Fabrication of a heterostructured Ag/AgCl/Bi2MoO6 plasmonic photocatalyst with efficient visible light activity towards dyes
Qing Yana,
Meng Sun*ab,
Tao Yana,
Mengmeng Lic,
Liangguo Yana,
Dong Weia and
Bin Du*a
aSchool of Resources and Environment, University of Jinan, Shandong Provincial Engineering Technology Research Center for Ecological Carbon Sink and Capture Utilization, Jinan 250022, P. R. China. E-mail: smlcu@163.com; bindu61@gmail.com; Fax: +86-531-82765969; Tel: +86-531-82769235
bFujian Provincial Key Laboratory of Photocatalysis-State Key Laboratory Breeding Base, FuzhouUniversity, Fuzhou 350002, P. R. China
cSchool of Civil Engineering and Architecture, University of Jinan, Jinan 250022, P. R. China
First published on 3rd February 2015
Abstract
A ternary Ag/AgCl/Bi2MoO6 plasmonic photocatalyst was successfully fabricated through a two-step synthesis method. The obtained samples were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and ultraviolet-visible diffuse reflectance spectroscopy (DRS). The DRS results showed that the visible light absorption of the Ag/AgCl/Bi2MoO6 composite had been greatly enhanced owing to the surface plasmonic resonance (SPR) of Ag0 particles. The SEM and TEM images showed that the obtained flower-like Bi2MoO6 microspheres were composed of nanoplates, on the surface of which Ag/AgCl particles were distributed. In the photocatalytic degradation of dyes, Ag/AgCl/Bi2MoO6 photocatalysts exhibited significantly enhanced activities compared with Bi2MoO6. It was also found that the components of Ag/AgCl/Bi2MoO6 have a great influence on the activity. Controlled experiments proved that the degradation of pollutants over Ag/AgCl/Bi2MoO6 was mainly ascribed to the strong oxidation ability of photo-generated holes. On the basis of band structure analysis and active species trapping experimental results, a photocatalytic mechanism is also proposed.
Introduction
Over the past years, the water pollution problem becomes more and more severe because of the rapid development of industrialization and urbanization. The organic pollutants in contaminated wastewater are usually found to be toxic synthetic dyes, which are harmful to the environment and human health. Thus, great efforts have been made to explore green and economic methods for treating the organic pollutants discharged into the environment. For example, numerous studies have focused on the photocatalytic degradation of stable organic pollutants,1–4 which are difficult to remove using traditional treatment methods. TiO2, as an efficient photocatalyst, has been widely studied and applied in the photocatalysis field because of its unique surface structure characteristics, high chemical stability, nontoxicity, and prefect photocatalytic activity.5–8 However, its wide band gap (3.2 eV for anatase TiO2) and low quantum efficiency have greatly hindered its practical application.9–11 Therefore, it is an urgent topic to develop visible-light driven photocatalysts for the practical utilization of solar energy.Recently, noble metals with localized surface plasmonic resonance properties have attracted considerable attention because of their strong absorption of visible light.12–15 Among which, Ag/AgCl-based composites have exhibited the most outstanding photocatalytic activity and stability.16,17 Huang et al. reported the synthesis of Ag/AgCl photocatalyst using ion-exchange method, which exhibited efficient activity for the degradation of methyl orange.18 In order to further improve its activity, many researchers have focused on the morphology-controlled fabrication of Ag/AgCl.16,19 Therefore, many Ag/AgCl-based hierarchical nanostructures, such as Ag/AgCl/WO3,20 AgCl/Ag/TaON,21 and Ag/AgCl/Bi20TiO32,22 have been fabricated and exhibited efficient activity. Furthermore, Ag/AgCl has also been successfully used to combine with flower-like semiconductors, such as Bi2WO6 (ref. 23) and BiOBr,24 and the photocatalytic activity has been greatly improved by the synergy effect of SPR effect driving from Ag nanoparticles and light scattering effect driving from flower-like structure.
In this work, Ag/AgCl has also been used to couple with a flower-like Bi2MoO6 photocatalyst via a two-step route. Bi2MoO6, with a narrow band gap of 2.5–2.8 eV, has been reported as an excellent photocatalyst from the viewpoint of utilizing visible light. It could serve as a solar-energy-conversion material for water splitting and the decomposition of organic compounds under visible-light irradiation.25–27 Unfortunately, the rapid recombination of electrons and holes after photo-excitation leads to the low efficiency of Bi2MoO6. Thus, it was used to couple with Ag/AgCl to enhance the visible light responsive photocatalytic activity. The obtained Ag/AgCl/Bi2MoO6 composites have exhibited efficient activities in photocatalytic decomposing of Rhodamine B (RhB) dye under visible light illumination. After 40 min of reaction, the degradation ratio of RhB was up to 99%. The activity improvement should be ascribed to the SPR effect of Ag0 and the formation of heterojunction, which respectively improved the visible light absorption and separation efficiency of photo-generated carriers.
Experimental section
Synthesis of flower-like Bi2MoO6 microspheres
All chemical reagents were analytical grade and used without further purifications. For the synthesis of flower-like Bi2MoO6 microspheres, 2 mmol of Bi(NO3)3·5H2O and 1 mmol of (NH4)6MoO4·4H2O were dissolved in 20 mL of ethylene glycol and stirred for 120 min. Then 50 mL of ethanol was added into it. The mixture was stirred for another 10 min. The resulting solution was transferred into a 100 mL Teflon-lined stainless steel autoclave and kept at 160 °C for 12 h. Subsequently, the autoclave was cooled to room temperature naturally. The obtained samples were filtered, washed with deionized water for several times, and finally dried at 80 °C in air.Synthesis of heterostructured Ag/AgCl/Bi2MoO6 composites
In a typical synthesis of Ag/AgCl/Bi2MoO6 composites, 0.4 g of Bi2MoO6 powders and 0.075 g of hexadecyltrimethylammoniumchloride (CTAC) were added into 200 mL of deionized water and stirred for 60 min. Then 2.0 mL of 0.1 M AgNO3 was quickly added into the above mixture. During this process, the excessive surfactant CTAC adsorbed onto the surface of Bi2MoO6 and induced Cl− to react with Ag+ to produce AgCl particles in the suspension. After 60 min of continuous stirring, the resulting suspension was then exposed to UV-vis light emitted by a 300 W Xe lamp for a certain time to deoxidize Ag+ to Ag0 on the surface of AgCl particles. The suspension was filtered, washed with deionized water, and dried at 80 °C for 12 h. The illumination time has great influence on the reduction of Ag+, the obtained samples illuminated for 10, 30, and 50 min were marked as AAB-10, AAB-30, and AAB-50, respectively.Characterization of photocatalysts
X-ray diffraction (XRD) patterns of the obtained products were recorded on a Bruker D8 Advance X-ray diffractometer under the conditions of generator voltage = 40 kV; generator current = 40 mA; divergence slit = 1.0 mm; Cu Kα (λ = 1.5406 Å). The morphologies and microstructures were examined with a Hitachi S-4800 scanning electron microscopy (SEM). The transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM) images were measured by a JEOL model JEM 2010 EX instrument at an accelerating voltage of 200 kV. Carbon-coated copper grid was used as the sample holder. The ultraviolet-visible diffuse reflectance spectroscopy (DRS) were measured at room temperature in the range of 200–700 nm on a UV-vis spectrophotometer (Cary 500 Scan Spectrophotometers, Varian, and U.S.A) equipped with an integrating sphere attachment. X-ray photoelectron spectroscopy (XPS) analysis was conducted on an ESCALAB 250 photoelectron spectroscopy (Thermo Fisher Scientific) at 3.0 × 10−10 mbar using Al Kα X-ray beam (1486.6 eV).Evaluation of photocatalytic performance
