A lotus root inspired implant system with fever responsive characteristics and 3D printing defined nano-antibiotic release patterns†
Xingwei
Ding‡
a,
Xiaoyi
He‡
a,
Chaowen
Xue
a,
Changwen
Wu
b,
Lin
Xie
a,
Tingtao
Chen
a,
Junchao
Wei
c,
Xigao
Cheng
d and
Xiaolei
Wang
*a
aInstitute of Translational Medicine, Nanchang University, Nanchang, Jiangxi 330088, P. R. China. E-mail: wangxiaolei@ncu.edu.cn
bCollege of Medical, Nanchang University, Nanchang, Jiangxi 330031, P. R. China
cDepartment of Chemistry, Nanchang University, Nanchang, Jiangxi 330031, P. R. China
dFirst Department of Orthopedics, Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi 330006, P. R. China
First published on 28th June 2016
Abstract
Lotus root imitated implant scaffolds with highly flexible drug release patterns were fabricated. Several critical release parameters could be conveniently prearranged through creating a precisely customized inner chamber via 3D printing. Furthermore, when postoperative infection caused hyperthermia, the inner drugs could be spontaneously released to inhibit the invasive pathogens.
In recent years, three-dimensional (3D) printing has been attracting much attention in a wide range of areas, such as engineering, art and medicine.1 Notably, the customization of bone implants has always been denoted as one of the most promising applications of 3D printing.2 So far, about 4.4 million patients are eager for bone transplants yearly on the global scale.3 Unfortunately, around 1.5% to 3% patients would acquire postoperative infection. Unnecessary pain and cost would emerged in vast majority of “innocent” patients if antibiotics were used blindly. Furthermore, the overuse of antibiotics would also induce serials of resistant strains and drastic side effects.4 To overcome this problem, inspired by the lotus root, a columnar vegetable with different sized inner chambers, we employed 3D printing technology to construct customized implantable devices with different shaped inner chambers. Theoretically, by varying the structure of inner chamber, several critical releasing parameters could be conveniently prearranged, including drug loading amount, rate and curve. Besides, the drug releasing could be triggered by using appropriate thermo-sensitive hydrogel as a cap (Fig. 1). Under ordinary situation, the releasing of the encapsulated drugs could be precisely controlled by external heating source, such as infrared radiation. In case of an emergency, these inner drugs could be released spontaneously by severe infection induced hyperthermia.
 | ||
| Fig. 1 Schematic illustration of the fabrication of a 3D drug delivery implants for silver nanoparticles release in vitro and in vivo. | ||
To demonstrate the unique advantages of 3D printing for creating implants with highly flexible drug release patterns, we first designed three implants with different inner structures. As shown in Fig. 2, the height and outer diameter of three designed implants were 11 mm and 6 mm, and the internal structures were designed as cylindrical cavity with a diameter of 3.3 mm (Cy3.3) and 3.6 mm (Cy3.6) respectively (Fig. 2a and b). The inner structure of the third implant was designed to a spherical cavity (Sp5) combined with two cylindrical cavities (Cy3) (Fig. 2c). To present the internal structure visually, blue ink was painted to show the cross section of the three internal cavities (Fig. 2d–f). What's more, the inner and outer diameters of printed implants were shown in Fig. 2g and h. As shown in Fig. S1,† the mechanic strength of Cy3.3, Cy3.6 and Sp5&Cy3 were 80 MPa, 78 MPa and 60 MPa separately, it suggests that the mechanic strength could also be modified by changing the inner structure.
To confirm the drug release curve could be regulated with the customized inner structures, fluorescein isothiocyanate (FITC), used as model drugs, was injected into the above three implants. The drug loading amount could be set through pre-designing the structure of cavity with alterable volume. The FITC loading amount and released efficiency of three designed implants were shown in Table S1.† The volume and drug loading amount were approximated between Cy3.6 and Sp5&Cy3. As shown in Fig. 2i, compared with Cy3.3 and Sp5&Cy3, the amount of released FITC from Cy3.6 at the initial time for 2 min was much higher due to a larger calibre of Cy3.6. Subsequently, we found that the loaded FITC in Sp5&Cy3 was burst released for about 10 min. Meanwhile, Cy3.3 and Cy3.6 presented a mild release curve. It could be interpreted that the spherical structure of Sp5&Cy3 endowed a bigger pressure. After incubation for 1 h, two tails of the releasing curve, from Cy3.6 and Sp5&Cy3, were tended to be joined ascribed to the similar drug loading capacity. The results revealed that the releasing curve of loading drug could be adjusted through designing the internal structure of implants with similar volume.
