.
- S. Tyagi, H. B. Baskey, R. Agarwala, V. Agarwala and T. Shami, Trans. Indian Inst. Met., 2011, 64, 271 CrossRef CAS.
- R. Ji, C. Cao, Z. Chen, H. Zhai and J. Bai, J. Mater. Chem. C, 2014, 2, 5944 RSC.
- Y. Hwang, Mater. Lett., 2006, 60, 3277 CrossRef CAS.
- D. Chen, G. S. Wang, S. He, J. Liu, L. Guo and M. S. Cao, J. Mater. Chem. A, 2013, 1, 5996 CAS.
- C. Wang, X. Han, P. Xu, X. Zhang, Y. Du, S. Hu, J. Wang and X. Wang, Appl. Phys. Lett., 2011, 98, 072906 CrossRef.
- L. Gan, S. Shang, C. W. M. Yuen, S.-X. Jiang and E. Hu, Appl. Surf. Sci., 2015, 351, 140 CrossRef CAS.
- G. He, J. Ding, J. Zhang, Q. Hao and H. Chen, Ind. Eng. Chem. Res., 2015, 54, 2862 CrossRef CAS.
- J. Sun, Y. Fu, P. Xiong, X. Sun, B. Xu and X. Wang, RSC Adv., 2013, 3, 22490 RSC.
- Y. Fu, H. Chen, X. Sun and X. Wang, Appl. Catal., B, 2012, 111, 280–287 CrossRef.
- Y. Fu and X. Wang, Ind. Eng. Chem. Res., 2011, 50, 7210 CrossRef CAS.
- Y. Fu, P. Xiong, H. Chen, X. Sun and X. Wang, Ind. Eng. Chem. Res., 2012, 51, 725 CrossRef.
- S. Bai, X. Shen, X. Zhong, Y. Liu, G. Zhu, X. Xu and K. Chen, Carbon, 2012, 50, 2337 CrossRef CAS.
- D. Zhang, Q. Wang, L. Wang and L. Zhang, J. Mater. Chem. A, 2015, 3, 3576 CAS.
- Z.-T. Hu, J. Liu, X. Yan, W.-D. Oh and T.-T. Lim, Chem. Eng. J., 2015, 262, 1022 CrossRef CAS.
- Z. Yang, Y. Wan, G. Xiong, D. Li, Q. Li, C. Ma, R. Guo and H. Luo, Mater. Res. Bull., 2015, 61, 292 CAS.
- M. Fu, Q. Jiao, Y. Zhao and H. Li, J. Mater. Chem. A, 2014, 2, 735 CAS.
- M. Fu, Q. Jiao and Y. Zhao, J. Mater. Chem. A, 2013, 1, 5577 CAS.
- M. Fu, Q. Jiao and Y. Zhao, Mater. Charact., 2013, 86, 303 CrossRef CAS.
- M. Zong, Y. Huang, H. Wu, Y. Zhao, Q. Wang and X. Sun, Mater. Lett., 2014, 114, 52 CrossRef CAS.
- W.-D. Xue, R. Zhao, X. Du, F.-W. Xu, M. Xu and K.-X. Wei, Mater. Res. Bull., 2014, 50, 285 CrossRef CAS.
- H.-L. Xu, H. Bi and R.-B. Yang, J. Appl. Phys., 2012, 111, 07A522 Search PubMed.
- M. Zong, Y. Huang, N. Zhang and H. Wu, J. Alloys Compd., 2015, 644, 491 CrossRef CAS.
- X.-J. Zhang, G.-S. Wang, W.-Q. Cao, Y.-Z. Wei, J.-F. Liang, L. Guo and M.-S. Cao, ACS Appl. Mater. Interfaces, 2014, 6, 7471 CAS.
- G.-S. Wang, Y. Wu, Y.-Z. Wei, X.-J. Zhang, Y. Li, L.-D. Li, B. Wen, P.-G. Yin, L. Guo and M.-S. Cao, ChemPlusChem, 2014, 79, 375 CrossRef CAS.
- S.-Q. Liu, B. Xiao, L.-R. Feng, S.-S. Zhou, Z.-G. Chen, C.-B. Liu, F. Chen, Z.-Y. Wu, N. Xu and W.-C. Oh, Carbon, 2013, 64, 197 CrossRef CAS.
- X. Jian, B. Wu, Y. Wei, S. X. Dou, X. Wang, W. He and N. Mahmood, ACS Appl. Mater. Interfaces, 2016, 8, 6101 CAS.
- W. S. Hummers Jr and R. E. Offeman, J. Am. Ceram. Soc., 1958, 80, 1339 Search PubMed.
- X. Zhang, Z. Zhou and C. Lu, RSC Adv., 2015, 5, 20186–20192 RSC.
- M. Zong, Y. Huang, Y. Zhao, X. Sun, C. Qu, D. Luo and J. Zheng, RSC Adv., 2013, 3, 23638–23648 RSC.
- Y. Zhao, X. Song, Q. Song and Z. Yin, CrystEngComm, 2012, 14, 6710 RSC.
