Reduction kinetics of hematite to magnetite under hydrothermal treatments
J. F. Lu and
C. J. Tsai*
National Tsing-Hua University, Department of Material Science and Engineering, Hsinchu, Taiwan 30013, Republic of China. E-mail: cjtsai@mx.nthu.edu.tw
First published on 23rd January 2015
Abstract
The phase transformation of α-Fe2O3 to Fe3O4 was observed by the hydrothermal treatment of a ferric solution at 160–220 °C with the addition of both KOH and EDA into the reaction system. The reactions began with the formation of α-Fe2O3 hexagonal plates followed by the phase transformation involving the dissolution of the α-Fe2O3 hexagonal platelets, the reduction of Fe3+ to Fe2+, and the nucleation and growth of new Fe3O4 polyhedral particles. The activation energies for the phase transformation of α-Fe2O3 to Fe3O4 under hydrothermal conditions are estimated to be 96.4 ± 11, 113.1 ± 5, and 118.3 ± 11 kJ mol−1 for the addition of 0.5, 1 and 1.5 ml of EDA, respectively, which are about the same for the typical phase transformation of α-Fe2O3 to Fe3O4 in a hydrogen atmosphere.
1. Introduction
The more stable phases of iron oxides are hematite (α-Fe2O3) and magnetite (Fe3O4). Hematite can be used in a lot of applications such as sensors,1 water photooxidation,2 drug delivery,3 lithium ion battery,4 pigmentation,5 and solar cell,6 and the application of magnetite can be found in biomedicine,7–11 magnetic devices,12 etc. Thus, studies about the nano/microstructures of iron oxides and their properties, which are related to the intrinsic structure and crystal shapes, have been intensively engaged, particularly for hematite and magnetite. Many researches have demonstrated the capability of using chemical syntheses to control the particle morphologies of the iron oxide by surfactants.13–26 Morphologies, such as wires,16 rods,17 tubes,18 rings,19 disks,20 cubes,21 spheres,22 hexagonal platelets,23,24 of α-Fe2O3 and polyhedral particles of Fe3O4 (ref. 25 and 26) have been synthesized successfully.The phase transformation between different iron oxides is technologically important due to the fact that the process provides alternative routes to produce unusual particle shapes for some phases.27 The phase transformation between hematite and magnetite is also a topic of geological interest.28
Several reaction routes were reported for the phase transformation between hematite and magnetite. The first route is the non-redox transformation of iron oxides, which was made possible by adding Fe2+ into solution to form Fe3O4 or removing Fe2+ out of the solution to reverse to α-Fe2O3.29 The second route is the redox reaction of iron oxides under oxidizing (oxygen) or reducing (hydrogen) condition.27,30–36 Viswanath et al. investigated the kinetics of the reduction of Fe2O3 to Fe3O4 by the isothermal differential thermal analysis method.30 Lin et al. used a temperature-programmed reduction method to obtain the activation energy for the transformation of Fe2O3 to Fe3O4 in H2 containing atmosphere, and the activation energy was reported to be 89.13 kJ mol−1.31 Wang et al. used thermal gravimetric analysis (TGA) to obtain an activation energy of 152.44 kJ mol−1 for the transformation. The relatively large activation energy was attributed to the high density of the particle structure.32 Tiernan et al. investigated the reduction of iron oxide and obtained its activation energy by constant rate thermal analysis (CRTA), and the value of the activation energy for the Fe2O3 to Fe3O4 transformation was 96 kJ mol−1.33
Yanagisawa et al. also showed that by controlling the mineralizer solutions, temperatures, and partial pressures of hydrogen in a hydrothermal system, phase transformation from α-Fe2O3 to Fe3O4 particles can be achieved, However, the activation energy was not reported.37 The third route is the redox reaction of iron oxide in hydrothermal conditions with a reducing agent such as sodium sulfite and diethylene glycol.38,39 Sapieszko et al. observed a similar phase transformation from α-Fe2O3 hexagonal platelets to octahedral Fe3O4 particles triggered by the addition of hydrazine, which was used as an anti-oxidant during the hydrothermal process.40 However, no details of the transformation process were discussed. The fourth route is the mechanochemical reaction, which involves ball-milling α-Fe2O3 with the addition of other oxides that decompose and assist the transformation of α-Fe2O3 to Fe3O4.41
Most of the phase transformation kinetics of hematite and magnetite were mainly conducted with solid iron oxides in gas ambient containing H2 molecules. In this experiment, we explored the phase transformation kinetics of hematite and magnetite in a solution environment containing ethylenediamine (EDA or en in ligand form) in alkaline solution under hydrothermal conditions. The role of different ions in the reaction system was explored, and the activation energies for the transformation in a solution environment are reported for the first time.