Hyperthermia is one of the commonest symptoms of postoperative infection.5 According to this characteristic, the inner drugs were sealed with 1-tetradecanol, a thermo-sensitive and biocompatible phase change material (phase-transition temperature = 39 °C),6 to realize auto-releasing. To demonstrate the drug release behaviour of this system, FITC loaded Cy3.6 was exposed to phosphate buffered saline (PBS) at 37 °C and 39 °C, respectively. As shown in Fig. 3b, only limited FITC could be determinate from implant at 37 °C for 30 min. It suggests that FITC loaded implant has a good drug retaining efficiency at 37 °C. On the other hand, FITC could be triggered to release when the implant kept at 39 °C within 25 min, and burst release followed due to the liquidation of 1-tetradecanol. It revealed that the FITC loaded implant with a thermo-sensitive cap could keep stable under relative intensive physiological environment, while release most FITC quickly when the temperature reached to 39 °C (hyperthermia induced by inflammation). The dynamic release process was shown in ESI video S1.† Because the FITC release process was so quick that we could not followed timely, the intensity of released FITC was monitored every 5 s and the results were present in the inset graph in Fig. 3b. It suggested that the process of burst release last about 45 s and the max released FITC amount was 95%. As shown in Fig. 3a, the process of drug release present visually at 37 °C and 39 °C. It also suggested that the designed implant could be triggered to release drugs by severe infection induced hyperthermia.
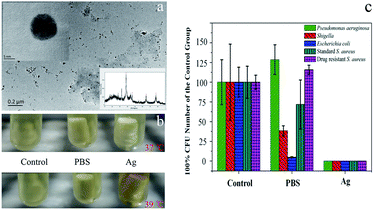
Postoperative infection is focused and resolved urgently after orthopaedic surgery.7 Silver nanoparticles (Ag NPs) are attracted much attention as a high efficient and broad spectrum antibacterial material recently.8 Here, Ag NPs was synthesized as the model of antibiotic agents. As shown in the inset photo of Fig. 4a, the corresponding X-ray diffraction (XRD) peak marked with asterisks proved that the Ag NPs were synthesized successfully. Transmission electron microscopy (TEM) images showed that the synthesized Ag NPs had well dispersed morphology with uniform round features (Fig. 4a). The size of Ag NPs could be measured from the inset photo of Fig. 4a, and the average diameter of Ag NPs were around 10.2 ± 2.4 nm (mean ± SD, n = 300) after statistical analysis. As shown in Fig. S2,† the prepared Ag NPs was also dispersed uniformly. To evaluate the antibacterial capability of the released Ag NPs from printed implants, PBS and Ag NPs loaded implants (Cy3.6) were incubated with S. aureus solution at different temperature. As shown in Fig. 4b, the changed color of S. aureus solution at 39 °C for 30 min revealed that Ag NPs were released from implant successfully. After co-cultured in orbital shaker for 6 h, the S. aureus solution was inoculated on the culture dish at 37 °C for 24 h. The results suggested that the released Ag NPs inhibited drastically the growth of S. aureus (Fig. S3†). What's more, Ag NPs loaded implant was cultured with three other common clinical bacteria (Pseudomonas aeruginosa, Shigella, Escherichia coli and drug resistant S. aureus) at 39 °C, the results reveal that the released Ag NPs also could inhibit these clinical bacteria effectively (Fig. 4c). Notably, The Pseudomonas aeruginosa and drug resistant S. aureus, as the most common and troublesome pathogenic bacterium in bone grafting, were extracted from 40 clinical infection cases. Conventional antibiotic therapy presents a limited effect to the above bacterium, therefore, the impressive antibiotic effect with Ag NPs reveals a delightful clinical significance. To further verify the feasibility of Ag NPs loaded implant scaffolds, the biosafety of Ag NPs was evaluated through intraperitoneal injection to mice (Fig. S4A†). 30 mice were injected with hundredfold amount of Ag NPs every day for 7 days. After treatment, most mice present certain weight loss at initial 2 days (after that the weight of mice gradually recovered to the lever of control), no mouse appear death 7 days later (Fig. S4B†). The results suggest that Ag NPs loaded implant scaffolds could be endowed practical value clinically due to broad-spectrum antibacterial effect and limited biotoxicity. Therefore, it is deserved to study further for clinical application.
