Gold nanoparticles supported on layered TiO2–RGO hybrid as an enhanced and recyclable catalyst for microwave-assisted hydration reaction†
Yunfeng Chenga,
Qingshan Zhaoab,
Yang Lia,
Wenchao Penga,
Guoliang Zhanga,
Fengbao Zhang*a and
Xiaobin Fan*a
aSchool of Chemical Engineering and Technology, State Key Laboratory of Chemical Engineering, Collaborative Innovation Center of Chemical Science and Engineering, Tianjin University, Tianjin 300072, China. E-mail: xiaobinfan@tju.edu.cn; fbzhang@tju.edu.cn
bCollege of Chemical Engineering, State Key Laboratory of Heavy Oil Processing, China University of Petroleum (East China), Qingdao 266580, China
First published on 2nd August 2016
Abstract
In this study, we synthesized a novel composite material (Au–TiO2–RGO) consisting of tiny gold nanoparticles (∼4.5 nm) grown on a layered titania (TiO2) and reduced graphene oxide (RGO) hybrid. After treatment with microwave and sulfuric acid, solid acid (SO42−/TiO2) was in situ formed on the surface of TiO2, and the resulting Au–SO42−/TiO2–RGO was determined as an enhanced catalyst for hydration reaction. The strong metal-support interaction (SMSI) between Au and TiO2 and the cooperative effect between Au and SO42−/TiO2 solid acid collectively account for the excellent performance. Moreover, due to the versatile RGO substrate, the catalyst could also be recycled and reused at least 5 times without obvious deactivation.
1 Introduction
The hydration reaction is very important in organic synthesis, as it can produce valuable carbonyl derivatives from accessible alkynyl substrates.1 In the last century, the most extensively employed catalytic systems for this reaction were mercury salts.2 Limited by their strong toxicity, green and environmentally friendly catalysts are therefore needed. New organometallic catalysts containing Ru,3 Rh,4 Pt,5 Au6–8 and other metals have recently been developed and applied successfully for this reaction. Although excellent catalytic activities have been achieved, the homogeneous feature limited their practical applications. These noble metal containing catalysts are expensive and difficult to separate from the reaction system for recycling. Therefore, developing efficient recyclable catalytic systems is an urgent task.Heterogeneous catalysis using supported gold nanoparticles (Au NPs) have been intensively explored in organic reactions with great success.9–12 The catalytic performance of Au NPs largely depends on the property of the support. In some cases, the support can go further than being simple carriers.13,14 They can also form strong interactions with Au NPs and affect the catalytic activity. On advantages of low cost, nontoxicity, structural stability and safety, titania (TiO2) is the most widely employed support for Au NPs in heterogeneous catalysis.15–18 It has been reported that Au and TiO2 can form strong support-metal interaction (SMSI) and promote the reactions in certain catalytic systems.19–21 Actually, to date, Au NPs supported on TiO2 nanoparticles (Au–TiO2) have exhibited high activity and selectivity in many important reactions, such as photocatalytic degradation reactions,22,23 hydrogen production reactions,24,25 hydrogenation reactions,26 oxidation reactions27–29 and so on.30,31
Considering its excellent catalytic performance, we speculated Au–TiO2 might be an effective heterogeneous catalyst for hydration reactions. However, despite of heterogeneous feature, the separation and recycle process are still difficult to be performed due to small particle size of the Au–TiO2 composite. To facilitate the recycle process, a more versatile support is needed for Au–TiO2. Considering the advantages in facilitating the dispersion and mass transfer of the supported catalysts in solvents,32 the two-dimensional reduced graphene oxide (RGO) possesses great potential as an ideal substrate for Au–TiO2. Actually, many papers have reported TiO2 supported on reduced graphene oxide (TiO2–RGO), and the composite has been successfully employed as catalysts for many reactions.33–35 The strong interaction between TiO2 and RGO36 confirms the excellent performance.
Hence, in this account, we designed a facile strategy to synthesize a novel composite (Au–TiO2–RGO) as a catalyst for hydration reactions. As seen in Scheme 1, TiO2 nanoparticles with anatase phase were firstly overlayed on the two-dimensional RGO nanosheets. Subsequently, tiny Au NPs with uniform dispersion and size dimension (∼4.5 nm) were deposited on the sandwich-like TiO2–RGO support. Finally, with the assistance of microwave and the addition of sulfuric acid, surface acid sites (SO42−/TiO2) with bridge bidentate structure was in situ formed, and the obtained Au–SO42−/TiO2–RGO served as an enhanced catalyst for hydration reaction. What's more, owing to the versatile RGO substrate, the catalyst could also be recovered and reused easily without significant decrease of activity.
2 Experimental
2.1 Preparation of Au–TiO2–RGO
Graphite oxide (GO) was prepared by the Hummers method,37 and GO was obtained and purified as described in previous studies.38 To prepare TiO2–RGO, 240 mg of GO and 3 mL of tetrabutyl titanate were dispersed in 100 mL isopropanol by sonication for 1 h. Subsequently, 3 mL of deionized water was added dropwise, and the mixture was kept stirring for another 2 h. The solution was then transferred into a 150 mL Teflon-lined stainless steel autoclave and heated at 180 °C for 8 h. GO could be reduced to RGO during the hydrothermal process.36 The as-prepared TiO2–RGO was then purified by centrifugation and washing with ethanol and deionized water three times, respectively. To prepare Au–TiO2–RGO, TiO2–RGO was firstly dispersed in 100 mL deionized water with sonication for 0.5 h. 0.5 g of glucose and 0.5 g of sodium citrate were then added into the suspension. After sonication for another 5 min, 2.5 mL of chloroauric acid (HAuCl4) (0.02 M) was added to this mixture, and it was stirred for 4 h at room temperature. The resulting mixture was centrifugated and washed with deionized water three times, followed by freeze-drying.2.2 Preparation of Au–SO42−/TiO2–RGO
The Au–SO42−/TiO2–RGO was obtained during the hydration reaction. 25 mg of Au–TiO2–RGO and 1.0 mmol of sulfuric acid were added to 5 mL of dioxane. The mixture was heated to 120 °C with the assistance of microwave and stirred for 2 h. The as-prepared Au–SO42−/TiO2–RGO was then purified by centrifugation and washing with ethanol and deionized water three times, respectively.2.3 Catalytic hydration reaction
The catalytic reactions were conducted in a microwave reactor. 0.25 mmol of phenylacetylene, 25 mg of Au–SO42−/TiO2–RGO (1 mol% equiv.), 0.5 mmol of deionized water and 1.0 mmol of sulfuric acid were added to 5 mL of dioxane. The mixture was heated to 120 °C with the assistance of microwave and stirred for 2 h. At the stated time, samples were taken from the reaction mixture and analyzed by gas chromatography (GC). For the recycling experiments, the reactions were carried out under the same conditions. After each run, the catalyst was recovered from the reaction mixture by centrifugation, washed with ethanol and deionized water three times, respectively, followed by freeze-drying, and then reused in next the run.2.4 Characterization
The X-ray diffraction (XRD) was performed on a Bruker-Nonius D8 FOCUS diffractometer. Transmission electron microscopic (TEM) and high resolution transmission electron microscopic (HRTEM) images were recorded using a JEM-2100F transmission electron microscope operating at 200 kV. X-ray photoelectron spectroscopy (XPS) measurements were performed on a PHI 1600 spectrometer (Perkin-Elmer). Fourier transform infrared (FTIR) spectroscopy was carried out on a Nicolet Nexus FTIR Spectrometer using KBr pellets method. NH3 Temperature-Programmed Desorption (NH3-TPD) was measured in a flow-type fixed-bed reactor at ambient pressure. 0.1 g sample was heated at 500 °C for 2 h and cooled to 120 °C in flowing He. At 120 °C, the sample was treated by sufficient pulses of NH3 till adsorption saturation, followed by a He purge for about 2 h. The temperature was raised up from 120 to 500 °C at a rate of 10 °C min−1 to desorb NH3 and maintained at 500 °C for 0.5 h. The gold loading of the Au–TiO2–RGO composites was spectrometer (ICPOES, Vista-MPX). The catalytic results were measured by an Agilent 6890N GC using the standard curve method.3 Results and discussion
3.1 Characterization of Au–TiO2–RGO
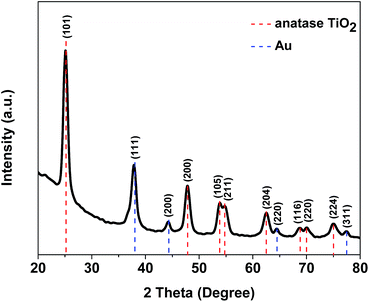
The X-ray diffraction (XRD) pattern of the composite is shown in Fig. 1. The specific diffraction peaks of anatase TiO2 and Au can be identified. They match well with the standard patterns of anatase TiO2 (JCPDS card no. 21-1272) and Au (JCPDS card no. 04-0784).Fig. 2a shows the transmission electron microscopic (TEM) image of the Au–TiO2–RGO sample. As can be seen, the RGO substrate is homogeneously and condensedly decorated with TiO2 nanoparticles. Au NPs are dotted around the TiO2 layer without any agglomeration. With narrow size distribution, the average size of Au NPs and TiO2 are 4.5 nm and 8.3 nm, respectively (Fig. 2c and d) the high resolution transmission electron microscopic (HRTEM) image in Fig. 2b proves a very close contact between the three components, and both of the characteristic finger lattices of TiO2 and Au can be observed. The measured regular d-spacing of 0.352 nm corresponds to the (101) face of the anatase TiO2, and the d-spacing of 0.235 nm is representative to the (111) face of the Au nanocrystals. As a control, TEM image of Au–TiO2 was also provided in the ESI (Fig. S1†). As can be seen, TiO2 and Au would agglomerate seriously in the absence of RGO. Therefore, the RGO substrate plays an important role on the homogeneous dispersion of the catalytic sites.
 | ||
| Fig. 2 (a) TEM and (b) HRTEM image of Au–TiO2–RGO; size distribution of (c) anatase TiO2 nanoparticles and (d) Au NPs. | ||
The X-ray photoelectron spectroscopy (XPS) spectra of Au–TiO2–RGO and Au–SO42−/TiO2–RGO are shown in Fig. 3. Au, Ti and C signals can be clearly observed in the full range XPS spectrum of Au–TiO2–RGO, and an additional signal of S appears in the spectrum of Au–SO42−/TiO2–RGO (Fig. 3a), indicating that Au–TiO2–RGO might be sulfated during the reaction. Meanwhile, the content of S and Au are estimated to be 4 atomic% and 0.2 atomic%, respectively. A further study reveals the S band can be divided into two peaks at binding energies of 168.5 and 169.6 eV, respectively, corresponding to sulfur in a six-oxidation state (S6+) (Fig. 3b).39 This result confirms the generation of SO42−/TiO2 solid acid in the reaction process. The Ti and Au band of Au–TiO2–RGO were also carefully analyzed. As seen in Fig. 3c, Ti 2p spectrum shows two peaks at 459.2 and 464.9 eV, corresponding to the binding energies of Ti 2p3/2 and Ti 2p1/2.40 Au 4f spectrum exhibits two typical peaks at 83.7 and 87.4 eV, which correspond to the binding energy of Au(0) (Fig. 3d). Compared with the general binding energies of 4f7/2 (84.0 eV) and 4f5/2 (87.7 eV) for Au(0),41 the peaks shift to lower value. Such phenomena reveals a charge transfer from TiO2 to Au, and the SMSI between them can facilitate the reaction rate.42 Moreover, compared with GO, C 1s XPS spectrum of Au–TiO2–RGO shows a significant decrease in the C–O–C and C–OH peaks at around 286.7 eV (Fig. S2†), which indicates the GO precursor is partially reduced to RGO in the hydrothermal process.36 To study the stability of the catalyst, the XPS spectrum of Au–SO42−/TiO2–RGO after 5 cycles is shown in Fig. S3.† The content of Au is estimated as 0.2 atomic%, confirming the stability of Au–SO42−/TiO2–RGO. Besides, S signal can also be seen, which further confirms the generation of SO42−/TiO2 solid acid. However, the content decreases to 1 atomic%, which might be caused by loss of acid sites upon reuse.
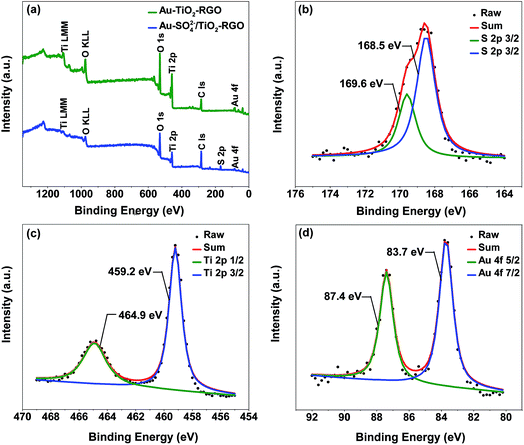 | ||
| Fig. 3 (a) Full range XPS spectra of Au–TiO2–RGO and Au–SO42−/TiO2–RGO; (b) S 2p XPS spectrum of Au–SO42−/TiO2–RGO; (c) Ti 2p and (d) Au 4f XPS spectrum of Au–TiO2–RGO. | ||
To further investigate the formation of surface SO42−/TiO2, Fourier transform infrared (FTIR) spectra of Au–TiO2–RGO and Au–SO42−/TiO2–RGO are provided in Fig. 4. The presence of peaks at around 3450 and 1400 cm−1 in both spectra should be due to the absorbed water.
The band at about 1630 cm−1 is attributed to in-plane vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C band, suggesting a partially reduction of GO.43 In the spectrum of Au–TiO2–RGO, the peak at 1220 cm−1 should be ascribed to the residual C–O groups of RGO. By contrast, characteristic frequencies of bidentate SO42− co-ordinated to Ti4+ at 1214, 1136, 1049, and 989 cm−1 can be clearly observed in Au–SO42−/TiO2–RGO. The result provides direct evidence on the successful introducing of sulphate groups to the TiO2 framework.44–46
C band, suggesting a partially reduction of GO.43 In the spectrum of Au–TiO2–RGO, the peak at 1220 cm−1 should be ascribed to the residual C–O groups of RGO. By contrast, characteristic frequencies of bidentate SO42− co-ordinated to Ti4+ at 1214, 1136, 1049, and 989 cm−1 can be clearly observed in Au–SO42−/TiO2–RGO. The result provides direct evidence on the successful introducing of sulphate groups to the TiO2 framework.44–46
The surface acidity of the Au–SO42−/TiO2–RGO is measured by NH3-TPD method. Four types of acid site can be observed in Fig. 5. The peak at 247 °C should be generated by weak adsorption in the low test temperature range. The peaks at 327 °C, 396 °C and 486 °C should correspond to the medium-strong acid sites, the strong acid sites, and the super strong acid sites, respectively.47 The ratio of four acidic peaks is 0.61/0.65/1/0.16, indicating the Brønsted acid property of Au–SO42−/TiO2–RGO.
3.2 Catalytic reaction
The catalytic performance of the prepared catalyst was systematically examined through the hydration of phenylacetylene. As microwave is an efficient way to improve catalytic performance, especially for solid acid involved reactions,48 microwave was employed. Table 1 summarizes the catalytic performance of different samples. In the absence of H2SO4, all of the Au–RGO, Au–TiO2, Au–TiO2–RGO were inactive (Entry 3, 5, 7). Pure H2SO4 without any catalyst showed very low activity for the reaction (Entry 1). Therefore, H2SO4 is an essential promoter for the reaction. With the addition of H2SO4, RGO alone was hardly active (Entry 2). When Au NPs was loaded (Au–RGO), the conversion and yield reached up to 64% and 48%, respectively (Entry 4). The result implied Au was a necessary active substance for hydration process. Au–TiO2 showed a better performance, resulting in 60% yield and 100% selectivity (Entry 6), which should be attributed to the SMSI as probed above. Remarkably, when Au–TiO2 was supported on RGO, namely Au–TiO2–RGO, a yield of 100% could be achieved (Entry 9). It is worth mentioning that TiO2–RGO exhibited a surprising conversion of 63%, but a low selectivity (Entry 8). This should be mainly ascribed to the significantly enhanced acidity of H2SO4 with the assistance of the SO42−/TiO2 solid acid formed during the reaction.49 So the enhanced performance of Au–TiO2–RGO should owe to not only the homogeneous dispersion and promoted SMSI arising from the RGO substrate, but also the in situ formed SO42−/TiO2 acid sites. To optimize the loading weight of Au, Au–TiO2–RGO with two more Au percentage were prepared (Entry 10, 11). It was found that Au–TiO2–RGO with 0.13 mmol g−1 gold loading showed the best catalytic activity. Controlling experiments with oil bath heating were also conducted. Au–TiO2–RGO gave a much lower conversion of 57% and yield of 49% within the same time (Entry 12). When the reaction time was extended to 16 h, the conversion and yield could just reach up to 95% and 89%, respectively (Entry 13).| Entry | Sample | Au (mmol g−1) | H2SO4 (mmol) | Conversion (%) | Yieldb (%) |
|---|---|---|---|---|---|
| a General conditions: phenylacetylene (0.25 mmol), H2O (0.5 mmol), dioxane (5 mL), catalyst (1 mol% Au), microwave heating at 120 °C, 2 h.b Calibrated yields determined by GC.c Au–TiO2–RGO with different gold loading.d Oil bath heating at 120 °C within 2 h and 16 h. | |||||
| 1 | Blank | — | 1.0 | 14 | 11 |
| 2 | RGO | — | 1.0 | 15 | 11 |
| 3 | Au–RGO | 0.16 | 0 | 0 | 0 |
| 4 | Au–RGO | 0.16 | 1.0 | 64 | 48 |
| 5 | Au–TiO2 | 0.14 | 0 | 0 | 0 |
| 6 | Au–TiO2 | 0.14 | 1.0 | 60 | 60 |
| 7 | Au–TiO2–RGO | 0.13 | 0 | 0 | 0 |
| 8 | TiO2–RGO | — | 1.0 | 63 | 37 |
| 9 | Au–TiO2–RGO | 0.13 | 1.0 | 100 | 100 |
| 10 | Au–TiO2–RGO-1c | 0.22 | 1.0 | 83 | 82 |
| 11 | Au–TiO2–RGO-2c | 0.07 | 1.0 | 89 | 89 |
| 12 | Au–TiO2–RGOd | 0.13 | 1.0 | 57 | 49 |
| 13 | Au–TiO2–RGOd | 0.13 | 1.0 | 95 | 89 |
To demonstrate the universality of the Au–SO42−/TiO2–RGO, different substrates with significant structural variation were proceeded (Table 2). Au–SO42−/TiO2–RGO shows excellent catalytic activity in the hydration of substrates with excellent yields (94–99%). It can be noted that the presence of substituent on the p-position of benzene had very little influence on the catalytic efficiencies, showing 100% conversion and 99% yield for the corresponding product (Entry 1). Importantly, when an unsymmetrical internal alkyne engaged in the reaction, one exclusive regioisomer was obtained in very high yield (94%, Entry 2). Aliphatic alkynes also achieved excellent yield of 96% (Entry 3 and 4). The catalytic results reveal that Au–SO42−/TiO2–RGO is a versatile catalyst for hydration reaction.
According to the catalytic results, a general mechanism has been proposed to explain this catalytic process (Scheme 2).50 First of all, Au forms complex with the triple bond of phenylacetylene. In this step, TiO2 bonds strong interactions with the Au NPs to decrease surface energy of the nanoparticles. So phenylacetylene can be more easily adsorbed to the active sites and accelerate the catalytic cycle.51 Besides, charge transfer from RGO to Au–TiO2 further regulates the strong interactions between Au and TiO2, yielding electron-rich Au NPs, which are easier to form higher valence gold species, thus rendering remarkable catalytic performance. Subsequently, regioselective addition of water happens, which is affected by the SMSI in Au–TiO2 and generates desired acetophenone. Finally, acid catalyzed reductive elimination of Au gives enol form, which rearranges into keto form. With the assistance of microwave, the in situ formed solid acid SO42−/TiO2 may greatly facilitate this step. Therefore, the enhanced catalytic performance should mainly attributed to the SMSI between Au and TiO2, the versatile RGO substrate, and the in situ formed SO42−/TiO2 acid sites.
After the reaction, the solid catalyst can be easily recovered from the reaction mixture by filtration or centrifugation. As shown in Fig. 6, the catalyst can be successively recycled for 5 times without obviously decrease of the activity and selectivity decreasing. According to the XPS results, we speculate the drop in activity should be mainly ascribed to the loss of acid sites. Metal leaching is also examined by ICP analysis after each recycle, and no Au and Ti can be detected, which is in accordance with the XPS analysis, revealing the high stability of the catalyst.
4 Conclusions
In summary, we have prepared a novel Au–TiO2–RGO composite with uniform and tiny Au NPs supported on layered TiO2–RGO support. After treatment with microwave and sulfuric acid, surface SO42−/TiO2 solid acid was in situ formed. The resulting Au–SO42−/TiO2–RGO was determined as an enhanced and recyclable catalyst for the hydration. The SMSI between Au and TiO2, versatile RGO substrate, and in situ formed SO42−/TiO2 account for the excellent performance. The novel catalyst design strategy is readily extended to other reaction systems with amazing catalytic performance.Acknowledgements
This study is supported by the National Natural Science Funds for Excellent Young Scholars (No. 21222608), Foundation for the Author of National Excellent Doctoral Dissertation of China (No. 201251), Program for New Century Excellent Talents in University (No. NCET-12-0392) and the Program of Introducing Talents of Discipline to Universities (No. B06006).Notes and references
- F. X. Zhu, W. Wang and H. X. Li, J. Am. Chem. Soc., 2011, 133, 11632–11640 CrossRef CAS PubMed.
- S. Wang, C. Miao, W. Wang, Z. Lei and W. Sun, Chin. J. Catal., 2014, 35, 1695–1700 CrossRef CAS.
- R. García-Álvarez, A. E. Díaz-Álvarez, P. Crochet and V. Cadierno, RSC Adv., 2013, 3, 5889–5894 RSC.
- L. Xu, L. Liu, Z. Wang and X. Fu, Chem. Commun., 2015, 51, 11896–11898 RSC.
- H. Yuzawa, S. Yoneyama, A. Yamamoto, M. Aoki, K. Otake, H. Itoh and H. Yoshida, Catal. Sci. Technol., 2013, 3, 1739–1749 CAS.
- F. Li, N. Wang, L. Lu and G. Zhu, J. Org. Chem., 2015, 80, 3538–3546 CrossRef CAS PubMed.
- A. Cavarzan, J. N. H. Reek, F. Trentin, A. Scarso and G. Strukul, Catal. Sci. Technol., 2013, 3, 2898–2901 CAS.
- T. Chen and C. Cai, Catal. Commun., 2015, 65, 102–104 CrossRef CAS.
- B. Demir, F. B. Barlas, E. Guler, P. Z. Gumus, M. Can, M. Yavuz, H. Coskunol and S. Timur, RSC Adv., 2014, 4, 34687–34695 RSC.
- E. A. Artiukha, A. L. Nuzhdin, G. A. Bukhtiyarova, S. Y. Zaytsev, P. E. Plyusnin, Y. V. Shubin and V. I. Bukhtiyarov, Catal. Sci. Technol., 2015, 5, 4741–4745 CAS.
- Y. Ma, S. Qing, Z. Gao, X. Mamat, J. Zhang, H. Li, W. Eli and T. Wang, Catal. Sci. Technol., 2015, 5, 3649–3657 CAS.
- A. Corma and H. Garcia, Chem. Soc. Rev., 2008, 37, 2096–2126 RSC.
- A. Arcadi, Chem. Rev., 2008, 108, 3266–3325 CrossRef CAS PubMed.
- X. Wan, W. Deng, Q. Zhang and Y. Wang, Catal. Today, 2014, 233, 147–154 CrossRef CAS.
- A. E. Lanterna, A. Elhage and J. C. Scaiano, Catal. Sci. Technol., 2015, 5, 4336–4340 CAS.
- K. Yang, C. Meng, L. Lin, X. Peng, X. Chen, X. Wang, W. Dai and X. Fu, Catal. Sci. Technol., 2016, 6, 829–839 CAS.
- Y. Choi, H. Kim, G. Moon, S. Jo and W. Choi, ACS Catal., 2016, 6, 821–828 CrossRef CAS.
- R. Daghrir, P. Drogui and D. Robert, Ind. Eng. Chem. Res., 2013, 52, 3581–3599 CAS.
- D. W. Goodman, Catal. Lett., 2005, 99, 1–4 CrossRef CAS.
- A. Naldoni, F. Riboni, M. Marelli, F. Bossola, G. Ulisse, A. D. Carlo, I. Pis, S. Nappini, M. Malvestuto and M. V. Dozzi, Catal. Sci. Technol., 2016, 6, 3220–3229 CAS.
- C. T. Campbell, Nat. Chem., 2012, 4, 597–598 CrossRef CAS PubMed.
- D. Pang, L. Qiu, R. Zhu and F. Ouyang, Chem. Eng. J., 2015, 270, 590–596 CrossRef CAS.
- A. Bumajdad, M. Madkour, Y. Abdel-Moneam and M. El-Kemary, J. Mater. Sci., 2013, 49, 1743–1754 CrossRef.
- Z. Bian, T. Tachikawa, P. Zhang, M. Fujitsuka and T. Majima, J. Am. Chem. Soc., 2013, 136, 458–465 CrossRef PubMed.
- J. Fang, L. Xu, Z. Zhang, Y. Yuan, S. Cao, Z. Wang, L. Yin, Y. Liao and C. Xue, ACS Appl. Mater. Interfaces, 2013, 5, 8088–8092 CAS.
- A. Corma and P. Serna, Science, 2006, 313, 332–334 CrossRef CAS PubMed.
- L. Liu, X. Gu, Y. Cao, X. Yao, L. Zhang, C. Tang, F. Gao and L. Dong, ACS Catal., 2013, 3, 2768–2775 CrossRef CAS.
- A. Mahmood and S. I. Woo, Korean J. Chem. Eng., 2013, 30, 1876–1881 CrossRef CAS.
- D. I. Enache, J. K. Edwards, P. Landon, B. Solsona-Espriu, A. F. Carley, A. A. Herzing, M. Watanabe, C. J. Kiely, D. W. Knight and G. J. Hutchings, Science, 2006, 311, 362–365 CrossRef CAS PubMed.
- M. M. Khan, S. A. Ansari, J. Lee and M. H. Cho, J. Ind. Eng. Chem., 2013, 19, 1845–1850 CrossRef CAS.
- C. Lin, Y. Song, L. Cao and S. Chen, ACS Appl. Mater. Interfaces, 2013, 5, 13305–13311 CAS.
- Q. Zhao, C. Bai, W. Zhang, Y. Li, G. Zhang, F. Zhang and X. Fan, Ind. Eng. Chem. Res., 2014, 53, 4232–4238 CrossRef CAS.
- W. Q. Fan, Q. H. Lai, Q. H. Zhang and Y. Wang, J. Phys. Chem. C, 2011, 115, 10694–10701 CAS.
- X. Liu, L. Pan, T. Lv and G. Zhu, RSC Adv., 2011, 47, 11984–11986 CAS.
- M. S. Shah, A. R. Park, K. Zhang, J. H. Park and P. J. Yoo, ACS Appl. Mater. Interfaces, 2012, 4, 3893–3901 Search PubMed.
- X. Y. Zhang, H. P. Li, X. L. Cui and Y. Lin, J. Mater. Chem., 2010, 20, 2801–2806 RSC.
- W. S. Hummers, R. E. Offeman, W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- Y. Li, X. Fan, J. Qi, J. Ji, S. Wang, G. Zhang and F. Zhang, Mater. Res. Bull., 2010, 45, 1413–1418 CrossRef CAS.
- G. X. Yu, D. L. Lin, Y. Hu, X. L. Zhou, C. L. Li, L. F. Chen and J. A. Wang, Catal. Today, 2011, 166, 84–90 CrossRef CAS.
- B. Hao, Y. Yan, X. Wang and G. Chen, Nanoscale, 2013, 5, 10472–10480 RSC.
- Y. Wang, J. Yu, W. Xiao and Q. Li, J. Mater. Chem. A, 2014, 2, 3847–3855 CAS.
- Z. Xu, J. Yu and G. Liu, Electrochem. Commun., 2011, 13, 1260–1263 CrossRef CAS.
- Q. Zhao, Y. Li, R. Liu, A. Chen, G. Zhang, F. Zhang and X. Fan, J. Mater. Chem. A, 2013, 1, 15039–15045 CAS.
- J. L. Ropero-Vega, A. Aldana-Pérez, R. Gómez and M. E. Niño-Gómez, Appl. Catal., A, 2010, 379, 24–29 CrossRef CAS.
- W. Li, F. Ma, F. Su, L. Ma, S. Zhang and Y. Guo, ChemSusChem, 2011, 4, 744–756 CrossRef CAS PubMed.
- X. Wang, J. C. Yu, P. Liu, X. Wang, W. Su and X. Fu, J. Photochem. Photobiol., A, 2006, 179, 339–347 CrossRef CAS.
- P. Chen, M. Du, H. Lei, Y. Wang, G. Zhang, F. Zhang and X. Fan, Catal. Commun., 2012, 18, 47–50 CrossRef CAS.
- B. Krishnakumar, R. Velmurugan and M. Swaminathan, Catal. Commun., 2011, 12, 375–379 CrossRef CAS.
- S. Liang, G. B. Hammond and B. Xu, Chem. Commun., 2015, 51, 903–906 RSC.
- M. Hassam and W. S. Li, Tetrahedron, 2015, 71, 2719–2723 CrossRef CAS.
- J. A. Rodriguez, P. J. Ramírez, A. Gian Giacomo, V. E. Francesc, E. Jaime, L. Ping, J. M. Ricart and I. Francesc, Angew. Chem., 2014, 53, 11270–11274 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation of Au–RGO, Au–TiO2, Au–TiO2–RGO-1 and Au–TiO2–RGO-2, TEM image of Au–TiO2, C 1s XPS spectra of GO and Au–TiO2–RGO, full range XPS spectrum of Au–SO42−/TiO2–RGO after 5 cycles. See DOI: 10.1039/c6ra08021a |
| This journal is © The Royal Society of Chemistry 2016 |