2. Experimental methods
Ferric nitrate (Fe(NO3)3·9H2O) (SHOWA, 99%), 1 mmol, was dissolved in 10 ml of distilled water to form a transparent yellow solution. Next, three different mineralizing agents were added to the ferric solution. The first mineralizing agent was a 10.67 M potassium hydroxide (KOH) or sodium hydroxide (NaOH) aqueous solution, 5 ml, which was added dropwisely into the ferric solution. The second one was 0.5 ml of EDA (TEDIA, 99%) which was added gradually to the ferric solution. The third type of mineralizing agent was the combination of KOH and EDA. The 10.67 M KOH solution, 5 ml, was added first followed by the addition of 0.5, 1, or 1.5 ml of EDA. After adding these mineralizing agents, brown Fe(OH)3 or FeOOH suspensions were obtained. Then, these solutions were stirred for 30 min before transferring the mixture into a Teflon-lined stainless steel autoclave of 40 ml capacity, followed by heat treatments at 160 °C to 220 °C for different times. After that, the autoclave was cooled down to room temperature in air. The precipitates were collected by centrifugation, washed with deionized water and ethanol several times to remove organic and impurities, and finally dried in air at 80 °C for 12 h.The as-synthesized powder was characterized by X-ray diffraction (XRD, BRUKER, D2) with Cu-Kα radiation, field emission scanning electron microscopy (JOEL, 6500 FESEM), transmission electron microscopy (TEM) (JOEL, TEM-2010), and Raman spectroscopy (Horiba Jobin Yvon, LABRAM HR 800 UV).
3. Results and discussions
Fig. 1 shows the typical SEM and TEM images of the iron oxide particles that were synthesized under the hydrothermal condition of 200 °C for 9 h in the ferric solution with three different mineralizing agents, KOH, EDA, and KOH/EDA. Fig. 1(a) shows the α-Fe2O3 hexagonal plates, which were obtained with the addition of KOH. The plates have an average size of about 10 μm in edge-length and about 500 nm in thickness. In Fig. 1(b), the hexagonal plate has the zone axis of [0001] and the six directions normal to the edge are 〈2![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0〉 and its other five equivalent directions. Fig. 1(c) shows the α-Fe2O3 hexagonal bipyramid particles obtained when EDA was added into the system. The average lateral size of the α-Fe2O3 particles is about 120 nm. In Fig. 1(d), the hexagonal bipyramid shows that the pyramid is pointed in the direction of 〈0001〉. According to previous studies, the bipyramidal structure is enclosed by {10
0〉 and its other five equivalent directions. Fig. 1(c) shows the α-Fe2O3 hexagonal bipyramid particles obtained when EDA was added into the system. The average lateral size of the α-Fe2O3 particles is about 120 nm. In Fig. 1(d), the hexagonal bipyramid shows that the pyramid is pointed in the direction of 〈0001〉. According to previous studies, the bipyramidal structure is enclosed by {10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1} crystal planes.42 Fig. 1(e) shows the Fe3O4 polyhedral particles obtained with the addition of both KOH and 1 ml of EDA into the reaction system. The Fe3O4 polyhedral particles, mainly octahedral in shape, have an average lateral size in the range of 5–25 μm. In Fig. 1(f), the Fe3O4 polyhedral particles are composed of a pure magnetite phase and the diffraction spots are identified to be the (202), (0
1} crystal planes.42 Fig. 1(e) shows the Fe3O4 polyhedral particles obtained with the addition of both KOH and 1 ml of EDA into the reaction system. The Fe3O4 polyhedral particles, mainly octahedral in shape, have an average lateral size in the range of 5–25 μm. In Fig. 1(f), the Fe3O4 polyhedral particles are composed of a pure magnetite phase and the diffraction spots are identified to be the (202), (0![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 2), (
2), (![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif)
![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0) planes and their equivalent planes under an incident electron beam along [
0) planes and their equivalent planes under an incident electron beam along [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11].
11].
The crystal structures of these iron oxide particles were analyzed by XRD as shown in Fig. 2(a). The phase was identified to be α-Fe2O3 when either KOH or EDA alone was added to the reaction system, despite the different morphologies. The diffraction peaks match the JCPDS card no. 33-0664, which is a rhombohedral structure with the space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c. The diffraction peaks of the polyhedral particles, which were obtained with the addition of both KOH and EDA into the reaction system, corresponds to the phase of Fe3O4, JCPDS card no. 19-0629, which is a face-centered cubic structure with the space group Fd
c. The diffraction peaks of the polyhedral particles, which were obtained with the addition of both KOH and EDA into the reaction system, corresponds to the phase of Fe3O4, JCPDS card no. 19-0629, which is a face-centered cubic structure with the space group Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m. The characteristic reflections in Fe3O4 phase and γ-Fe2O3 phase are about the same.43 Here, the diffraction peaks of the (221), (210) and (213) planes for the γ-Fe2O3 phase does not exist. To further clarify the phase of the polyhedral particles, the Raman spectra of the α-Fe2O3 hexagonal plates and Fe3O4 polyhedral particles are shown in Fig. 2(b). α-Fe2O3 can be characterized by the peaks at around 225, 247, 299, 412, 497, 613, and 1320 cm−1. The peaks at 306, 538 and 668 cm−1 are attributed to Fe3O4. For γ-Fe2O3 particles, Raman peaks should be observed at around 350, 500, 700, 1400, and 1560 cm−1.44–46 The lack of Raman peaks at the high wavenumbers (>1000 cm−1) for the bottom part of Fig. 2(b) clearly indicates that the particles are Fe3O4. The appearance of the Fe3O4 phase during reaction is a clear evidence that the valence change from Fe3+ to Fe2+ during the reaction must occur due to the fact that Fe2+ ions are required to occupy the octahedral sites of Fe3O4. It is interesting to know that the particles obtained from the reaction systems with the addition of KOH and EDA alone have the same phase but difference shapes. One would assume that the reaction system with the addition of both KOH and EDA would produce particles with maybe different shapes but still maintain the phase of α-Fe2O3. However, the results show that the particles that we obtained have a different phase, Fe3O4.
m. The characteristic reflections in Fe3O4 phase and γ-Fe2O3 phase are about the same.43 Here, the diffraction peaks of the (221), (210) and (213) planes for the γ-Fe2O3 phase does not exist. To further clarify the phase of the polyhedral particles, the Raman spectra of the α-Fe2O3 hexagonal plates and Fe3O4 polyhedral particles are shown in Fig. 2(b). α-Fe2O3 can be characterized by the peaks at around 225, 247, 299, 412, 497, 613, and 1320 cm−1. The peaks at 306, 538 and 668 cm−1 are attributed to Fe3O4. For γ-Fe2O3 particles, Raman peaks should be observed at around 350, 500, 700, 1400, and 1560 cm−1.44–46 The lack of Raman peaks at the high wavenumbers (>1000 cm−1) for the bottom part of Fig. 2(b) clearly indicates that the particles are Fe3O4. The appearance of the Fe3O4 phase during reaction is a clear evidence that the valence change from Fe3+ to Fe2+ during the reaction must occur due to the fact that Fe2+ ions are required to occupy the octahedral sites of Fe3O4. It is interesting to know that the particles obtained from the reaction systems with the addition of KOH and EDA alone have the same phase but difference shapes. One would assume that the reaction system with the addition of both KOH and EDA would produce particles with maybe different shapes but still maintain the phase of α-Fe2O3. However, the results show that the particles that we obtained have a different phase, Fe3O4.
 | ||
| Fig. 2 (a) The corresponding XRD patterns for the samples shown in Fig. 1. (b) The Raman spectra of α-Fe2O3 micro-plates obtained with the KOH mineralizing agent and Fe3O4 particles obtained with both KOH and EDA mineralizing agents. | ||
The phase transformation process of α-Fe2O3 to the Fe3O4 was further investigated by varying the heat treatment times of the reaction systems with the addition of both KOH and EDA and the hydrothermal temperature still fixed at 200 °C. Fig. 3(a) shows that after 2 h of growth, the main phase of the particles is α-Fe2O3 hexagonal plates. The edge of the hexagonal plate is not as straight as that obtained for the reaction system with KOH only. As the reaction times increased to 3, 5, and 7 h, as shown in Fig. 3(b)–(d), respectively, one can observe that the original hexagonal plate started to dissolve and become smaller and evolved to plates with an irregular shape. At the same time, small octahedron particles appeared and gradually became larger as the reaction time increased. At the reaction time of 8 h, as shown in Fig. 3(e), the observed particles are mainly polyhedron in shape and small amount of plate-like particles still existed. Finally, all particles were in the form of polyhedron when the reaction time was increased to 9 h, as shown in Fig. 3(f). In order to check whether the Fe3O4 particles could be directly transformed by the dehydration of iron hydroxide, we also examined the end product of iron hydroxides with only EDA or only KOH solution added and hydrothermally treated at the same conditions. Both cases produced the α-Fe2O3 phase only. Hence, the result indicated that the reaction processes involved four steps: (1) the reaction systems rapidly transformed Fe(OH)3 or FeOOH to α-Fe2O3 hexagonal plates under the hydrothermal conditions, (2) the α-Fe2O3 hexagonal plates dissolved gradually, (3) the reduction process caused valence transition from Fe3+ to Fe2+, and (4) the Fe3O4 particles started to nucleate then finally grew to form polyhedral particles.
