Efficient hydroarylation and hydroalkenylation of vinylarenes by Brønsted acid catalysis†
Muwen Liuab,
Jinlong Zhanga,
Hui Zhouab,
Huameng Yanga,
Chungu Xia*a and
Gaoxi Jiang*a
aState Key Laboratory for Oxo Synthesis and Selective Oxidation, Suzhou Research Institute of LICP, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Lanzhou, 730000, China. E-mail: cgxia@lzb.ac.cn; gxjiang@licp.cas.cn; Fax: +86-512-62872775
bGraduate University of Chinese Academy of Sciences, Beijing, China
First published on 3rd August 2016
Abstract
Brønsted acid Tf2NH alone catalyzed Friedel–Crafts-type hydroarylation and head-to-tail hydroalkenylation of vinylarenes under mild reaction conditions have been realized, providing a readily scalable, metal-free, and practical access to the 1,1-diarylalkane scaffolds and trans-1,3-diaryl-1-butenes in high yields and excellent regioselectivities.
The direct catalytic hydroarylation and hydroalkenylation of vinylarenes are highly atom-economical and fundamental methods for the synthesis of 1,1-diarylalkane (branched) scaffolds, which are core fragments existing in a number of complex natural and synthetic molecules. These compounds always display some biological activities and are potential therapeutic agents against cancer, smallpox, and insomnia, as well as other diseases (Fig. 1).1 Hence, much attention has been paid to achieve such transformations and numerous elegant developments have been reported (Scheme 1). According to the mechanism, the catalytic reactions can be classified into two major types: (1) hydroarylation through C–H activation by transition metal catalysts,2,3 and (2) Friedel–Crafts-type alkylation in the presence of Lewis or Brønsted acid.4 The former method usually requires a directing group on the arene and preferentially affords the linear adduct, with a few exceptions.5 In contrast, 1,1-diarylethanes are generally obtained from Friedel–Crafts-type hydroarylation due to the stability of positive charge on the α-position of styrene that develops upon Lewis acid coordination4a–g or Brønsted acid protonation4h–k (Scheme 1, A and B). Recently, Pospech group reported a hydroarylation of styrene derivatives catalyzed by phosphate with indoles as reactive partner. However, the presence of hydroxyl group was necessary for forming more reactive quinonemethide-like intermediate to accomplish the whole reaction (Scheme 1, C).6 With the advent of modern ‘super acids’, especially triflimide (Tf2NH, pKa = 0.67 in HOAc7), we recognized that hydroarylation of simple vinyl arenes might be catalyzed by the strong Brønsted acid via the generation of the intermediate B (Scheme 1, i).
Additionally, hydroalkenylation of olefins is one of the current interesting and useful protocols to synthesize essential intermediates for fine and industrial chemicals.8 Generally, such reactions could be carried out following three ways to date: head-to-head (h–h),9 head-to-tail (h–t)10 and tail-to-tail (t–t).11 Since a new allylic carbon stereogenic center is formed, the h–t dimerization is considered more attractive. Although a range of metal catalytic systems have been developed for this transformation: Dawans10a and Yi10i disclosed that nickel-based catalyst could catalyze dimerization of styrenes. Shirakawa10e and Cheng10f reported that the catalyst containing metal center of palladium and cobalt also showed highly catalytic activity to the reaction. Other development was linked to the discovery that the cooperation of iron and silver salts10k to facilitate the formation of trans-1,3-diaryl-1-butenes. Although these methods are efficient, the need of expensive phosphorus ligands and additives, as well as generation of oligomers or polymers was a huge drawback. Therefore, in view of the demand for most simple and less expensive processes, the dimerization of styrenes catalyzed by organic catalyst, especially easily accessible Brønsted acids, is a very promising alternative to above-mentioned methodologies. And we suppose that the benzyl cation generated under the catalysis of Tf2NH could be captured by vinylarenes themselves through nucleophilic addition, followed by deprotonation to form the dimerized product (Scheme 1, ii). In this paper, we describe the successful results obtained in both intramolecular Friedel–Crafts hydroarylation and hydroalkenylation of vinylarenes catalyzed by Tf2NH alone under mild reaction conditions.12
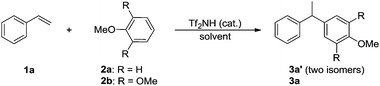
The initial investigation was carried out using a model reaction between styrene 1a and anisole 2a (Table 1). We found that the Friedel–Crafts hydroarylation could take place with Tf2NH as a catalyst. The screening of solvents showed that dioxane was superior to other solvent and the product was obtained in 71% yield at 80 °C with 4 mol% of Tf2NH (entries 1–5). Lowering the temperature to 60 °C resulted in 38% yield (entry 6). Because of the challenge to separate two isomers 3a′ of 2- and 4-regioselectivities with anisole 2a, and more importantly, for the development of a practical access to analogues of potential therapeutic agents I–III (Fig. 1),1 we finally selected 1,2,3-trimethoxybenzene 2b as the reactant to continue our study (entries 7–9), and the further improved reaction condition was obtained in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of arene 2b to 1a at 90 °C with a 4 mol% catalyst loading that led to 75% yield of product 3a exclusively (entry 9). No reaction occurred in the absence of acid catalyst (entry 10).
1 molar ratio of arene 2b to 1a at 90 °C with a 4 mol% catalyst loading that led to 75% yield of product 3a exclusively (entry 9). No reaction occurred in the absence of acid catalyst (entry 10).
| Entry | Substrate 2 | Tf2NH (%) | Solvent | t (°C) | Yieldb (%) |
|---|---|---|---|---|---|
a Reaction conditions: 1a (0.2 mmol) and 2 (0.4 mmol) in 1.0 mL of solvent for 12 h in a sealed tube.b Isolated yield.c The reaction was conducted in a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of arene 2b (0.5 mmol) to styrene 1a (0.1 mmol).d In the absence of acid, no reaction determined by GC. 1 molar ratio of arene 2b (0.5 mmol) to styrene 1a (0.1 mmol).d In the absence of acid, no reaction determined by GC. |
|||||
| 1 | 2a | 2 | Et2O | rt | Trace |
| 2 | 2a | 2 | THF | 80 | 66 |
| 3 | 2a | 2 | Cyclohexane | 80 | 62 |
| 4 | 2a | 2 | Dioxane | 80 | 68 |
| 5 | 2a | 4 | Dioxane | 80 | 71 |
| 6 | 2a | 4 | Dioxane | 60 | 38 |
| 7 | 2b | 4 | Dioxane | 80 | 68 |
| 8c | 2b | 4 | Dioxane | 80 | 73 |
| 9c | 2b | 4 | Dioxane | 90 | 75 |
| 10d | 2b | — | Dioxane | 90 | n.r. |
With the optimized reaction conditions established, we then examined the substrate scope of the hydroarylation between various vinylarenes 1 and 2b (Table 2). Significantly, besides 1a, the transformations took place smoothly to assemble (1-(3,4,5-trimethoxyphenyl)ethyl)-arenes exclusively with other substituted vinylarenes and self-dimerization of 1 was tremendously suppressed. Basically, the relatively electron-rich styrenes were more reactive than electron-deficient ones. For instance, styrenes 1b and 1c with ortho-/para-methyl substituent converted into the corresponding adducts 3b and 3c in 86% and 82% yields, respectively (entries 2 and 3). Compound 3d was readily obtained in 88% yield employing 4-tert-butyl styrene 1d as the substrate (entry 4). The best result was obtained by introducing two methyl groups on 2,5-position of the benzene ring to furnish 3e in 92% yield (entry 5). When electron-withdrawing halogen atom was attached to the aromatic ring in styrenes, the good outcomes was gained. 4-Halogen-containing (F, Cl, and Br) styrenes 1f–h were all compatible with this transformation and cleanly led to 3f–h in 75–50% yields (entries 6–8).
To highlight the potential application of the highly atom-economic hydroarylation process, we conducted the reactions of 2-vinylnaphthalene 1i with 2b (Scheme 2, eqn (1)), and 1f with benzofuran 2c and benzo[b]thiophene 2d to gram-scale (eqn (2)). Accordingly, treating 1i (3.0 mmol, 0.462 g) with 2b (15.0 mmol, 5 equiv.), and 1f (6.0 mmol, 0.73 g) with 2c (5.0 mmol, 0.59 g) and 2d (5.0 mmol, 0.67 g) to the standard reaction conditions, readily provided the potent of tubulin polymerization 3i,1d 3j, and 3k in 80–84% yields. Notably, 3k is a very useful material for the synthesis of anti-insomnia agent benzothiophene IV.1d
During the period of our studies on above hydroarylation between vinylarenes and anisole, it was found that a small quantity of homodimerized products generated. We reasoned that such dimerization of styrene derivatives might be also catalyzed by Brønsted acids (Table 3). Examination of usual Brønsted acids in THF at 80 °C revealed that Tf2NH is optimal which promoted the homodimerization successfully to provide the desired product 4a in 70% (entries 1–4). Changing the solvent THF to a mixed solvent of THF and cyclohexane in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio increased the yield up to 82% (entry 5). Enlarging the Tf2NH-loading to 6 mol% didn't improve the reaction (entry 6). A sharp decrease of yield was observed from 80 °C to 60 °C, while raising the temperature to 90 °C didn't afford improvement in the yield (entries 7 and 8).
1 volume ratio increased the yield up to 82% (entry 5). Enlarging the Tf2NH-loading to 6 mol% didn't improve the reaction (entry 6). A sharp decrease of yield was observed from 80 °C to 60 °C, while raising the temperature to 90 °C didn't afford improvement in the yield (entries 7 and 8).
| Entry | Acid (mol%) | Solvent | t (°C) | Yieldb (%) | |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), acid (4–6 mol%), solvent (1 mL), 60–90 °C, 12 h.b Isolated yield.c Detected by GC analysis. | |||||
| 1 | p-TSA (4) | THF | 80 | — | |
| 2 | TFA (4) | THF | 80 | — | |
| 3 | CF3SO3H (4) | THF | 80 | 60 | |
| 4 | Tf2NH (4) | THF | 80 | 70 | |
| 5 | Tf2NH (4) | THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexane (3 cyclohexane (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 82 | |
| 6 | Tf2NH (6) | THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexane (3 cyclohexane (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 81 | |
| 7 | Tf2NH (4) | THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexane (3 cyclohexane (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60 | <10c | |
| 8 | Tf2NH (4) | THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexane (3 cyclohexane (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
90 | 80 | |
Next, the scope and generality of this homodimerization were explored using various commercially available vinylarenes (Table 4). Besides styrene, both alkyl-substituted and halogenated vinylarenes were all able to participate in the dimerization to provide smoothly the corresponding products. Methyl group in ortho- and para-position had no apparent effect on the yields of products (entries 1–2). Notably, tert-butyl substituent could also tolerate the reaction conditions and provided 4d in 82% yield (entry 3). Gratifyingly, the fluorinated substrates underwent well the process, and gave the trans-1,3-diaryl-1-butenes 4f, and 4j–k in good yields regard less of the substituted position (entries 4–6). Yields of more than 60% were obtained when Cl and Br substituted vinylarenes were used as reaction substrates (entries 7 and 8). With 1-vinylnaphthalene 1l as substrate, the satisfied yield was achieved (entry 9). Prop-1-en-2-ylbenzene 1m and the bicyclic compounds 1n and 1H-indene 1o are also compatible with this transformation, providing 4m–o in acceptable yields (entries 10–12). It is noteworthy that the current homo-hydroalkenylation of 1o is easily scaled up to 10 mmol scale, producing 0.88 g of 4o in 76% yield (entry 12).
| Entry | Substrate | Product | Yieldb (%) |
|---|---|---|---|
a Reaction conditions: 1 (0.2 mmol), Tf2NH (4 mol%), 1.0 mL mixed solvent of THF and cyclohexane with a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio, 80 °C, 12 h.b Isolated yield.c 10 mmol of 1o run the reaction to give 0.88 g of 4o. 1 volume ratio, 80 °C, 12 h.b Isolated yield.c 10 mmol of 1o run the reaction to give 0.88 g of 4o. |
|||
| 1 | 2-Me, 1b | 4b | 82 |
| 2 | 4-Me, 1c | 4c | 91 |
| 3 | 4-tert-Butyl, 1d | 4d | 82 |
| 4 | 2-F, 1j | 4j | 78 |
| 5 | 3-F, 1k | 4k | 70 |
| 6 | 4-F, 1f | 4f | 90 |
| 7 | 4-Cl, 1g | 4g | 60 |
| 8 | 4-Br, 1h | 4h | 85 |
| 9 | 2-Vinylnaphthalene, 1l | 4l | 80 |
| 10 | Prop-1-en-2-ylbenzene, 1m | 4m | 84 |
| 11 | 1,2-Dihydronaphthalene, 1n | 4n | 75 |
| 12c | 1H-indene, 1o | 4o | 76 |
Remarkably, the cross-hydroalkenylation process occurred efficiently (Table 5). Treatment of 1f (5.0 equiv.) with another vinylarenes, including 1b, 1d, 1p, and 1g, to the standard reaction conditions afforded the desired products 5b–e in 55–89% yields.
In conclusion, we described a readily scalable, mild, and efficient method for hydroarylation and head-to-tail hydroalkenylation of vinylarenes with Brønsted acid Tf2HN as the same only catalyst, which provides an easy access to bioactive 1,1-diarylalkane scaffold and trans-1,3-diaryl-1-butenes. This is the first example of highly regioselective hydroarylation and hydroalkenylation with only one organic catalyst.
Acknowledgements
Financial support from Hundred Talent Program of Chinese Academy of Sciences (CAS) is gratefully acknowledged.Notes and references
- (a) G. Beaton, W. J. Moree, F. Jovic, T. Coon and J. Yu, US Pat., 2006/0014797 A1, 2006; (b) M. Alami, S. Messaoudi, A. Hamze, O. Provot, J. D. Brion, J.-M. Liu, J. Bignon and J. Bakala, Patent WO 2009/147217 A1, 2009; (c) A. V. Cheltsov, M. Aoyagi, A. Aleshin, E. C.-W. Yu, T. Gilliland, D. Zhai, A. A. Bobkov, J. C. Reed, R. C. Liddington and R. Abagyan, J. Med. Chem., 2010, 53, 3899 CrossRef CAS PubMed; (d) B. L. H. Taylor, E. C. Swift, J. D. Waetzig and E. R. Jarvo, J. Am. Chem. Soc., 2011, 133, 389 CrossRef CAS PubMed.
- For reviews, see: (a) F. Kakiuchi and N. Chatani, Adv. Synth. Catal., 2003, 345, 1077 CrossRef CAS; (b) F. Kakiuchi and T. Kochi, Synthesis, 2008, 3013 CrossRef CAS; (c) D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624 CrossRef CAS PubMed.
- For selected examples, see: (a) S. Murai, F. Kakiuchi, S. Sekine, Y. Tanaka, A. Kamatani, M. Sonoda and N. Chatani, Nature, 1993, 36, 529 CrossRef; (b) F. Kakiuchi, S. Sekine, Y. Tanaka, A. Kamatani, M. Sonoda, N. Chatani and S. Murai, Bull. Chem. Soc. Jpn., 1995, 68, 62 CrossRef CAS; (c) T. Matsumoto, R. A. Periana, D. J. Taube and H. Yoshida, J. Mol. Catal. A: Chem., 2002, 180, 1 CrossRef CAS; (d) C.-H. Jun, C. W. Moon, J.-B. Hong, S.-G. Lim, K.-Y. Chung and Y.-H. Kim, Chem.–Eur. J., 2002, 8, 485 CrossRef CAS PubMed; (e) K. L. Tan, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2002, 124, 13964 CrossRef CAS PubMed; (f) R. Martinez, R. Chevalier, S. Darses and J. P. Genet, Angew. Chem., Int. Ed., 2006, 45, 8232 CrossRef CAS PubMed; (g) R. Martinez, J. P. Genet and S. Darses, Chem. Commun., 2008, 3855 RSC; (h) K. Jaesung, O. Youhwa, J. Yousung and C. Sukbok, J. Am. Chem. Soc., 2012, 134, 17778 CrossRef PubMed; (i) G. E. M. Crisenza, N. G. McCreanor and J. F. Bower, J. Am. Chem. Soc., 2014, 136, 10258 CrossRef CAS PubMed; (j) G. E. M. Crisenza, O. O. Sokolova and J. F. Bower, Angew. Chem., Int. Ed., 2015, 54, 14866 CrossRef CAS PubMed.
- For selected examples with Lewis acid catalysis, see: (a) J. Kischel, I. Jovel, K. Mertins, A. Zapf and M. Beller, Org. Lett., 2006, 8, 19 CrossRef CAS PubMed; (b) H.-B. Sun, B. Li, R. Hua and Y. Yin, Eur. J. Org. Chem., 2006, 4231 CrossRef CAS; (c) M. Rueping, B. J. Nachtsheim and T. Scheidt, Org. Lett., 2006, 8, 3717 CrossRef CAS PubMed; (d) Z. Zhang, X. Wang and R. A. Widenhoefer, Chem. Commun., 2006, 3717 RSC; (e) S. Hajra, B. Maji and S. Bar, Org. Lett., 2007, 9, 2783 CrossRef CAS PubMed; (f) Y. Yamamoto and K. Itonaga, Chem.–Eur. J., 2008, 14, 10705 CrossRef CAS PubMed; (g) K. M. Reddy, N. S. Babu, P. S. S. Prasad and N. Lingaiah, Catal. Commun., 2008, 9, 2525 CrossRef CAS. With Brønsted acid catalysis, see: (h) A. E. Cherian, G. J. Domski, J. M. Rose, E. B. Lobkovsky and G. W. Coates, Org. Lett., 2005, 7, 5135 CrossRef CAS PubMed; (i) X. Zhao, Z. Yu, T. Xu, P. Wu and H. Yu, Org. Lett., 2007, 9, 5263 CrossRef CAS PubMed; (j) B. Das, M. Krishnaiah, K. Laxminarayana, K. Damodar and D. N. Kumar, Chem. Lett., 2009, 38, 42 CrossRef CAS; (k) T. P. Pathak, J. G. Osiak, R. M. Vaden, B. E. Welm and M. S. Sigman, Tetrahedron, 2012, 68, 5203 CrossRef CAS PubMed. With other organic catalysts, see: (l) C.-M. Chu, W.-J. Huang, J.-T. Liu and C.-F. Yao, Tetrahedron Lett., 2007, 48, 6881 CrossRef CAS; (m) R. D. Patil, G. Joshi and S. Adimurthy, Monatsh. Chem., 2010, 141, 1093 CrossRef CAS.
- (a) Y. Uchimaru, Chem. Commun., 1999, 1133 RSC; (b) Y. Nakao, N. Kashihara, K. S. Kanyiva and T. Hiyama, J. Am. Chem. Soc., 2008, 130, 16170 CrossRef CAS PubMed; (c) T. Mukai, K. Hirano, T. Satoh and M. Miura, J. Org. Chem., 2009, 74, 6410 CrossRef CAS PubMed; (d) Y. Nakao, N. Kashihara, K. S. Kanyiva and T. Hiyama, Angew. Chem., Int. Ed., 2010, 49, 4451 CrossRef CAS PubMed; (e) S. Pan, N. Ryu and T. Shibata, J. Am. Chem. Soc., 2012, 134, 17474 CrossRef CAS PubMed; (f) L.-P. Sheng and N. Yoshikai, Angew. Chem., Int. Ed., 2013, 52, 1240 CrossRef PubMed; (g) Z. Yang, H. Yu and Y. Fu, Chem.–Eur. J., 2013, 19, 12093 CrossRef CAS PubMed; (h) J. Dong, P.-S. Lee and N. Yoshikai, Chem. Lett., 2013, 42, 1140 CrossRef CAS; (i) G. E. M. Crisenza, N. G. McCreanor and J. F. Bower, J. Am. Chem. Soc., 2014, 136, 10258 CrossRef CAS PubMed; (j) P.-S. Lee and N. Yoshikai, Org. Lett., 2015, 17, 22 CrossRef CAS PubMed; (k) M. Y. Wong, T. Yamakawa and N. Yoshikai, Org. Lett., 2015, 17, 442 CrossRef CAS PubMed.
- I. Fleischer and J. Pospech, RSC. Adv., 2015, 5, 493 RSC.
- (a) The pKa Values in glacial acetic acid were measured by 1H NMR spectroscopy, see: B. M. Rode, A. Engelbrecht and J. Schantl, Z. Phys. Chem., 1973, 253, 17 CAS; (b) The pKa Values of Tf2NH and HOTf in glacial acetic acetic acid are 7.8 and 4.2, respectively, see: J. Foropoulos and D. D. DesMarteau, Inorg. Chem., 1984, 23, 3720 CrossRef CAS.
- (a) Y. Chauvin and H. Olivier, in Applied Homogeneous Catalysis with Organometallic Compounds, ed. B. Cornils and W. Herrmann, VCH, New York, 1996, vol. 1, p. 258 Search PubMed; (b) J. Skupinska, Chem. Rev., 1991, 91, 613 CrossRef CAS; (c) T. Alderson, E. Jenner and R. V. Lindsey, J. Am. Chem. Soc., 1965, 87, 5638 CrossRef CAS; (d) M. Brookhart, P. S. White and G. M. Direnzo, J. Am. Chem. Soc., 1996, 118, 6225 CrossRef; (e) B. L. Small and A. J. Marcucci, Organometallics, 2001, 20, 5738 CrossRef CAS; (f) B. L. Small, Organometallics, 2003, 22, 3178 CrossRef CAS; (g) T. Mitsudo, T. Suzuki, S.-W. Zhang, D. Imai, K. Fujita, T. Manabe, M. Shiotsuki, Y. Watanabe, K. Wada and T. Kondo, J. Am. Chem. Soc., 1999, 121, 1839 CrossRef CAS.
- T. Kondo, D. Takagi, H. Tsujita, Y. Ura, K. Wada and T. Mitsudo, Angew. Chem., Int. Ed., 2007, 46, 5958 CrossRef CAS PubMed.
- (a) F. Dawans, Tetrahedron Lett., 1971, 12, 1943 CrossRef; (b) A. Sen, T.-W. Lai and R. R. Thomas, J. Organomet. Chem., 1988, 358, 567 CrossRef CAS; (c) Z. Jiang and A. Sen, Organometallics, 1993, 12, 1406 CrossRef CAS; (d) T. Tsuchimoto, S. Kamiyama, R. Negoro, E. Shirakawa and Y. Kawakami, Chem. Commun., 2003, 852 RSC; (e) T. Tsuchimoto, S. Kamiyama, R. Negoro, E. Shirakawa and Y. Kawakami, Chem. Commun., 2003, 852 RSC; (f) C.-C. Wang, P.-S. Lin and C.-H. Cheng, Tetrahedron Lett., 2004, 45, 6203 CrossRef CAS; (g) C.-C. Wang, P.-S. Lin and C.-H. Cheng, Tetrahedron Lett., 2004, 45, 6203 CrossRef CAS; (h) G. W. Kabalka, G. Dong and B. Venkataiah, Tetrahedron Lett., 2004, 45, 2775 CrossRef CAS; (i) C. Yi, R. Hua and H.-X. Zeng, Catal. Commun., 2008, 9, 85 CrossRef CAS; (j) J. R. Cabrero-Antonino, A. Leyva-Prez and A. Corma, Adv. Synth. Catal., 2010, 352, 1571 CrossRef CAS; (k) H. Ma, Q. Sun, W. Li, J. Wang, Z. Zhang, Y. Yang and Z. Lei, Tetrahedron Lett., 2011, 52, 1569 CrossRef CAS.
- (a) G. Wu, A. L. Rheingold and R. F. Heck, Organometallics, 1987, 6, 2386 CrossRef CAS; (b) G. Wu, S. J. Geib, A. L. Rheingold and R. F. Heck, J. Org. Chem., 1988, 53, 3238 CrossRef CAS; (c) R. N. Das, K. Sarma, M. G. Pathak and A. Goswami, Synlett, 2010, 2908 CAS; (d) C.-Y. Ho and L. He, Angew. Chem., Int. Ed., 2010, 49, 9182 CrossRef CAS PubMed.
- A recent review on strong Brønsted acids as catalysts, see: T. Akiyama and K. Mori, Chem. Rev., 2015, 115, 9277 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization data for new compounds. See DOI: 10.1039/c6ra16627j |
| This journal is © The Royal Society of Chemistry 2016 |