- D. Moitra, S. Hazra, B. K. Ghosh, R. K. Jani, M. K. Patra, S. R. Vadera and N. N. Ghosh, RSC Adv., 2015, 5, 51130 RSC.
- H. Lu, W. Zheng and Q. Jiang, J. Phys. D: Appl. Phys., 2007, 40, 320 CrossRef CAS.
- Z. Tang, C. Sorensen, K. Klabunde and G. Hadjipanayis, Phys. Rev. Lett., 1991, 67, 3602 CrossRef CAS PubMed.
- R. Frison, G. Cernuto, A. Cervellino, O. Zaharko, G. M. Colonna, A. Guagliardi and N. Masciocchi, Chem. Mater., 2013, 25, 4820 CrossRef CAS.
- Y. Zhan, F. Meng, X. Yang, R. Zhao and X. Liu, Mater. Sci. Eng., B, 2011, 176, 1333 CrossRef CAS.
- D. Moitra, B. Ghosh, M. Chandel, R. Jani, M. Patra, S. Vadera and N. Ghosh, RSC Adv., 2016, 6, 14090 RSC.
- Y. Naito and K. Suetake, IEEE Trans. Microwave Theory Tech., 1971, 19, 65 CrossRef.
- Y. Li, R. Yi, A. Yan, L. Deng, K. Zhou and X. Liu, Solid State Sci., 2009, 11, 1319 CrossRef CAS.
- K. Chen, C. Xiang, L. Li, H. Qian, Q. Xiao and F. Xu, J. Mater. Chem., 2012, 22, 6449 RSC.
- R. Che, C. Zhi, C. Liang and X. Zhou, Appl. Phys. Lett., 2006, 88, 3105 Search PubMed.
- S. Zhang, Q. Jiao, Y. Zhao, H. Li and Q. Wu, J. Mater. Chem. A, 2014, 2, 18033 CAS.
- L. Guo-Min, W. Lian-Cheng and X. Yao, Chin. Phys. B, 2014, 23, 088105 CrossRef.
- N. Gandhi, K. Singh, A. Ohlan, D. Singh and S. Dhawan, Compos. Sci. Technol., 2011, 71, 1754 CrossRef CAS.
- K. Khan, J. Supercond. Novel Magn., 2013, 27, 453 CrossRef.
- L. Wang, Y. Huang, X. Sun, H. Huang, P. Liu, M. Zong and Y. Wang, Nanoscale, 2014, 6, 3157 RSC.
- S. Hazra, B. K. Ghosh, M. K. Patra, R. K. Jani, S. R. Vadera and N. N. Ghosh, Powder Technol., 2015, 279, 10 CrossRef CAS.
- X. Huang, J. Zhang, W. Rao, T. Sang, B. Song and C. Wong, J. Alloys Compd., 2016, 662, 409 CrossRef CAS.
- M. Verma, A. P. Singh, P. Sambyal, B. P. Singh, S. Dhawan and V. Choudhary, Phys. Chem. Chem. Phys., 2015, 17, 1610 RSC.
- J. Zhao, J. Yu, Y. Xie, Z. Le, X. Hong, S. Ci, J. Chen, X. Qing, W. Xie and Z. Wen, Sci. Rep., 2016, 6, 20496 CrossRef PubMed.
- X. Liu, H. Guo, Q. Xie, Q. Luo, L.-S. Wang and D.-L. Peng, J. Alloys Compd., 2015, 649, 537 CrossRef CAS.
- B. Qu, C. Zhu, C. Li, X. Zhang and Y. Chen, ACS Appl. Mater. Interfaces, 2016, 8, 3730 CAS.
- K. Singh, A. Ohlan, V. H. Pham, R. Balasubramaniyan, S. Varshney, J. Jang, S. H. Hur, W. M. Choi, M. Kumar and S. Dhawan, Nanoscale, 2013, 5, 2411 RSC.
- Z. Durmus, A. Durmus and H. Kavas, J. Mater. Sci., 2015, 50, 1201 CrossRef CAS.
- P. Kubelka, Z. Tech. Phys., 1931, 12, 593 Search PubMed.
- J. Li, Q. Xiao, L. Li, J. Shen and D. Hu, Appl. Surf. Sci., 2015, 331, 108 CrossRef CAS.
- S. Zhuang, X. Xu, B. Feng, J. Hu, Y. Pang, G. Zhou, L. Tong and Y. Zhou, ACS Appl. Mater. Interfaces, 2013, 6, 613 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1 EDAX spectra of (A) synthesized CoFe2O4 nanoparticle and (B) CoFe2O4–RGO nanocomposite, Fig. S2: these C/C0 vs. Irradiation time plots show that photocatalysis reactions of dyes do not occur in absence of CF–RGO and H2O2. Table S1: microwave absorption properties of various ferrites and ferrite based composites, Table S2: comparison of photocatalytic activity of 75CF–25RGO nanocomposite with those of the other reported RGO–ferrite and RGO based nanocomposites. See DOI: 10.1039/c6ra17384e |
| This journal is © The Royal Society of Chemistry 2016 |