Photocatalytic reactions were conducted in a customized reactor. The visible light photocatalytic activity of Ag/AgCl/Bi2MoO6 composites were evaluated by the degradation of dyes, a 300 W xenon lamp (PLS-SXE300C, Beijing Perfect Light Co. Ltd., Beijing) with two cutoff filters (420 nm < λ < 800 nm) has been used as light source. In each experiment, 100 mg of photocatalyst was added into 100 mL of RhB dye aqueous solution (2 × 10−5 M). Before irradiation, the suspensions were magnetically stirred in dark for 60 min to ensure the establishment of an adsorption–desorption equilibrium between the photocatalyst and dye. During reaction under visible light irradiation, 3 mL of suspension was sampled and centrifuged to remove the photocatalyst particles at given time intervals. The resulting clear liquor was analyzed on a PerkinElmer UV-vis spectrophotometer (model: Lambda 35) to record the concentration changes of dyes. The percentage of degradation is reported as C/C0. C is the absorption of RhB absorption spectrum at 554 nm. And C0 is the absorption of the starting concentration when adsorption–desorption equilibrium is achieved.Results and discussion
Structural characterization
Fig. 1 shows the XRD patterns of the obtained Bi2MoO6, Ag/AgCl and Ag/AgCl/Bi2MoO6 photocatalysts. As we can see, all the diffraction peaks of the obtained Bi2MoO6 sample located at 10.9, 23.6, 28.2, 32.5, 36.0, 46.8, 55.4, and 58.4° can be assigned to orthorhombic-phase Bi2MoO6 (JCPDS no. 76-2388). No impurity has been detected from the diffraction patterns. For the obtained Ag/AgCl sample, five characteristic diffraction peaks located at 27.8, 32.2, 46.2, 54.8, and 57.5° have been observed, which indexed to the (111), (200), (220), (311), and (222) planes of cubic phase AgCl (JCPDS no. 31-1238). In addition, a very weak diffraction peak of cubic phase Ag (JCPDS no. 65-2871) located at 38.1° has also been observed, this result was in accordance with the experimental phenomena that the color of AgCl changed from white to gray during light irradiation. As for the Ag/AgCl/Bi2MoO6 photocatalysts, it is difficult to distinguish which diffraction peak was ascribed to Ag/AgCl or Bi2MoO6 because of the overlapping of their main diffraction peaks (see Fig. 1).Morphology and microstructure
The morphologies of Bi2MoO6, Ag/AgCl and Ag/AgCl/Bi2MoO6 composites have been characterized by SEM. Fig. 2a shows that the obtained Bi2MoO6 exhibited flower-like microsphere morphology. The microspheres with average diameter of 1–2 μm were composed of large amount of nanoplates. Meanwhile, Fig. 2b shows that the obtained Ag/AgCl exhibited particle morphology with smaller sizes of 400–500 nm compared with Bi2MoO6. Even smaller Ag nanoparticles (average diameter of 50 nm) have also been observed on the surface of AgCl. Fig. 2c shows that when Ag/AgCl was coupled with Bi2MoO6, the Ag/AgCl particles would be decorated on the surfaces of flower-like Bi2MoO6 structures. The element composition of the Ag/AgCl/Bi2MoO6 composites has been measured using energy-dispersive X-ray (EDX) spectroscopy, and the corresponding result was shown in Fig. 2d. It shows that the composites are mainly consisted of Bi, O, Mo, Cl, and Ag elements. | ||
| Fig. 2 SEM images of the as-prepared sample: (a) pure Bi2MoO6, (b) Ag/AgCl, (c) Ag/AgCl/Bi2MoO6 composite, and (d) corresponding EDX spectrum for Ag/AgCl/Bi2MoO6 composite. | ||
The morphology and nanostructures of Ag/AgCl/Bi2MoO6 composite were further investigated by TEM and HRTEM. Fig. 3a shows that the Ag/AgCl/Bi2MoO6 composite possessed microsphere morphology, and the average diameter of the microspheres was about 1.5 μm. The HRTEM image showing clear lattice planes was shown in Fig. 3b. The uniform fringe with an interval of 0.327 nm was indexed to the (140) facet of Bi2MoO6. Another set of clear fringes with an inter-planer spacing of 0.277 nm corresponded to the (200) lattice plane of AgCl, while the fringe of 0.204 nm could be ascribed to the (200) facet of Ag. Fig. 3b implies that obvious interfaces between Bi2MoO6 and Ag/AgCl can be observed. This result suggests that the heterojunction is actually formed at the interfaces of those materials. The elemental mapping has been performed to investigate the distribution of Ag/AgCl over Ag/AgCl/Bi2MoO6 composites. Fig. 3 also displays the elemental mapping images for the Ag/AgCl/Bi2MoO6 sample. The cyan, red, and yellow-brown color of the images was respectively acquired at the K-line spectra of Ag, Cl, and O elements, while that for the magenta and green color was respectively acquired at the L-line spectra of Bi and Mo elements. This result provides solid evidence that Ag/AgCl were successfully combined with Bi2MoO6.
DRS and BET surface analysis
The UV-vis diffuse reflectance spectra (DRS) have been performed to investigate the optical properties of the as-prepared samples. As shown in Fig. 4a, the absorption edge of Bi2MoO6 was estimated to 485 nm showing a strong absorption in the UV range, while Ag/AgCl exhibited strong light absorption in the visible light region. When Ag/AgCl combined with Bi2MoO6, the absorption edges of Ag/AgCl/Bi2MoO6 composites were red-shifted. The visible light adsorption has also been greatly enhanced compared with pure Bi2MoO6. Fig. 4b shows the nitrogen adsorption isotherm for the as-synthesized AAB-30 and Bi2MoO6 sample, which exhibited the stepwise adsorption and desorption (type-IV isotherm). The pore-size distribution plot (inset) showed that the samples had exhibited a mean pore diameter of 16 nm with a narrow distribution of pore size. The BET specific surface area of AAB-30 was 50.4 m2 g−1, larger than that of Bi2MoO6 (35.7 m2 g−1).XPS characterization
The XPS characterization was performed to investigate the chemical composition of Ag/AgCl/Bi2MoO6 sample and identify the chemical state of the Ag element. The results have been added into the revised manuscript. Fig. 5a shows the XPS survey spectra of AAB-30 sample. As we can see, the sample contains Bi, Mo, O, Ag, Cl and C elements. The C element should be attributed to the adventitious hydrocarbon from the XPS instrument itself. Fig. 5b shows that Ag 3d peak locates at the binding energy of 367.2 eV. Compared with that for bulk Ag (368.2 eV), it shows a peak shifts to the lower binding energy. Very similar phenomenon has also been reported,28–30 and the shift of the binding energy of Ag 3d should be primarily attributed to the interactions among the Ag, AgCl and Bi2MoO6.Photocatalytic activity tests
To investigate the influences of Ag/AgCl on the photocatalytic activities, Ag/AgCl/Bi2MoO6 composites prepared under different conditions have been used to decompose RhB in water under visible light irradiation. Fig. 6 shows the photocatalytic degradation results of RhB over pure Bi2MoO6, Ag/AgCl, and Ag/AgCl/Bi2MoO6 composites. As we can see, all of the Ag/AgCl/Bi2MoO6 composites have exhibited efficient activities, which were higher than that for pure Bi2MoO6 or Ag/AgCl. After 45 min of visible light irradiation, the degradation ratio of RhB over Ag/AgCl and Bi2MoO6 was only about 70 and 20%, respectively. As for the Ag/AgCl/Bi2MoO6 composites, the AAB-30 sample exhibited the best activity, and the degradation ratio of RhB was nearly to 100%. That is to say, the amount of Ag/AgCl has great influence on the photocatalytic activity. Fig. 7 shows the temporal evolution of the spectral changes of RhB mediated by AAB-30. It exhibited that the absorption peak of RhB located at 554 nm gradually blue-shifted to 499 nm within 30 min of reaction. This hypsochromic shift of absorption maximum was caused by the N-deethylation of RhB during irradiation, which has been confirmed by Watanabe and co-workers.31,32 After that the peak decreased in the following degradation process and finally disappeared. | ||
| Fig. 6 Degradation rates of RhB under visible light irradiation using Bi2MoO6, Ag/AgCl, and Ag/AgCl/Bi2MoO6 composites. | ||
 | ||
| Fig. 7 The absorption spectra of RhB in the presence of AAB-30 under exposure to visible light (420 nm < λ < 800 nm). | ||
The reaction kinetics study shows that the photocatalytic degradation of RhB dye over those photocatalysts followed pseudo-first-order kinetic model. The rate constant (k) can be calculated by the formula of −ln(C/C0) = kt.33 k is kinetic constant (min−1), while t represents the irradiation time (min). Fig. 8 showed the kinetic plots for RhB degradation over different photocatalysts. The rate constant of the photocatalytic degradation of RhB over AAB-30 photocatalyst is 0.1295 min−1, which is much higher than that of Bi2MoO6 (0.0036 min−1), Ag/AgCl (0.0200 min−1), or other Ag/AgCl/Bi2MoO6 composites (0.0435 and 0.0639 min−1 for AAB-10 and AAB-50, respectively). Thus, Ag/AgCl/Bi2MoO6 composite photocatalysts exhibited the enhanced photocatalytic activities, among of which AAB-30 was the most efficient. That is to say, the irradiation time played an important role in improving the activities, with too long or too short illumination time should be avoided. With a shorter illumination time as to 10 min, only a very small amount of Ag0 would be produced over the sample, leading to weak light absorption ability and a lower activity. However, when the illumination time was longer than 50 min, too much of AgCl would be photo-reduced to form Ag/AgCl nano-clusters. The Ag/AgCl clusters might further agglomerated and shaded the active sties on the surface of Bi2MoO6.34,35 Therefore, the degradation rates would be depressed accordingly.
Fig. 9 showed the photocatalytic degradation of RhB at different dosages of AAB-30 photocatalyst under visible light irradiation. It was obviously that the degradation rate of RhB was accordingly improved when the amount of AAB-30 added was increased from 0.025 to 0.100 g, and nearly 100% of RhB has been decomposed within 30 min of reaction. The activity increased with photocatalyst concentration enhancement was a characteristic of heterogeneous catalysis. This phenomenon can be rationalized in relation to the amount of surface active cites as well as the light penetration into the suspensions.
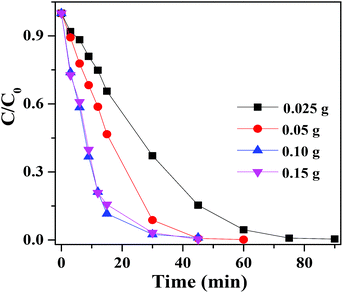 | ||
| Fig. 9 Photocatalytic degradation of RhB with different dosages of AAB-30 under the visible light (420 nm < λ < 800 nm) irradiation. | ||
The stability of a photocatalyst is very important for its practical application. In this work, Ag/AgCl/Bi2MoO6 composite photocatalyst was recycled for four times in the photocatalytic degradation experiments. Fig. 10 showed the life-time test results. The photocatalytic activity of AAB-30 composite only deteriorated slightly in the reuse cycles. Fig. 11 showed the XRD patterns of AAB-30 composites before and after RhB degradation. It illustrated that the crystal structure of the AAB-30 photocatalyst had not changed during the photocatalytic reaction. In order to study the degradation mechanism of RhB over AAB-30 photocatalyst, diagnostic experiments have also been performed with different active scavenger added. Herein, tert-butanol (TBA), p-benzoquinone (PBQ), and oxalate was respectively used as hydroxyl radical, superoxide radical, and hole scavengers.36,37 Fig. 12 presented the influences of active species (such as ˙OH, ˙O2−, and hVB+) on the degradation rates of RhB. In the presence of AO (1.0 mL added), the peak blue-shift of RhB became very slow during the degradation process, meaning that the N-deethylation of RhB becoming more difficult. What was worse, the blue-shift absorption peak of RhB did not disappear after 90 min of reaction, but observed with relatively higher peak intensity. When PBQ was added into the system, a very similar situation was observed, but the degradation rate was further decreased. However, when certain amount of TBA was added into the system, it could hardly affect the N-deethylation process and degradation rates of RhB. That is to say, during the degradation process of RhB, ˙O2− and hVB+ played more important role compared with other active species.
 | ||
| Fig. 10 Photocatalytic performances of AAB-30 composite photocatalyst in the first four reuse cycles. | ||
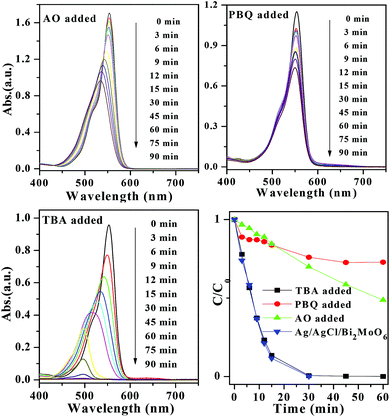 | ||
| Fig. 12 The absorption spectra of RhB in the presence of AAB-30 under exposure to visible light (420 nm < λ < 800 nm) with different active scavenger added, such as AO, PBQ, and TBA. | ||
As discussed above, Ag/AgCl/Bi2MoO6 composites have exhibited enhanced photocatalytic activity for decomposing RhB dye compared with pure Bi2MoO6. This activity enhancement should be ascribed to two main reasons, including their suitable band potentials and the SPR effect of Ag. According to the band gap structure of Ag/AgCl/Bi2MoO6 and the effects of scavengers, a possible pathway for the photocatalytic activity enhancement mechanism of Ag/AgCl/Bi2MoO6 photocatalyst was proposed (shown in Scheme 1). Bi2MoO6 possessed a band gap of 2.58 eV, and the conduction band (CB) and valence band (VB) energy levels are ca. −0.13 and 2.45 eV (vs. NHE).26 The positive potential endows the VB holes of Bi2MoO6 with high oxidation ability. That is to say, the holes generated over could directly react with pollutants absorbed on its surface. As for Ag/AgCl, it has been reported as an efficient visible light photocatalyst result in the strong surface plasmon resonance of metallic Ag0 species. With a large band gap of 3.26 eV, AgCl cannot be excited by visible light, but its lower CB potential (−0.06 vs. NHE) enabled the plasmon-induced electrons over Ag to transfer to the CB of AgCl.26 On the other hand, metallic Ag specie could strongly absorb visible light because of its surface plasmon resonance effect, which was also beneficial for activity enhancement. The electrons transferred to the surface of AgCl could be further captured by dissolved oxygen to produce ˙O2−. Because the CB potential of Bi2MoO6 was more negative than that of Ag+/Ag (+0.799 eV vs. NHE), the CB electrons over Bi2MoO6 should transfer to the VB of metallic Ag and captured by the holes generated, leaving the VB holes generated over Bi2MoO6 directly to react with surface absorbed pollutants.
 | ||
| Scheme 1 Photocatalytic degradation mechanism for dyes over Ag/AgCl/Bi2MoO6 composites under visible light irradiation. | ||
Conclusions
Novel visible-light responsive Ag/AgCl/Bi2MoO6 plasmonic photocatalyst was successfully fabricated through a two-step synthesis method. After combination of Ag/AgCl, the Ag/AgCl/Bi2MoO6 photocatalyst possessed a significantly enhanced visible light activity in decomposing dyes. The highest degradation efficiency was observed for the AAB-30 sample. The activity enhancement was mainly attributed to the SPR effect of Ag0, and high separation efficiency of electron–hole pairs on their heterojunction interfaces. A possible photocatalytic degradation mechanism was proposed based on the band potentials of Ag/AgCl and Bi2MoO6. This method was expected to be extended for other Ag/AgCl loaded materials, which might have potential applications in removing pollutants.Acknowledgements
This work was financially supported by the Scientific Research Reward Fund for Excellent Young and Middle-Aged Scientists of Shandong Province (BS2012HZ001), National Natural Science Foundation of China (no. 21103069, 21075052, 21175057, and 40672158), and Scientific Research Foundation for Doctors of University of Jinan (XBS1037 and XKY1043).References
- H. B. Wu, H. H. Hng and X. W. Lou, Adv. Mater., 2012, 24, 2567 CrossRef CAS PubMed.
- P. Wang, B. Huang, X. Qin, X. Zhang, Y. Dai, J. Wei and M. H. Whangbo, Angew. Chem., Int. Ed., 2008, 47, 7931 CrossRef CAS PubMed.
- W. Zhou, Z. Yin, Y. Du, X. Huang, Z. Zeng, Z. Fan, H. Liu, J. Wang and H. Zhang, Small, 2013, 9, 140 CrossRef CAS PubMed.
- Y. Bi, H. Hu, S. Ouyang, Z. Jiao, G. Lu and J. Ye, J. Mater. Chem., 2012, 22, 14847 RSC.
- Y. Ou, J. Lin, S. Fang and D. Liao, Catal. Commun., 2007, 8, 936 CrossRef CAS PubMed.
- B. Jiang, C. Tian, Q. Pan, Z. Jiang, J. Q. Wang, W. Yan and H. Fu, J. Phys. Chem. C, 2011, 115, 23718 CAS.
- S. Liu, J. Yu and M. Jaroniec, J. Am. Chem. Soc., 2010, 132, 11914 CrossRef CAS PubMed.
- X. Chen, L. Liu, P. Y. Yu and S. S. Mao, Science, 2011, 331, 746 CrossRef CAS PubMed.
- X. X. Yao and X. H. Liu, J. Hazard. Mater., 2014, 280, 260 CrossRef CAS PubMed.
- R. Dong, B. Tian, C. Zeng, T. Li, T. Wang and J. Zhang, J. Phys. Chem. C, 2013, 117, 213 CAS.
- C. An, S. Peng and Y. Sun, Adv. Mater., 2010, 22, 2570 CrossRef CAS PubMed.
- Z. W. Seh, S. Liu, M. Low, S. Y. Zhang, Z. Liu, A. Mlayah and M. Y. Han, Adv. Mater., 2012, 24, 2310 CrossRef CAS PubMed.
- X. X. Yao and X. H. Liu, J. Mol. Catal. A: Chem., 2014, 393, 30 CrossRef CAS PubMed.
- L. Han, P. Wang, C. Zhu, Y. Zhai and S. Dong, Nanoscale, 2011, 3, 2931 RSC.
- P. A. DeSario, J. J. Pietron, D. E. DeVantier, T. H. Brintlinger, R. M. Stroud and D. R. Roli-son, Nanoscale, 2013, 5, 8073 RSC.
- M. Zhu, P. Chen, W. Ma, B. Lei and M. Liu, ACS Appl. Mater. Interfaces, 2012, 4, 6386 CAS.
- Y. Tang, Z. Jiang, G. Xing, A. Li, P. D. Kanhere, Y. Zhang, T. C. Sum, S. Li, X. Chen, Z. Dong and Z. Chen, Adv. Funct. Mater., 2013, 23, 2932 CrossRef CAS.
- P. Wang, B. Huang, X. Qin, X. Zhang, Y. Dai, J. Wei and M. H. Whangbo, Angew. Chem., Int. Ed., 2008, 47, 7931 CrossRef CAS PubMed.
- Y. Bi and J. Ye, Chem. Commun., 2009, 43, 6551 RSC.
- D. Chen, T. Li, Q. Chen, J. Gao, B. Fan, J. Li, X. Li, R. Zhang, J. Sun and L. Gao, Nanoscale, 2012, 4, 5431 RSC.
- J. Hou, C. Yang, Z. Wang, Q. Ji, Y. Li, G. Huang, S. Jiao and H. Zhu, Appl. Catal., B, 2013, 142–143, 579 CrossRef CAS PubMed.
- J. Hou, Z. Wang, C. Yang, W. Zhou, S. Jiao and H. Zhu, J. Phys. Chem. C, 2013, 117, 5132 CAS.
- L. S. Zhang, K. H. Wong, Z. G. Chen, J. C. Yu, J. C. Zhao, C. Hu, C. Y. Chan and P. K. Wong, Appl. Catal., A, 2009, 363, 221 CrossRef CAS PubMed.
- H. F. Cheng, B. B. Huang, P. Wang, Z. Y. Wang, Z. Z. Lou, J. P. Wang, X. Y. Qin, X. Y. Zhang and Y. Dai, Chem. Commun., 2011, 47, 7054 RSC.
- Y. Shimodaira, H. Kato, H. Kobayashi and A. Kudo, J. Phys. Chem. B, 2006, 110, 17790 CrossRef CAS PubMed.
- J. Zhang, C. G. Niu, J. Ke, L. F. Zhou and G. M. Zeng, Chem. Commun., 2015, 59, 30 CAS.
- X. Zhao, J. H. Qu, H. J. Liu and C. Hu, Environ. Sci. Technol., 2007, 41, 6802 CrossRef CAS.
- Y. H. Zheng, L. R. Zheng, Y. Y. Zhan, X. Y. Lin, Q. Zheng and K. M. Wei, Inorg. Chem., 2007, 46, 6980 CrossRef CAS PubMed.
- D. D. Lin, H. Wu, R. Zhang and W. Pan, Chem. Mater., 2009, 21, 3479 CrossRef CAS.
- W. W. Lu, S. Y. Gao and J. J. Wang, J. Phys. Chem. C, 2008, 112, 16792 CAS.
- T. Watanabe, T. Takizawa and K. Honda, J. Phys. Chem., 1977, 81, 1845 CrossRef CAS.
- T. Takizawa, T. Watanabe and K. Honda, J. Phys. Chem., 1978, 82, 1391 CrossRef CAS.
- W. S. Wang, H. Du, R. X. Wang, T. Wen and A. W. Xu, Nanoscale, 2013, 5, 3315 RSC.
- J. D. Zhuang, W. X. Dai and P. Liu, Langmuir, 2010, 26, 9686 CrossRef CAS PubMed.
- Y. H. Liang, S. L. Lin, L. Liu, J. S. Hu and W. Q. Cui, Appl. Catal., B, 2015, 164, 192 CrossRef CAS PubMed.
- Y. Yang, W. Guo, Y. Guo, Y. Zhao, X. Yuan and Y. Guo, J. Hazard. Mater., 2014, 271, 150 CrossRef CAS PubMed.
- X. X. Yao and X. H. Liu, J. Hazard. Mater., 2014, 280, 260 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |