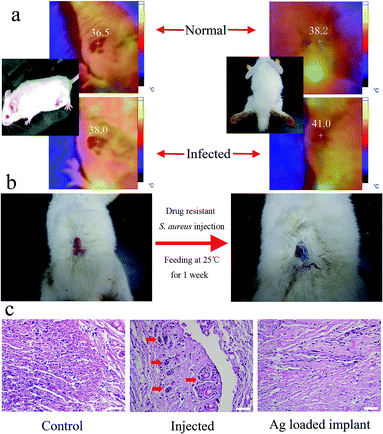
To certify that hyperthermia would be induced by bacterial infection, which could be used to trigger drug release, KM mice and rabbits were infected by S. aureus via subcutaneous injection at the site of wound. After injected and fed at 25 °C for 3 days, both of KM mice and rabbits present obvious hyperthermia with the elevated temperature of 2–3 °C (Fig. 5a), compared with the normal temperature of mice and rabbits are 36.5 °C and 38 °C respectively.9 So, it is reasonable to hypothesis that the loaded drugs could be triggered to release in the body of rabbits (or human) with severe infection caused hyperthermia. To confirm our hypothesis, blue ink loaded implant with the thermo-sensitive cap (1-tetradecanol) was embedded subcutaneously in rabbit, at the same time, 1 mL drug resistant S. aureus solution was injected at the site of wound. Interestingly, the tissue at the site of wound was stained by blue ink shown in Fig. 5b after remove stitch one week later. It suggested that blue ink was auto-released with the phase change of 1-tetradecanol ascribed to hyperthermia induced by inflammation. Furthermore, Ag NPs were loaded into implant instead of blue ink to evaluate the antibacterial capability in vivo. As shown in Fig. S5 and 5c,† H&E staining of skin slices showed that the inflammation in skin had been relieved when treated with Ag NPs loaded implant. This result further confirmed that the loaded drug (Ag NPs) could be auto-released to deal with severe infection. In addition, to evaluate the cytocompatibility of materials, CCK-8 assay was performed (Fig. S6†). It could be found that the 3T3 cell viability decreased slightly with time increasing, which suggests that PLA have a good cytocompatibility to 3T3 cells.
In summary, inspired by lotus root, we used 3D printing to construct implant scaffolds with customize structured inner chambers. Without complex chemical combinations, several drug releasing parameters could be regulated conveniently, including drug loading amount, drug releasing area, sequence, rate and curve. One innovation of this study was the introduction of appropriate thermo-sensitive phase change material to realize inflammation-triggered release properties. As a promising model of the loaded antibiotics, small sized Ag NPs were synthesized to inhibit several pathogens, including drug-resistant strains derived from practical clinical cases. To the best of our knowledge, this is the first example of an unplugged implant system with automatic local inflammation triggered release properties. This concept, though still in infancy, opened a window for individualizing accurate local antibacterial treatment according to each patient's postoperative situation, which essentially reduced the indiscriminate use of antibiotics. From a long-term point of view, the development of this strategy will decrease the emergence of drug resistant bacteria. The ongoing research will then focus on the incorporation of “stem cells therapeutic unit” into the proposed implant system.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21103159 and 21461015 to Xiaolei Wang); National Natural Science Foundation of China (81503364 and 31560264 to Tingtao Chen); National Natural Science Foundation of China (51463013 to Junchao Wei); Natural Science Foundation of Jiangxi Province (KJLD14010 and 20153BCB23035 to Xiaolei Wang) and Natural Science Foundation of Jiangxi Province (20161BAB215203 and GJJ150194 to Xingwei Ding).Notes and references
- X. Wang, X. Cai, Q. Guo, T. Zhang, B. Kobe and J. Yang, Chem. Commun., 2013, 49, 10064 RSC; T. A. Campbell and O. S. Ivanova, Nano Today, 2013, 8, 119 CrossRef CAS; L. S. Dimas, G. H. Bratzel, I. Eylon and M. J. Buehler, Adv. Funct. Mater., 2013, 23, 4629 CrossRef; S. V. Murphy and A. Atala, Nat. Biotechnol., 2014, 32, 773 CrossRef PubMed; U. Kalsoom, A. Peristyy, P. N. Nesterenko and B. Paull, RSC Adv., 2016, 6, 38140 RSC; J. M. Zhang, E. Q. Li, A. A. Aguirre-Pablo and S. T. Thoroddsen, RSC Adv., 2016, 6, 2793 RSC.
- S. Bose, M. Roy and A. Bandyopadhyay, Trends Biotechnol., 2012, 30, 546 CrossRef CAS PubMed; S. Bose, S. Vahabzadeh and A. Bandyopadhyay, Mater. Today, 2013, 16, 496 CrossRef; M. O. Wang, C. E. Vorwald, M. L. Dreher, E. J. Mott, M. H. Cheng, A. Cinar, H. Mehdizadeh, S. Somo, D. Dean and E. M. Brey, Adv. Mater., 2015, 27, 138 CrossRef PubMed; H. W. Kang, S. J. Lee, I. K. Ko, C. Kengla, J. J. Yoo and A. Atala, Nat. Biotechnol., 2016, 34, 312 CrossRef PubMed.
- X.-f. Chen and X.-l. Li, J. Med. Hypotheses Ideas, 2013, 7, 54 CrossRef CAS; D. A. Prawel, M. J. Kipper, K. C. Popat and S. P. James, Mater. Lett., 2013, 97, 81 CrossRef.
- Y.-H. Yang, S.-G. Fu, H. Peng, A.-D. Shen, S.-J. Yue, Y.-F. Go, L. Yuan and Z.-F. Jiang, Pediatr. Infect. Dis. J., 1993, 12, 986 CrossRef CAS PubMed; P. Bi, S. Tong and K. A. Parton, Soc. Sci. Med., 2000, 50, 1445 CrossRef PubMed.
- F. S. Aghdas, H. Akhavizadegan and A. Aryanpoor, Surg. Infect., 2006, 7, 367 CrossRef PubMed; J. C. Pile, Cleve Clin J Med, 2006, 73, S62 CrossRef PubMed; A. P. Czaplicki, J. E. Borger, J. R. Politi, B. T. Chambers and B. C. Taylor, J. Arthroplasty., 2011, 26, 1387 CrossRef PubMed; J. Gutierrez, A. Smith, P. Geavlete, H. Shah, A. R. Kural, M. de Sio, J. H. A. Sesmero, A. Hoznek, J. de la Rosette and C. P. S. Group, World J. Urol., 2013, 31, 1135 CrossRef PubMed.
- A. A. Aydın, Sol. Energy Mater. Sol. Cells, 2013, 113, 44 CrossRef CAS; J. Li, Y. Hu, Y. Hou, X. Shen, G. Xu, L. Dai, J. Zhou, Y. Liu and K. Cai, Nanoscale, 2015, 7, 9004 RSC.
- C. H. Halpern, G. W. Mitchell, A. Paul, D. R. Kramer, K. R. McGill, D. Buonacuore, M. Kerr, J. L. Jaggi, J. J. Stern and G. H. Baltuch, Am. J. Infect. Control, 2012, 40, 431 CrossRef PubMed; S. Koutsoumbelis, A. P. Hughes, F. P. Girardi, F. P. Cammisa, E. A. Finerty, J. T. Nguyen, E. Gausden and A. A. Sama, J. Bone Jt. Surg., 2011, 93, 1627 Search PubMed; B. Mraovic, D. Suh, C. Jacovides and J. Parvizi, J. Diabetes Sci. Technol., 2011, 5, 412 CrossRef PubMed.
- Z.-m. Xiu, Q.-b. Zhang, H. L. Puppala, V. L. Colvin and P. J. Alvarez, Nano Lett., 2012, 12, 4271 CrossRef CAS PubMed; X. Cai, M. Lin, S. Tan, W. Mai, Y. Zhang, Z. Liang, Z. Lin and X. Zhang, Carbon, 2012, 50, 3407 CrossRef.
- H. Sareh, M. E. Tulapurkar, N. G. Shah, I. S. Singh and J. D. Hasday, Cell Stress Chaperones, 2011, 16, 297 CrossRef CAS PubMed; C. Gordon, J. Therm. Biol., 2012, 37, 654 CrossRef; C. Nasui, G. Nathanael, E. Miller, J. Belik, A. Crawley, R. Weiss, G. Detzler, A. Zhong, R. Moineddin and A. Doria, Rheumatology, 2012, 2, 2161 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra10652h |
| ‡ These authors contributed equally and should be considered co-first authors. |
| This journal is © The Royal Society of Chemistry 2016 |