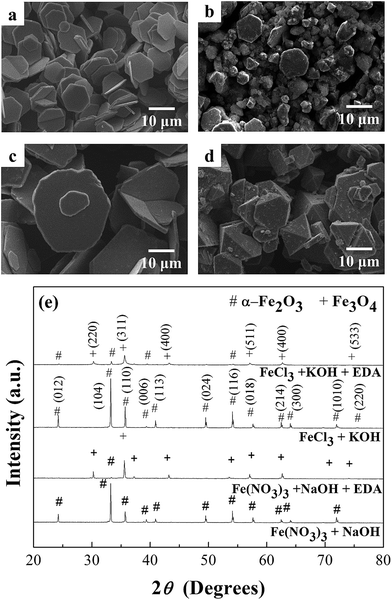
In order to understand the role of K+ ions and NO3− ions on the phase transformation process, the precursor of Fe(NO3)3 was replaced by FeCl3 to check the effect of NO3− on the reaction process. Moreover, the KOH mineralizing agent was substituted by NaOH with the same molarity to examine its effect on the reaction process. The hydrothermal conditions were maintained the same at 200 °C for 9 h. Four cases were investigated: the first and second cases were solutions with the FeCl3 precursor and the addition of either KOH only or both KOH and EDA (1 ml). The third and fourth cases were solutions with the Fe(NO3)3 precursor and the addition of either NaOH only or both NaOH and EDA (1 ml). Fig. 4(a) shows that the α-Fe2O3 hexagonal plates were obtained when the reaction system consisted of FeCl3 and KOH. The phase transformation from α-Fe2O3 hexagonal plates to Fe3O4 polyhedral particles still occurred when the reaction system consisted of FeCl3, KOH, and EDA, as shown in Fig. 4(b). The shape of the polyhedral particles is more irregular in this case. Fig. 4(c) shows that the irregular α-Fe2O3 plates were formed when the reaction system constituted of Fe(NO3)3 and NaOH. The phase transformation from irregular α-Fe2O3 plates to Fe3O4 polyhedral particles still occurred with the addition of EDA into the reaction system, as shown in Fig. 4(d). The XRD patterns, shown in Fig. 4(e), confirmed the related phases. It can be seen that the α-Fe2O3 plates were not completely reduced to Fe3O4 particles in the case of the reaction system containing both NaOH and EDA. Thus K+ ions and NO3− ions are not directly involved in the reduction process of Fe3+ to Fe2+. However, the transformation process is faster with the presence of KOH in the reaction system than that with NaOH, which might be due to the pH values. The pH value is 11.02 for EDA, 13.17 for NaOH and EDA, and 15.26 for KOH and EDA in the reaction systems. The KOH has the fastest phase transformation rate followed by the NaOH. No phase transformation was observed for pure EDA within the limited reaction time used in the experiment. This phenomenon indicates that the pH value may affect the kinetics of the reduction process of Fe3+ to Fe2+. Moreover, the transformation process is faster with the presence of NO3− ions in the reaction system than that with Cl− ions. The role that NO3− ions played in the phase transformation was explored by Lu et al.,47 and they found that there exist a certain amount of HNO3 that could induce the maximum rate for phase transformation. The slow phase transformation rate observed for small and large amounts of HNO3 may be attributed to the limiting dissolution of α-Fe2O3 and the NO3− ions can be an oxidant to further suppress the reduction of iron ions in system.
To investigate the role of pH values, first, different amounts of HNO3 were added into the solution with the Fe(NO3)3 precursor, KOH and EDA under hydrothermal conditions at 200 °C for 9 h, as shown in Fig. 5(a). The phase transformation rates decrease gradually when the amount of HNO3 increases from 0 to 0.3 ml and the pH values decrease from 15.26 to 15.71. Second, we used 9 mg of the pre-synthesized α-Fe2O3 hexagonal plates and added HNO3, 0.19 ml, to match the concentration of NO3− provided by the Fe(NO3)3 precursor in the previous experiments, and 10.67 M KOH with different amounts of EDA, ranging from 0.5 to 2.0 ml, heat-treated at 200 °C for 7 h, as shown in Fig. 5(b). The pH values varied from 15.38 for 0.5 ml EDA to 15.16 for 2.0 ml. The results show that the phase transition rate is fast when the solution contains a large amount of EDA, although the pH value decreases slightly from 15.38 to 15.12. Third, we used 9 ml of the pre-synthesized α-Fe2O3 hexagonal plates and added 0.19 ml of HNO3 and 1 ml of EDA with different molalities of KOH and heated to 200 °C for 7 h. As shown in Fig. 5(c), the results show that the phase transition rates are faster when the solution contains a higher molality of KOH. The pH value changes from 14.39 to 15.33 as the molality of KOH in the solution changes from 5.33 M to 10.67 M. If one examines the pH values for the cases of 0.5 HNO3 in Fig. 5(a) and 5.33 M KOH in Fig. 5(c), the phase transformation rate became very slow, even slower than the solution with the Fe(NO3)3 precursor, NaOH and EDA, which has even lower pH value. Thus, this phenomenon simply explained by pH value dependence is too simple. Rather, the results showed that hydroxide ions are a necessary ingredient for the phase transformation. The reason that pure EDA does not trigger the phase transformation is probably due to the fact that there are no hydroxide ions in the reaction system. The hydroxide ions can react with the hydrogen atoms of EDA and allow the Fe ion to be chelated with the EDA ligand and the reduction occurs. Thus, in a solution with fixed ion species, the more the hydroxide ions, the faster the phase transformation is a suitable explanation. However, when the species of the ions in a solution changes (e.g. K+ is changed to Na+), the interactions between all the species are changed as well. As we know, even α-Fe2O3 formation using the Fe(NO3)3 precursor and NaOH or Fe(NO3)3 and KOH has different rates and shapes. Although increasing the amount of EDA decreases the pH value, which should decrease the transformation rate, more EDA provides more reaction sites for the reduction process. Therefore, the overall transformation rate still increases.
Fig. 6(a) shows the curves of the transformed fraction of magnetite (Fe3O4) as a function of reaction time. Using the Avrami equation, 1 − exp(−ktn), where k is the reaction constant, t is the reaction time, and n is the exponent of the reaction, one can fit, relatively well, the corresponding experimental data of the magnetite fraction obtained by the hydrothermal treatment with 1 ml EDA at 200 °C for different times. In this case, the entire process includes the time required for the appearance of α-Fe2O3. The time required for the initial appearance of α-Fe2O3 hexagonal plates was about 3600 s, and α-Fe2O3 hexagonal plates were produced completely at about 7200 s under hydrothermal treatment with 1 ml of EDA at 200 °C. Thus the appearance of α-Fe2O3 is a much faster process than the phase transformation process that follows. The curve could be fitted by setting 7200 s as 0 s for the beginning of the phase transformation, as shown in Fig. 6(b). The fitted values of n are close to 2 and 3. The fraction of α-Fe2O3 and Fe3O4 were determined by XRD measurements in conjunction with the Rietveld method, as shown in Fig. 6(c). One can further acquire sigmoid-shaped curves at different temperatures and estimate the activation energies for the phase transformation in the reaction conditions with 0.5, 1, and 1.5 ml of EDA, which is shown in Fig. 7.
The values of the apparent activation energy for the reduction of hematite to magnetite were estimated based on the Arrhenius equation, which is the following:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 450 s for 0.5 ml of EDA and 8102 s for 1.5 ml of EDA. However, the activation energies do not change significantly. This further confirms that increasing the amount of EDA ligand mainly increases the available reaction sites for the reduction of iron ions. However, detail mechanism is not affected.
450 s for 0.5 ml of EDA and 8102 s for 1.5 ml of EDA. However, the activation energies do not change significantly. This further confirms that increasing the amount of EDA ligand mainly increases the available reaction sites for the reduction of iron ions. However, detail mechanism is not affected.
The values of the activation energy obtained in this work, and other results reported previously, which were based on kinetics studies within H2 containing ambient, are listed in Table 1. Because the activation energies for the α-Fe2O3 to Fe3O4 transformation obtained in this experiment are close to those obtained for the typical phase transformation in H2 containing ambient, one could conjecture that H2 might be produced locally during the hydrothermal treatment, which triggers the valence change from Fe3+ to Fe2+. With all these different environments, different locations for nuclei formation and different growth dimensions, the activation energies obtained for the transformation are all roughly the same. This may indicate that the slowest kinetic process that controlled the transformation is actually the reduction process of Fe3+ to Fe2+. Tiernan et al. concluded from the shape of the CRTA profile, that nucleation and diffusion are not the rate-controlling process for the reduction step of Fe2O3 to Fe3O4.33
| References | Nucleation model | Qr (kJ mol−1) | Method |
|---|---|---|---|
| Lin et al.31 | Unimolecular reaction | 89.13 | Linear heating rate ∼ 300 °C |
| Wang et al.32 | Formation and growth of nuclei (2D) | 152.44 | Isothermal 290–310 °C |
| Tiernan et al.33 | Phase boundary | 96 | CRTA rate jump 100–600 °C |
| Wang et al.34 | Phase-boundary-controlled reaction (3D) | 107 | 250–350 °C |
| Shimokawabe et al.35 | Formation and growth of nuclei (1D) | 74–117 | Linear heating rate 500–1200 °C |
| Wimmers et al.36 | Formation and growth of nuclei | 124 | Linear heating rate 287–417 °C |
| This work | Formation and growth of nuclei (3D) | 96–118 | Isothermal 160–220 °C |
4. Conclusions
α-Fe2O3 nano/micro hexagonal platelets can be successfully reduced to octahedral Fe3O4 particles with EDA in alkaline solution under a low temperature hydrothermal process. The entire reaction consists of two stages. The first stage is the rapid formation of α-Fe2O3 hexagonal plate, triggered by KOH, followed by the phase transformation stage, which includes three steps: (1) the dissolution of the α-Fe2O3 hexagonal platelets, (2) the reduction of Fe3+ to Fe2+, and (3) the nucleation and growth of new Fe3O4 polyhedral particles. The Avrami equation can be used to describe the transformation kinetics and the activation energies obtained are 96.4 ± 11, 113.1 ± 5, and 118.3 ± 11 kJ mol−1 for the reaction systems with the addition of 0.5, 1, and 1.5 ml of EDA, respectively. This is the first reported activation energy for the hematite to magnetite phase transformation in hydrothermal conditions, and the values are right in the range of those obtained from the typical solid reduction in H2 containing environment.Acknowledgements
The authors acknowledge the support from National Science Council through grant no. 101-2221-E-007-061-MY2.Notes and references
- Y. Wang, J. Cao, S. Wang, X. Guo, J. Zhang, H. Xia, S. Zhang and S. Wu, Facile Synthesis of Porous α-Fe2O3 Nanorods and Their Application in Ethanol Sensors, J. Phys. Chem. C, 2008, 112, 17804–17808 CAS.
- F. L. Souza, K. P. Lopes, E. Longo and E. R. Leite, The Influence of the Film Thickness of Nanostructured α-Fe2O3 on Water Photooxidation, Phys. Chem. Chem. Phys., 2009, 11, 1215–1219 RSC.
- P. C. Wu, W. S. Wang, Y. T. Huang, H. S. Sheu, Y. W. Lo, T. L. Tsai, D. B. Shieh and C. S. Yeh, Porous Iron Oxide Based Nanorods Developed as Delivery Nanocapsules, Chem.–Eur. J., 2007, 13, 3878–3885 CrossRef CAS PubMed.
- Y. Zou, J. Kan and Y. Wang, Fe2O3-Graphene Rice-on-Sheet Nanocomposite for High and Fast Lithium Ion Storage, J. Phys. Chem. C, 2011, 115, 20747–20753 CAS.
- F. Z. Dong, D. S. Ling, J. J. Chun, G. Y. Zheng, P. Y. Li and H. Y. Chun, Hierarchical Assembly of SnO2 Nanorod Arrays on α-Fe2O3 Nanotubes: A Case of Interfacial Lattice Compatibility, J. Am. Chem. Soc., 2005, 127, 13492–13493 CrossRef PubMed.
- S. M. Reda, Synthesis of ZnO and Fe2O3 Nanoparticles by Sol–Gel Method and Their Application in Dye-Sensitized Solar Cells, Mater. Sci. Semicond. Process., 2010, 13, 417–425 CrossRef CAS PubMed.
- S. Zhang, X. Chen, C. Gu, Y. Zhang, J. Xu, Z. Bian, D. Yang and N. Gu, The Effect of Iron Oxide Magnetic Nanoparticles on Smooth Muscle Cells, Nanoscale Res. Lett., 2009, 4, 70–77 CrossRef CAS.
- M. Kallumadil, M. Tada, T. Nakagawa, M. Abe, P. Southern and Q. A. Pankhurst, Suitability of Commercial Colloids for Magnetic Hyperthermia, J. Magn. Magn. Mater., 2009, 321, 1509–1513 CrossRef CAS PubMed.
- D. Thapa, V. R. Palkar, M. B. Kurup and S. K. Malik, Properties of Magnetite Nanoparticles Synthesized Through a Novel Chemical Route, Mater. Lett., 2004, 58, 2692–2694 CrossRef CAS PubMed.
- D. Zhang, Z. Liu, S. Han, C. Li, B. Lei, M. P. Stewart, J. M. Tour and C. Zhou, Magnetite (Fe3O4) Core–Shell Nanowires: Synthesis and Magnetoresistance, Nano Lett., 2004, 4, 2151–2155 CrossRef CAS.
- M. K. Yu, Y. Y. Jeong, J. Park, S. Park, J. W. Kim, J. J. Min, K. Kim and S. Jon, Drug-Loaded Superparamagnetic Iron Oxide Nanoparticles for Combined Cancer Imaging and Therapy In Vivo, Angew. Chem., Int. Ed., 2008, 47, 5362–5365 CrossRef CAS PubMed.
- H. Zeng, J. Li, J. P. Liu, Z. L. Wang and S. Sun, Exchange-Coupled Nanocomposite Magnets by Nanoparticle Self-Assembly, Nature, 2002, 420, 395–398 CrossRef CAS PubMed.
- G. Y. Zhang, Y. Y. Xu, D. Z. Gao and Y. Q. Sun, α-Fe2O3 Nanoplates: PEG-600 Assisted Hydrothermal Synthesis and Formation Mechanism, J. Alloys Compd., 2011, 509, 885–890 CrossRef CAS PubMed.
- W. Yin, X. Chen, M. Cao, C. Hu and B. Wei, α-Fe2O3 Nanocrystals: Controllable SSA-Assisted Hydrothermal Synthesis, Growth Mechanism, and Magnetic Properties, J. Phys. Chem. C, 2009, 113, 15897–15903 CAS.
- L. Liu, H. Z. Kou, W. Mo, H. Liu and Y. Wang, Surfactant-Assisted Synthesis of Alpha-Fe2O3 Nanotubes and Nanorods with Shape-Dependent Magnetic Properties, J. Phys. Chem. B, 2006, 110, 15218–15223 CrossRef CAS PubMed.
- A. G. Nasibulin, S. Rackauskas, H. Jiang, Y. Tian, P. R. Mudimela, S. D. Shandakov, L. I. Nasibulina, J. Sainio and E. I. Kauppinen, Simple and Rapid Synthesis of α-Fe2O3 Nanowires Under Ambient Conditions, Nano Res., 2009, 2, 373–379 CrossRef CAS PubMed.
- R. Ramesh, K. Ashok, G. M. Bhalero, S. Ponnusamy and C. Muthamizhchelvan, Synthesis and Properties of α-Fe2O3 Nanorods, Cryst. Res. Technol., 2010, 45, 965–968 CrossRef CAS.
- Z. Zhang, M. F. Hossain and T. Takahashi, Self-Assembled Hematite (α-Fe2O3) Nanotube Arrays for Photoelectrocatalytic Degradation of Azo dye under Simulated Solar Light Irradiation, Appl. Catal., B, 2010, 95, 423–429 CrossRef CAS PubMed.
- X. Hu, J. C. Yu, J. Gong, Q. Li and G. Li, α-Fe2O3 Nanorings Prepared by a Microwave-Assisted Hydrothermal Process and Their Sensing Properties, Adv. Mater., 2007, 19, 2324–2329 CrossRef CAS.
- D. Chen and L. Gao, A Facile Route for High-Throughput Formation of Single-Crystal α-Fe2O3 Nanodisks in Aqueous Solutions of Tween 80 and Triblock Copolymer, Chem. Phys. Lett., 2004, 395, 316–320 CrossRef CAS PubMed.
- W. Qin, C. Yang, R. Yi and G. Gao, Hydrothermal Synthesis and Characterization of Single-crystalline α-Fe2O3 Nanocubes, J. Nanomater., 2011, 159259 CAS.
- G. Liu, Q. Deng, H. Wang, D. H. L. Ng, M. Kong, W. Cai and G. Wang, Micro/Nanostructured α-Fe2O3 Spheres: Synthesis, Characterization, and Structurally Enhanced Visible-Light Photocatalytic Activity, J. Mater. Chem., 2012, 22, 9704–9713 RSC.
- D. Nishino, A. Nakafuji, J. M. Yang and D. Shindo, Precise Morphology Analysis on Platelet-type Hematite Particles by Transmission Electron Microscopy, ISIJ Int., 1998, 38, 1369–1374 CrossRef CAS.
- D. Peng, S. Beysen, Q. Li, Y. Sun and L. Yang, Hydrothermal Synthesis of Monodisperse α-Fe2O3 Hexagonal Platelets, Particuology, 2010, 8, 386–389 CrossRef CAS PubMed.
- W. Yu, T. Zhang, J. Zhang, X. Qiao, L. Yang and Y. Liu, The Synthesis of Octahedral Nanoparticles of Magnetite, Mater. Lett., 2006, 60, 2998–3001 CrossRef CAS PubMed.
- Z. Li, M. Kawasita, N. Araki, M. Mitsumori, M. Hiraoka and M. Doi, Preparation of Magnetic Iron Oxide Nanoparticles for Hyperthermia of Cancer in a FeCl2–NaNO3–NaOH Aqueous System, J. Biomater. Appl., 2011, 25, 643–661 CrossRef CAS PubMed.
- X. Zhang, Y. Niu, X. Meng, Y. Li and J. Zhao, Structural Evolution and Characteristics of the Phase Transformations Between α-Fe2O3, Fe3O4 and γ-Fe2O3 Nanoparticles under Reducing and Oxidizing Atmospheres, CrystEngComm, 2013, 15, 8166–8172 RSC.
- T. Otake, D. J. Wesolowski, L. M. Anovitz, L. F. Allard and H. Ohmoto, Mechanisms of Iron Oxide Transformations in Hydrothermal Systems, Geochim. Cosmochim. Acta, 2010, 74, 6141–6156 CrossRef CAS PubMed.
- H. Ohmoto, Nonredox Transformations of Magnetite–Hematite in Hydrothermal Systems, Econ. Geol., 2003, 98, 157–161 CrossRef.
- R. P. Viswanath, B. Viswanathan and M. V. C. Sastri, Kinetics of Reduction of Fe2O3 to Fe3O4 by the Constant Temperature Differential Thermal Analysis Method, Thermochim. Acta, 1976, 16, 240–244 CrossRef CAS.
- H. Y. Lin, Y. W. Chen and C. Li, The Mechanism of Reduction of Iron Oxide by Hydrogen, Thermochim. Acta, 2003, 400, 61–67 CrossRef CAS.
- J. H. Wang, Z. C. Tao, Y. Yang, J. Chang, H. W. Xiang and Y. W. Li, Reduction Kinetics of α-Fe2O3 Microspheres, Chin. J. Process Eng., 2007, 7, 288–292 CAS.
- M. J. Tiernan, P. A. Barnes and G. M. B. Parkes, Reduction of Iron Oxide Catalysts: The Investigation of Kinetic Parameters Using Rate Perturbation and Linear Heating Thermoanalytical Techniques, J. Phys. Chem. B, 2001, 105, 220–228 CrossRef CAS.
- H. Wang, Y. Yang, B. S. Wu, J. Xu, M. Y. Ding, H. L. Wang, W. H. Fan, H. W. Xiang and Y. W. Li, Hydrogen Reduction Kinetics Modeling of a Precipitated Iron Fischer–Tropsch Catalyst, J. Mol. Catal. A: Chem., 2009, 308, 96–107 CrossRef CAS PubMed.
- M. Shimokawabe, R. Furuichi and T. Ishii, Influence of the Preparation History of α-Fe2O3 on Its Reactivity for Hydrogen Reduction, Thermochim. Acta, 1979, 28, 287–305 CrossRef CAS.
- O. J. Wimmers, P. Arnoldy and J. A. Moulijn, Determination of the Reduction Mechanism by Temperature-Programmed Reduction: Application to Small Fe2O3 Particles, J. Phys. Chem., 1986, 90, 1331–1337 CrossRef CAS.
- K. Yanagisawa and N. Yamasaki, Reduction of Haematite to Magnetite under Controlled Hydrothermal Conditions with Hydrogen gas, J. Mater. Sci., 1991, 26, 473–478 CrossRef CAS.
- J. Ge, Y. Hu, M. Biasini, W. P. Beyermann and Y. Yinty, Superparamagnetic Magnetite Colloidal Nanocrystal Clusters, Angew. Chem., Int. Ed., 2007, 46, 4342–4345 CrossRef CAS PubMed.
- S. Qu, H. Yang, D. Ren, S. Kan, G. Zou, D. Li and M. Li, Magnetite Nanoparticles Prepared by Precipitation from Partially Reduced Ferric Chloride Aqueous Solutions, J. Colloid Interface Sci., 1999, 215, 190–192 CrossRef CAS PubMed.
- R. S. Sapieszko and E. Matijewic, Preparation of Well-Defined Colloidal Particles by Thermal Decomposition of Metal Chelates, J. Colloid Interface Sci., 1980, 74, 405–422 CrossRef CAS.
- H. Wang, Q. He, B. Yao, G. Wen, F. Wang, Y. Xu, Y. Li, J. Li, C. Zhou, J. Wang, G. Li, L. Shan and J. Chen, Transformation of α-Fe2O3 to Fe3O4 Realized by Mechanochemical Reaction of α-Fe2O3 and SrCO3, Metall. Mater. Trans. B, 2012, 43, 1574–1578 CrossRef CAS PubMed.
- B. Lv, Z. Liu, H. Tian, Y. Xu, D. Wu and Y. Sun, Single-Crystalline Dodecahedral and Octodecahedral α-Fe2O3 Particles Synthesized by a Fluoride Anion-Assisted Hydrothermal Method, Adv. Funct. Mater., 2010, 20, 3987–3996 CrossRef CAS.
- Y. B. Khollam, S. R. Dhage, H. S. Potdar, S. B. Deshpande, P. P. Bakare, S. D. Kulkarni and S. K. Date, Microwave Hydrothermal Preparation of Submicron-Sized Spherical Magnetite (Fe3O4) Powders, Mater. Lett., 2002, 56, 571–577 CrossRef CAS.
- L. Slavov, M. V. Abrashev, T. Merodiiska, C. Gelev, R. E. Vandenberghe, I. Markova-Deneva and I. Nedkovt, Raman Spectroscopy Investigation of Magnetite Nanoparticles in Ferrofluids, J. Magn. Magn. Mater., 2010, 322, 1904–1911 CrossRef CAS PubMed.
- D. L. A. de Faria, S. Venancio Silva and M. T. de Oliveira, Raman Microspectroscopy of Some Iron Oxides and Oxyhydroxides, J. Raman Spectrosc., 1997, 28, 873–878 CrossRef CAS.
- H. Monika, Raman Spectroscopy of Iron Oxides and (Oxy)hydroxides at Low Laser Power and Possible Applications in Environmental Magnetic Studies, Geophys. J. Int., 2009, 177, 941–948 CrossRef PubMed.
- J. F. Lu and C. J. Tsai, Hydrothermal Phase Transformation of Hematite to Magnetite, Nanoscale Res. Lett., 2014, 9, 230 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |