Enhanced thermal, mechanical and fire-retarding properties of polystyrene sulphonate-grafted-nanosilica/polypropylene composites
M. M. Hassan*ac and
K. Koyamaab
aVenture Business Laboratory, Yamagata University, 4-3-16 Jonan, Yonezawa, Yamagata 992-8510, Japan
bDepartment of Polymer Science and Engineering, 4-3-16 Jonan, Yonezawa, Yamagata 992-8510, Japan
cFood & Biobased Products Group, AgResearch Limited, Cnr Springs Road and Gerald Street, Lincoln, Christchurch 7647, New Zealand. E-mail: Mahbubul.hassan@agresearch.co.nz
First published on 22nd January 2015
Abstract
Isotactic polypropylene (iPP) nanocomposites have been prepared by melt blending PP with polystyrene sulphonate-grafted-silica nanoparticles (pSS-g-nanosilica) at 190 °C. The weight% of pSS-g-nanosilica was varied from 1 to 5. The unmodified and pSS-g-nanosilica were characterised by transmission electron microscopy, Fourier transform infra-red spectroscopy, X-ray diffraction and UV-vis spectroscopy. The produced nanocomposites were characterised by differential scanning calorimetry, scanning electron microscopy, thermogravimetric analysis, cone calorimetry, limiting oxygen index (LOI) determination and tensile strength analysis. It was found that the modified silica nanoparticles were fully dispersed in the iPP matrix. The maximum increase in tensile strength and melting/crystallisation temperatures were observed at the 2 weight% pSS-g-nanosilica loading, but the fire retardancy increased proportionally with an increase in nanosilica loading in the iPP matrix. The LOI of the nanocomposites was improved from 19.5 to 22, and the peak heat release rate was reduced from 28.49% to 59.13%, depending upon the weight% of pSS-g-nanosilica that was present.
1. Introduction
Nanocomposites of thermoplastic polymers are attracting considerable appeal because of their unique properties, such as improved rigidity, toughness, impact strength and heat deflection.1–4 They show not only improved mechanical properties but also excellent barrier properties, improved scratch resistance, better optical clarity, higher heat distortion temperature, lower coefficient of thermal expansion and reduced weight compared to the non-nanocomposite counterparts.5–8 Thermoplastic nanocomposites are already the 'superstars' of the plastic industry. They can offer tremendous opportunities through the unprecedented enhancement of physical and mechanical properties pushing the performance well beyond the domain of known composite technology. Nanoparticles have significantly larger surface to volume ratios over microparticles, which allow them to be in more intimate contact with the surplus area of a given polymer with the same volume fraction, and thus considerably affect the mechanical properties of the composites prepared. Nanocomposite technologies are applicable to a very broad range of polymers, including polyamides, polyimides, polycarbonates, acrylics, polystyrene, poly olefins, vinyl polymers, elastomers, adhesives, epoxies, and other thermo-setting resins, bolstering their various useful properties.9–14 Nanoparticles used include silica, zinc oxide, titanium, silver, copper, gold, iron, and different types of clays.15–18To achieve the best benefits, the nanoparticles have to be dispersed in the polymer matrix at nano levels without agglomeration, and the surface of nanoparticles has to be made compatibles with the polymer matrix as well; otherwise, instead of benefitting they may make poor composites with impaired mechanical properties. However, achieving a homogeneous dispersion of nano particle in a polymer matrix is a very difficult task due to the strong tendency of nanoparticles to agglomerate. Silica nanoparticles aggregate because of hydrogen bond formation between two hydroxyl groups of two silica nanoparticles. As a result, sometimes nanoparticle-filled polymers contain a number of loosened clusters of particles and exhibit properties even poorer than conventional particle-filled polymers.19 To break down these nano-particle aggregates and to produce nanostructural composition, many researchers focus on the approaches of surface treatment with a silane coupling agent as well as in situ polymerisation of monomers in the presence of nanoparticles and radiation grafting techniques.19–22 Nanoparticles also need special surface treatment to become compatible with a polymer matrix. Various surface treatments are reported in the published articles19–21 but no citation about polymeric electrolyte modified nanoparticles exists.
It is also advantageous to have fire-retardancy in thermoplastic composites because in the case of fire, there is a risk of severe burns to body parts that are in contact with the composite materials. Recently, it was reported that a thin coating of poly(vinyl sulphonic) acid of the order of a few nanometres in thickness improved the thermal stability and fire retardancy of a polyurethane foam.23 The presence of sulphur in poly(vinyl sulphonic) acid produces non-flammable sulphur dioxide at high temperatures, acting as a fire retardant. It is also known that sulphur species can destroy free-radicals produced during pyrolysis that otherwise accelerate the conversion of carbon into carbon dioxide.24,25 It is assumed that polystyrene sulphonate (pSS) may show fire-retardancy similar to polyvinyl sulphonic acid. In this article, we report the modification of silica nanoparticles by graft polymerisation with pSS electrolyte polymer and its composites with polypropylene.
2. Experimental
2.1 Materials
General purpose isotactic-polypropylene homo polymer (type K1014) with a melt flow rate of 4.7 g/10 min at 230 °C (the weight-averaged molecular weight = 225![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, number-averaged molecular weight = 50
000, number-averaged molecular weight = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 223 and polydispersity, Mw/Mn = 4.48, and density = 0.90 g cm−3) was kindly supplied by Chisso Petrochemicals Ltd (Japan). The colloidal silica nanoparticles used was NYACOL 2034DI, which was supplied by Eka Chemicals AB (Sweden). Styrene sulphonate (SS), vinyltrimethoxysilane (VTMS), potassium perhydroxy sulphate and acetic acid were purchased from Sigma-Aldrich Limited (USA) and were of analytical reagent grade.
223 and polydispersity, Mw/Mn = 4.48, and density = 0.90 g cm−3) was kindly supplied by Chisso Petrochemicals Ltd (Japan). The colloidal silica nanoparticles used was NYACOL 2034DI, which was supplied by Eka Chemicals AB (Sweden). Styrene sulphonate (SS), vinyltrimethoxysilane (VTMS), potassium perhydroxy sulphate and acetic acid were purchased from Sigma-Aldrich Limited (USA) and were of analytical reagent grade.
2.2 Surface modification of silica nanoparticles
A predetermined quantity of colloidal silica (10 g) was placed in a three-neck round bottom flask, which was configured with nitrogen purging system and magnetic stirrer. Then, 90 ml water was added and a homogenous dispersion of nanosilica in water was prepared by ultrasonic mixing for 30 min. Then, 3 g VTMS was added to it and the pH was adjusted to 9.0 with ammonia solution. The mixture was stirred for 2 hours, after which 5 g SS dissolved in 100 ml water was added. The pH was adjusted to 4.5 with acetic acid and sodium acetate. The flask was then placed in a glycerine bath pre-heated at 70 °C. Pre-dissolved potassium persulphate initiator in water was injected drop by drop over 5 minutes and polymerisation was continued for 2 hours under a nitrogen atmosphere, which produced a fully transparent solution of pSS-block-pVTMS grafted on silica nanoparticles (denoted as pSS-g-nanosilica). The modified silica nanoparticles were recovered by centrifuging at 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and were re-dispersed in water and recovered by centrifuging, which removed most of the un-grafted pSS, and only trace amounts of monomeric styrene sulphonate and VTMS remained. This grafted nanoparticles are denoted here as pSS-g-nanosilica. The formation mechanism of pSS-g-nanosilica is shown in Scheme 1.
000 rpm and were re-dispersed in water and recovered by centrifuging, which removed most of the un-grafted pSS, and only trace amounts of monomeric styrene sulphonate and VTMS remained. This grafted nanoparticles are denoted here as pSS-g-nanosilica. The formation mechanism of pSS-g-nanosilica is shown in Scheme 1.
 | ||
| Scheme 1 Schematic illustration of the formation of VTMS-bonded-nanosilica followed by the formation of pSS-g-nanosilica. | ||
2.3 Characterisation of pSS-grafted silica nanoparticles
The modified silica nanoparticles were characterised by UV-vis spectroscopy, Fourier transform infra-red (FTIR) spectroscopy, X-ray diffraction (XRD) and transmission electron microscopy (TEM). The unmodified and pSS-grafted silica nanoparticles were diluted with water and UV-visible spectroscopy was carried out on a Nicolet Evolution 100 spectrophotometer (Thermo Electron Corporation, Waltham, USA) from 200 to 710 nm wavelengths using a 10 mm quartz cuvette. A small quantity of dried unmodified and modified silica nanoparticles were mixed with potassium bromide (KBr) and pellets were produced. They were then scanned on a Shimadzu FT-IR spectrometer (model Prestige 21, Shimadzu Corporation, Japan) at a resolution of 4 cm−1 for 32 scans in the range from 450 to 4500 cm−1. A small quantity of colloidal solution of unmodified and pSS-grafted silica nanoparticles was sprayed on a hydrophilic copper grid, which was then dried in a dust-free environment for 48 h. The nanoparticle-coated copper grid was then scanned using an FEI transmission electron microscope (model: Morgagni 268D, FEI Inc., Oregon, USA). Micrographs were obtained by using an SIS/Olympus Megapixel III digital camera mounted above a phosphor screen. Unmodified and pSS-g-nanosilica particles were analysed using an X-ray diffractometer to determine their crystal structure as well as the crystallinity. Diffraction patterns of the samples were collected on a MiniFlex X-ray diffraction instrument (Rigaku Corporation, Tokyo, Japan) using a Ni-filtered Cu Kα radiation, λ = 1.54178 Å. The samples were scanned over a 2θ range of 3–100° with a step-scan of 0.02° per step.2.4 Preparation of PP nanocomposites
Before making composites, the iPP polymer chips and the pSS-g-nanosilica were dried under vacuum at 85 °C overnight. Nanocomposites were prepared by manually mixing the pre-weighted quantities of iPP and the pSS-grafted silica nanoparticles by melt compounding the mixture at 190 °C under a nitrogen atmosphere using a Brabender Plasticorder (Brabender GmbH & Co. KG, Duisburg, Germany) equipped with a static mixer of 50 ml capacity. The screw speed was maintained at 90 rpm. The application levels of silica were varied from 1 to 5 weight%. Blending was continued for 10 minutes because the torque graph showed that 10 minutes were sufficient for the optimum homogeneous mixing of iPP and pSS-g-nanosilica.2.5 Differential scanning calorimetry
The non-isothermal crystallisation and the subsequent melting behaviour of neat iPP and its nanocomposites were examined on a Perkin-Elmer 7 differential scanning calorimeter (DSC) equipped with an intercooler. The samples were heated from room temperature to 300 °C at a rate of 5 °C min−1 and held for 5 minutes, then cooled to 0 °C at 5 °C min−1 and held for 5 minutes, and again heated to 300 °C at 5 °C min−1 to minimise the effect of static heat.2.6 Mechanical properties and fractured morphology
1.5 mm thick plates were prepared by compression moulding at 140 °C for 5 minutes at 10 MPa pressure and then slow cooling to facilitate crystallisation. Tensile tests were performed to examine the tensile strength and elongation at break according to the ASTM standard test method D638-03. The size of the samples was 100 × 10 × 1.5 mm3. The gauge length was 50 mm and the crosshead speed was 20 mm min−1. An extensometer was employed to determine the elongation of the samples. Tests were carried out in an environmentally conditioned room maintained at 20 ± 1 °C and 65% ± 1% RH. The tensile strength was measured by an Instron Universal Testing machine (model: 4204, Instron Inc., Norwood, USA). The 12 identical samples of each blend composition and control were measured, and the average values are reported here.The morphology of the fractured surface of neat iPP and its various nanocomposites were evaluated by scanning electron microscopy (SEM). The grafted polymers were extruded through an extruder as a strand, which was then fractured after placing it in liquid nitrogen. The fractured surfaces were sputter-coated with gold and scanned in a JEOL SEM (model: JSM-6100, JEOL Ltd., Tokyo, Japan).
2.7 Thermal stability and fire-retardancy
Thermogravimetric analysis (TGA) was carried out to evaluate the thermal stability of neat iPP and with 2 and 5 weight% pSS-g-nanosilica-filled iPP nanocomposites. TGA was carried out on a Seiko DSC/TG Analyser (model: SSC 5000, Seiko Instruments Inc., Japan) from room temperature to 520 °C at a heating rate of 10 °C min−1 under a nitrogen environment. All TGA runs employed nitrogen (99.99% pure and food-grade) as the purge gas for the furnace at a constant flow rate of 100 ml min−1. For each run, 10 mg of sample was loaded in a platinum pan and was heated to 520 °C at a linear rate of 10 °C min−1 under constant nitrogen gas flow (100 ml min−1), held for 10 min, and slowly cooled to room temperature by switching off the heater. The thermogravimetric (TG) and differential thermal analysis (DTA) curves were recorded simultaneously along with the increasing temperature.1 mm thick film was produced from 10 g each of neat iPP and 1, 2, 3, 4 and 5 weight% pSS-g-nanosilica-filled iPP nanocomposites by compression moulding at 190 °C under 10 MPa pressure for 10 minutes and then slowly cooled to room temperature. Samples were prepared by compression moulding 20 g of neat iPP and various nanocomposites into a square plate by a mini hot press (Toyo Seiki Kogyo Co. Ltd. (Japan)). The moulded sheet was used to measure the fire-retardancy of iPP and its various nanocomposites by measuring the limiting oxygen index according to the ASTM Test Method D2863-00: Standard Test Method for measuring the minimum oxygen concentration to support candle-like combustion of plastics (oxygen index). A sheet of nanocomposites was clamped to a holder of the cylindrical glass housing and were burnt to candle-like burning at a certain ratio of nitrogen and oxygen flow. Combustion study was performed using a cone calorimeter (PL Thermal Sciences Ltd, Epsom, UK) according to the ASTM Test Method E1354: Cone calorimeter at an incident flux of 35 kW m−2. Exhaust flow was set at 24 l s−1 and the spark was continuous until the sample ignited.
3. Results
3.1 Characterisation of pSS-g-nanosilica
Nanoparticles of silica possess considerably different properties compared to the conventional silica particles, and these differences usually occur because of the difference in size, surface characteristics and structural defects.19 Previous studies show that the presence of inorganic fillers does not hinder the polymerisation reaction of monomers.20,21 In this work, we also found that pSS was successfully grafted on silica nanoparticles and the grafting efficiency was approximately 35%.To study the effect of grafting of pSS on the dispersion behaviour and optical properties of silica nanoparticles, UV-vis absorption measurements were performed. Fig. 1 shows the UV-vis spectra of unmodified nanosilica, pSS and pSS-g-nanosilica. The UV spectrum of unmodified silica nanoparticles does not show any peak. However, pSS and pSS-g-nanosilica show a peak at 219 nm, which is a characteristic peak of sulphonated benzene of polystyrene sulphonate. The visible spectrum of pSS does not show any peak but the pSS-grafted nanosilica shows a peak at 330 nm, which is specific to Si–O–Si bonds and confirms the presence of silica nanoparticles. The visible range spectra of both the modified and pSS-g-nanosilica were similar except that the latter showed a peak at 475 nm, which could be attributed to the grafted pSS.
Fig. 2(a) shows the FTIR spectra of unmodified nanosilica, pSS and pSS-g-nanosilica. The spectrum of unmodified silica nanoparticles shows a broad peak in the range of 1000–1330 cm−1 and sharp peaks at 800, 1630 and 1877 cm−1. It also shows a small peak at 2295 cm−1 and a broad peak at 3445 cm−1. The absorption band at 802 cm−1 represents the symmetric stretching vibration of Si–O–Si, and the band at 1100 cm−1 represents the asymmetric vibrations of Si–O–Si.26 The absorption bands between 800 and 1260 cm−1 have been described as a superimposition of various SiO2 peaks and Si–OH bonding. The peaks at 1630 and 1877 cm−1 could be due to the bending vibrations of the O–H bonds of the water absorbed by the nanoparticles.27 The broad peak at 3445 cm−1 could be attributed to OH groups due to the absorption of water by silica nanoparticles. Moreover, the spectra of pSS and pSS-g-nanosilica show new bands at 676, 1010, 1040, 1132, 1184, 1412, 1452, 1496, 1634, 2856 and 2933 cm−1 that are not present in the spectrum of the unmodified nanosilica. Their spectra also show a sharper peak at 3450 cm−1 rather than the broad absorption band shown by the unmodified nanosilica, which represents the asymmetric –OH vibrations.28 The spectra of both pSS and pSS-grafted-nanosilica show peaks at 775 and 835 cm−1 that represent ortho and para-substituted benzene, respectively, which indicates that the pSS is a mixture para and ortho-substituted forms. The peaks at 1010, 1040 and 1184 cm−1 could be attributed to sulphonate groups attached to benzene. The absorption band at 1132 cm−1 can be attributed to the sulphonate anion attached to a phenyl ring. The absorption bands at 2856 and 2933 cm−1 correspond to the C–H stretching vibration of methylene (–CH2) and aryl groups in polystyrene sulphonate, respectively.28–30 The results obtained suggest that pSS was successfully grafted onto nanosilica.
 | ||
| Fig. 2 FTIR spectra (a) and wide angle XRD patterns (b) of unmodified nanosilica, pSS and pSS-g-nanosilica. | ||
Fig. 2(b) shows the XRD patterns of unmodified nanosilica, pSS and pSS-g-nanosilica. The XRD patterns shown by unmodified nanosilica, pSS and pSS-g-nanosilica are considerably similar. However, unmodified nanosilica shows a broad peak at 21° but both pSS and pSS-g-nanosilica show a broad peak at 20°, which indicates that both the types of silica nanoparticles and pSS are amorphous.
Fig. 3 shows TEM images of unmodified and pSS-g-silica nanoparticles before breaking the dispersion. It can be seen that the untreated and both types of pSS-grafted silica nanoparticles formed agglomeration. However, the pSS-g-silica nanoparticles formed small size agglomerates compared to the untreated nanosilica. The size of the untreated nanosilica was found to be approximately 20 nm and the size of the silica nanoparticles after the grafting with pSS increased to approximately 22.5 nm. TEM reveals that the size of aggregated nanoparticles in the case of the pSS-grafting was considerably smaller compared to the size of the aggregated untreated silica nanoparticles.
3.2 Characterisation of iPP nanocomposites
The synthesis of nanocomposites involved the dispersion of dried pSS-grafted nanosilica in the iPP matrix by melt compounding. We observed that the addition of nanosilica to iPP at 190 °C increased the viscosity of the melted iPP within a few minutes as the torque increased, and after 10 minutes of mixing, the torque started decreasing, suggesting the start of the degradation of iPP. Therefore, mixing was continued only for 10 min.It is necessary to identify whether the addition of modified silica nanoparticles to the iPP matrix has any effect on the microstructure of iPP, which may affect the performance of the iPP nanocomposites. The iPP is a semi-crystalline thermoplastic polymer whose properties are related to its melting and crystallisation behaviour. Any change in the melting behavior of the iPP nanocomposites compared to the neat iPP reflects the nucleating effect of the silica nanoparticles. Fig. 4 shows the DSC melting curves of the second heating cycle of neat iPP and its nanocomposites with various weight% of pSS-g-nanosilica. The narrow change in melting peak with 1 to 5 weight% of modified silica nanoparticles during heating suggests that the more perfect lamellae were formed with a narrow size distribution.31 The melting point of the neat iPP was 167 °C. The highest shift of the melting point was observed for the 2 weight% of the iPP, which was 168.2 °C (Table 1) and the lowest shift was observed for the 5 weight% of the iPP. The width of the melting point peak, which reflects the lamellar thickness distribution, also decreased with the addition of the modified silica nanoparticles. There was a small melting peak between 153.6 and 154.2 °C in the case of various weight% pSS-grafted nanosilica-filled iPP composites. It was assigned to the β-phase crystals of iPP, which are known to have a melting peak around 145 °C.26
 | ||
| Fig. 4 DSC heating (top) and cooling (bottom) curves of PP and its nanocomposites at a heating rate of 10 °C min−1. | ||
| Samples | Thermal parameters | ||||
|---|---|---|---|---|---|
| Melting point (°C) | Crystallisation temp. (°C) | IPDT (°C) | LOI (%) | PHRR (kW m−2) | |
| Neat iPP | 167.0 | 116.2 | 456.0 | 18 | 1384.1 |
| 99% iPP/1% pSS-g-nanosilica | 167.7 | 119.6 | — | 19.5 | — |
| 98% iPP/2% pSS-g-nanosilica | 168.2 | 121.3 | 466.0 | 20.3 | 989.70 |
| 97% iPP/3% pSS-g-nanosilica | 167.8 | 120.7 | — | 21.0 | — |
| 96% iPP/4% pSS-g-nanosilica | 167.9 | 120.6 | — | 21.5 | — |
| 95% iPP/5% pSS-g-nanosilica | 167.4 | 119.7 | 479.0 | 22.0 | 565.6 |
The crystallisation curves of the iPP and its nanocomposites with pSS-g-nanosilica is shown in Fig. 5. The crystallisation temperature of the iPP nanocomposites shifted to the higher crystallisation temperatures compared to the neat iPP, indicating the nucleating effect of the modified nanosilica. The highest shift of the crystallisation temperature was observed for the iPP nanocomposite filled with 2 weight% of the modified nanosilica. The crystallisation temperature of the neat iPP was 116.2 °C, which was shifted to 121.4 °C for the 2 weight% pSS-g-nanosilica-filled iPP nanocomposite (Table 1). The results obtained showed that the addition of pSS-g-nanosilica to iPP positively affected its melting and crystallisation temperatures.
 | ||
| Fig. 5 Effect of increase in weight% of pSS-g-nanosilica on the tensile strength (top) and elongation at break (bottom) of iPP nanocomposites. | ||
It is well-known that the mechanical properties of nanocomposites are enhanced compared to those of the polymer.32 Fig. 5 shows the tensile strength and elongation of neat iPP and its nanocomposites with pSS-g-nanosilica. With an increase in pSS-g-nanosilica content, the tensile strength of the nanocomposites increased first and then the tensile strength started decreasing with an increase in the nanosilica content. Interfacial interaction between the phase of a thermoplastic polymer and the nanosilica in a thermoplastic composite governs the stress transfer efficiency and extent of matrix deformation, which eventually determines the mechanical performance of the composite.15 In general, in the case of a particle-filled composite, the tensile strength and the elongation decreases with an increase in filler loading because of the poor interfacial adhesion between the thermoplastic polymer and the filler and because of the agglomeration of the nanoparticles.33 However, it was found that in the case of compatible nanoparticles, the tensile strength increased with an increase in nanoparticle content.19
Fig. 5 shows that in the case of the iPP/pSS-g-nanosilica composite, the tensile strength increased with an increase in pSS-g-nanosilica loading up to 2 weight%, after which the tensile strength started decreasing with an increase in filler loading. However, in the case of unmodified nanosilica, the tensile strength decreased initially with an increase in nanosilica content up to 2 weight%, and then started increasing slightly with an increase in nanosilica loading up to 3 weight% (which was considerably lower compared to the tensile strength of the neat iPP); after this negligible increase in tensile strength up to 5 weight% was observed.
Similarly in the case of pSS-g-nanosilica, normalised elongation initially increased up to 2 weight% of nanosilica loading and then started decreasing with an increase in nanosilica loading up to 5 weight%; however, the elongation observed for 5 weight% of pSS-g-nanosilica was considerably higher than the elongation observed by the neat iPP. However, in the case of unmodified nanosilica, the normalised elongation decreased up to 2 weight% of unmodified nanosilica; after this the elongation slightly increased and then again decreased with an increase in nanosilica loading. The elongation observed at 5% weight fraction nanosilica was considerably low compared to the elongation shown by the neat iPP.
The tensile strength shown by the unmodified iPP was 34.3 MPa, which increased to 41.6 MPa for the iPP nanocomposite with 2 weight% pSS-g-nanosilica. Similarly, the normalised elongation to break shown by neat iPP was 1%, which increased to 1.6% for its nanocomposite with 2 weight% pSS-g-nanosilica. Moreover, the highest tensile strength shown by the PP/unmodified nanosilica was 33.7 MPa at 1 weight% loading of pSS-g-nanosilica, which was smaller than the tensile strength shown by the neat PP. The lowest strength shown by the PP/unmodified nanosilica was 31.8 MPa at 2 weight% loading. The mechanical properties shown by the pSS-g-nanosilica composites were consistent with the change in melting and crystallisation temperatures due to the increase in pSS-g-nanosilica loading. The obtained results indicate that the increase in pSS-g-nanosilica loading more than 2 weight% of iPP increased agglomeration and formed voids in the microstructure of the PP/pSS-g-nanosilica composites, which caused poor dispersion of nanosilica in the polymer matrix. Therefore, SEM analysis was carried out to observe the microstructure of various nanocomposites and whether they formed any microvoids.
3.3 Fractured morphology of nano-silica in nanocomposites
Fig. 6 shows the SEM micrographs of the impact-fractured surface of PP/silica nanocomposites. Neat iPP has a relatively smooth surface and exhibits no sign of deformation or drawing. However, pSS-g-nanosilica-filled iPP composites at various weight% show clear signs of plastic deformation. The fractured surface shows elongated matrix segments. Rong et al. found extensive cavitation sites formed by a number of matrix-fibrillated circles in the fractured surface of PP filled with polystyrene-g-nanosilica, and the probable cause of this behaviour according to them could be the debonding of the modified nanoparticles from the matrix due to the interfacial stress concentrations.19 However, in the case of PP nanocomposites loaded with 2% pSS-g-nanosilica, no micro or nanovoids of that type were visible in the fractured surface, as shown in Fig. 6. This indicates that nano level dispersion was achieved without any agglomeration of the modified nanoparticles, which caused high tensile strength. However, in the case of 5% pSS-g-nanosilica/PP composites, some nano-size voids are visible, which suggest that at 5% loading some aggregation of pSS-g-nanosilica occurred resulting in a decrease in tensile strength.On comparing the SEM micrographs of the fractured surfaces, it is evident that the iPP nanocomposites with 2 weight% pSS-g-nanosilica had undergone a stronger plastic deformation characterised by more fibrillar structure on the matrix compared to the neat iPP and iPP filled with 5 weight% modified nanosilica. The higher magnified images show that in the case of 2 weight% modified nanosilica-filled composite, nanoparticles were uniformly distributed, but for the 5 weight%, the modified nanoparticles formed few hundred nanometre long spindle-like agglomerations. The SEM images reveal that the 2 weight% modified nanosilica-composite produced a uniform distribution of nanosilica in the iPP matrix, whereas at 5 weight% loading, modified silica formed agglomerations, which reduced the tensile strength and elongation of the iPP nanocomposites.
3.4 Thermal stability and fire retardancy
The iPP nanocomposites with various amounts of pSS-g-nanosilica showed higher thermal stability, char residue, LOI and peak heat release rate compared to the neat iPP. The thermal decomposition behaviour of neat iPP and its nanocomposites is shown in Fig. 7. The onset degradation temperature of the nanocomposites were higher than the degradation temperature of the neat iPP. The nanocomposites showed higher thermal stability compared to neat iPP. This could be the result of an interaction between organic iPP and organic–inorganic pSS-g-nanosilica. From the TG curve, it can be seen that the weight loss of nanocomposites decreased with an increase in the weight% of pSS-g-nanosilica. The differential thermal analysis (DTA) curve shows that weight loss occurred in one stage, from 403 to 478, 406 to 487 and 412 to 502 °C for the neat iPP, iPP/1% pSS-g-nanosilica and iPP/2% pSS-g-nanosilica, respectively. Rong et al. also observed that the addition of polystyrene-grafted nanosilica to an iPP matrix increased the thermal stability and increased the char residue of the neat PP.19 The thermal degradation of iPP showed only one peak of backbone decomposition at 456 °C, which increased to 466 and 479 °C for 1 and 3 weight% of pSS-g-nanosilica, respectively. This indicates that pSS-g-nanosilica slowed down the thermal decomposition of PP through the destruction of free-radicals by the sulphur species present in the modified nanosilica, which was also found by other researchers.24,25 It can also be seen that the fraction of char residue increased with an increase of pSS-g-nanosilica loading in the iPP nanocomposites. This means that the thermal stability of the nanocomposites was better than that shown by the neat iPP during the high temperature degradation period. The most likely explanation is that the higher decomposition temperature of the pSS-g-nanosilica provides the formation of char during combustion, as it is evident from the TGA; the char residue increased with an increase in the weight% of the modified nanosilica.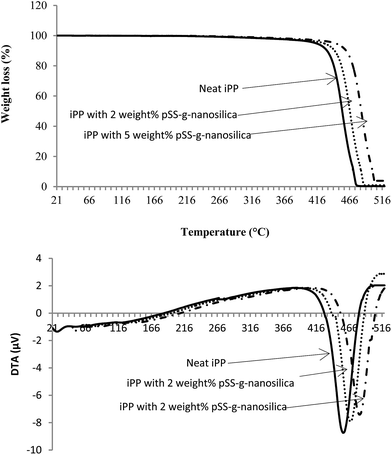 | ||
| Fig. 7 TG (top) and DTA (bottom) curves of neat iPP and its nanocomposites with 2 and 5 weight% pSS-g-nanosilica-filled iPP nanocomposites at a heating rate of 10 °C min−1. | ||
The integral procedure decomposition temperature (IPDT) proposed by Doyle involves the volatile parts of the polymeric materials, which is used to estimate the inherent thermal stability of a polymer.34 It can be seen that the IPDT of the neat iPP was 456 °C, which considerably increased with an increase in the weight% of pSS-g-nanosilica, indicating the increase in thermal stability with increase in nanosilica loading (Table 1). Fu and Qutubuddin also found that the addition of modified nanoclay to polystyrene increased its thermal stability.18
LOI values are used to determine the fire retardancy of a material. The minimum percentage of oxygen in a nitrogen/oxygen mixture to sustain a candle-like burning of a material is taken as the LOI. We used LOI test to evaluate the fire retarding performance of neat iPP and its nanocomposites with 1, 2, 3, 4, and 5 weight% of pSS-g-nanosilica. Fig. 8(a) shows the effect of increase in weight% of pSS-g-nanosilica in the iPP nanocomposites. Neat iPP is a flammable polymer with a low LOI value of 18. However, by the addition of pSS-g-nanosilica to iPP matrix, the LOI value of iPP was increased. The LOI value was directly related to the pSS-g-nanosilica content in the iPP nanocomposites, which was increased with an increase in the nanosilica content. Table 1 shows that the highest LOI (22.0) was achieved for the 5 weight% of the pSS-g-nanosilica in the iPP composites. The increase in LOI greatly increased when the modified nanosilica content was increased up to 2%, after which only marginal improvement in the LOI value was achieved.
 | ||
| Fig. 8 Effect of increase in weight% of pSS-grafted nanosilica on the LOI (top) and heat release rate (bottom) of neat iPP and iPP nanocomposites. | ||
The combustion properties of the iPP/pSS-g-nanosilica composites were characterised by means of a cone calorimeter. Heat release rate (HRR) curves for neat iPP and iPP with 2 and 5 weight% of pSS-g-nanosilica at 35 kW m−2 heat flux are shown in Fig. 8(b). The peak heat flux for the neat iPP was 1384 kW m−2, which was reduced to 989.7 and 565.6 kW m−2 for the 2 and 3 weight% loading of pSS-g-nanosilica, respectively (Table 1). The highest reduction in HRR was 59.13%, which was achieved for the nanocomposite containing 5% pSS-g-nanosilica. The results obtained indicate that the addition of pSS-g-nanosilica can decrease the HRR of the iPP matrix.
4. Discussion
In this work, we demonstrated that the addition of pSS-g-nanosilica to PP improved the fire retardancy of PP, as assessed by thermogravimetric analysis, LOI measurement and cone calorimetry. Silicone compounds have been investigated for improving the fire retardancy of polymers. The flame retardancy arises partly from the property that silicone compounds have in ‘diluting’ the more combustible organic components and partly from the intumescent property as the siliceous residues can form a barrier of char, which is a poor heat conductor, and thus heat transfer is retarded.35 Despite their relatively low combustibility, the use of silicone compounds as reactive fire retardants in fire retarding polymers has not been well investigated. Polyhedral oligomeric silsesquioxanes (POSS) have been explored as both additives and reactive components in thermoplastics and thermosets.36,37 POSS was found effective as a fire retardant at considerably low loadings. Modified and unmodified nanoclay (e.g. montmorillonite, which is a phyllosilicate group of minerals) as well as unmodified fumed silica have been investigated to improve the thermal stability and fire retarding properties of various thermoplastic polymers.38–40Zhu et al. found that the addition of nanoclay to polystyrene reduced its PHRR from 28% to 58% depending upon the amount of nanoclay present in the nanocomposites.38 Kashiwagi et al. examined the mechanism of flame retardancy of a nanoclay-filled PA-6 nanocomposites.41 They observed that a thin layer was formed on the top of the samples during a cone calorimeter experiment. The char layer was tough and it grew throughout the combustion, yielding a rigid multicellular char-brick. The TEM analysis of the combustion char layer revealed that the char layer was a multi-layered silicate structure. In this work, we found that by applying considerably lower loadings of pSS-g-nanosilica as compared to the loading of nanoclay used by Zhu et al.,38 a similar level of flame retardancy can be achieved. The intumescent property of the nanosilica did not contribute alone in the high fire retardancy exhibited by the pp/pSS-g-nanosilica composites. The high reduction in HRR achieved for the pSS-g-nanosilica/PP nanocomposites indicates that the grafted pSS also took part in the reduction of HRR along with the intumescent property of the silica nanoparticles. Possibly, sulphur dioxide gas was produced during combustion of the pSS-g-nanosilica, which diluted the flame. Moreover, the produced sulphur species destroyed hydroxyl free-radicals formed during combustion, as found by several researchers.24,25 As a result fire retardancy considerably improved after grafting pSS onto nanosilica.
5. Conclusions
The surface of silica nanoparticles was modified by grafting pSS onto it. The surface modified silica nanoparticles showed an excellent dispersion in the iPP matrix during melt compounding. DSC data revealed that the addition of 2 weight% silica to iPP showed the maximum effect, and increasing nanosilica loading beyond that level showed less benefit than the benefits achieved at 2 weight%. The tensile strength and elongation data also showed that the 2 weight% PSS-g-nanosilica addition showed the highest positive effect, which is consistent with the DSC data. Due to the heterogeneous nucleation by pSS, the pSS-grafted-nanosilica enhances the dispersion of the nanoparticles through enhanced interfacial adhesion, which leads to increase in the tensile strength and elongation capabilities. Cone calorimetry, LOI and TGA data confirm that the addition of pSS-g-nanosilica considerably improved the fire retarding properties of iPP as the LOI of the composites increased and the PHRR was also reduced.Acknowledgements
The authors wish to acknowledge help received from Professor Hideyuki Tagaya of the Department of Chemical Engineering for thermogravimetric analysis.References
- X. L. Xie, R. K. Y. Li, Q. X. Liu and Y. W. Mai, Structure–property relationship of in situ PMMA modified nanosized antimony trioxide filled poly(vinyl chloride) nanocomposites, Polymer, 2004, 45, 2793 CrossRef CAS PubMed.
- M. Bailly and M. Kontopoulou, Preparation and characterisation of thermoplastic olefin/nanosilica composites using a silane-grafted polypropylene, Polymer, 2009, 50, 2472 CrossRef CAS PubMed.
- L. F. Cai, Z. Y. Lin and H. Qian, Dispersion of nanosilica in monomer casting nylon6 and its effect on the structure and properties of composites, eXPRESS Polym. Lett., 2010, 4, 397 CrossRef CAS.
- J.-M. Raquez, Y. Habibi, M. Murariu and P. Dubois, Polylactide (PLA)-based nanocomposites, Prog. Polym. Sci., 2013, 38, 1504 CrossRef CAS PubMed.
- M. Pannirselvam, A. Genoves, M. C. Jollands, S. N. Bhattacharya and R. A. Shanks, Oxygen barrier property of polypropylene-polyether treated clay nanocomposites, eXPRESS Polym. Lett., 2008, 2, 429 CrossRef CAS.
- E. Barna, B. Bommer, J. Kürsteiner, A. Vital, O. V. Trzebiatowski, W. Koch, B. Schmid and T. Graule, Innovative scratch proof nanocomposites for clear coating, Composites, Part A, 2005, 36, 473 CrossRef PubMed.
- G. R. Sharma, C. Lind and M. R. Coleman, Preparation and properties of polyimide nanocomposites with negative thermal expansion nanoparticle filler, Mater. Chem. Phys., 2012, 137, 448 CrossRef CAS PubMed.
- F. Huang, Y. Liu, X. Zhang, G. Wei, J. Gao, Z. Song, M. Zhang and J. Qiao, Effect of elastomeric nanoparticles on toughness and heat resistance of epoxy resins, Macromol. Rapid Commun., 2002, 23, 786 CrossRef CAS.
- J. Wang, Y. Chen and Q. Jin, Preparation and characteristics of a novel silicone rubber nanocomposite based on organophilic montmorillonite, High Perform. Polym., 2006, 18, 325 CrossRef CAS PubMed.
- L. Wu, D. Cao, Y. Huang and B. Li, Poly(L-lactic acid)/SiO2 nanocomposites via in situ melt polycondensation of L-lactic acid in the presence of acidic silica sol: Preparation and characterisation, Polymer, 2008, 49, 742 CrossRef CAS PubMed.
- T. G. Gopakumar, J. A. Lee, M. Kontopoulou and J. S. Parent, Influence of clay exfoliation on the physical properties of montmorillonite/polyethylene composites, Polymer, 2002, 43, 5483 CrossRef CAS.
- S. Lu, H. Huang and K. Wu, Silicate/poly(dimethylsiloxane) nanocomposite pervaporation membrane for acetic acid/water separation, J. Mater. Res., 2001, 16, 3053 CrossRef CAS.
- S. Wang, L. Chen and Y. Tong, Structure–property relationship in chitosan-based biopolymer/montmorillonite nanocomposites, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 686 CrossRef CAS.
- S. Yuen, C. M. Ma, C. Chiang, C. Teng and Y. Yu, Poly(vinyltrimethoxysilane) modified MWCNT/polyimide nanocomposites – preparation, morphological, mechanical and electrical properties, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 803 CrossRef CAS.
- C. L. Wu, M. Q. Zhang, M. Z. Rong and K. Friedrich, Tensile performance improvement of low nanoparticles filled-polypropylene composites, Compos. Sci. Technol., 2002, 62, 1327 CrossRef CAS.
- E. Tang, H. Liu, L. Sun, E. Zheng and G. Cheng, Fabrication of zinc oxide/poly(styrene) grafted nanocomposite latex and its dispersion, Eur. Polym. J., 2007, 43, 4210 CrossRef CAS PubMed.
- H. Zhao and R. K. Y. Li, A study on the photo-degradation of zinc oxide (ZnO) filled polypropylene nanocomposites, Polymer, 2006, 47, 3207 CrossRef CAS PubMed.
- X. Fu and S. Qutubuddin, Polymer–clay nanocomposites: exfoliation of organophilic montmorillonite nanolayers in polystyrene, Polymer, 2001, 42, 807 CrossRef CAS.
- M. Z. Rong, M. Q. Zhang, Y. X. Zheng, H. M. Zeng, R. Walter and K. Friedrich, Structure–property relationships of irradiation grafted nano-inorganic particle filled polypropylene composites, Polymer, 2001, 42, 167 CrossRef CAS.
- J. W. Kim, L. U. Kim and C. K. Kim, Size control of silica nanoparticles and their surface treatment for fabrication of dental nanocomposites, Biomacromolecules, 2007, 8, 215 CrossRef CAS PubMed.
- Y. Shin, D. Lee, K. Lee, K. H. Ahn and B. Kim, Surface properties of silica nanoparticles modified with polymers for polymer nanocomposite applications, J. Ind. Eng. Chem., 2008, 14, 515 CrossRef CAS PubMed.
- L. F. Cai, Y. L. Mai, M. Z. Rong, W. H. Ruan and M. Q. Zhang, Interfacial effects in nano-silica/polypropylene composites fabricated by in situ chemical blowing, eXPRESS Polym. Lett., 2007, 1, 2 CrossRef CAS.
- G. Laufer, C. Kirkland, A. B. Morgan and J. C. Grunlan, Exceptionally flame retardant sulphur-based multilayer nanocoating for polyurethane prepared from aqueous polyelectrolyte solutions, ACS Macro Lett., 2013, 2, 361 CrossRef CAS.
- M. W. Beach, N. G. Rondah, R. D. Froese, B. B. Gerhart, J. G. Green, B. G. Stobby, A. G. Shmakov, V. M. Shvartsberg and O. P. Korobeinchev, Studies of degradation enhancement of polystyrene by flame retardant additives, Polym. Degrad. Stab., 2008, 93, 1664 CrossRef CAS PubMed.
- M. R. Zachariah and O. I. Smith, Experimental and numerical studies of sulphur chemistry in H2/O2/SO2 flames, Combust. Flame, 1987, 69, 125 CrossRef CAS.
- N. D. Singho and M. R. Johan, Complex impedance spectroscopy of silica nanoparticles via sol–gel method, Int. J. Electrochem. Sci., 2012, 7, 5604 CAS.
- C. O. Metin, L. W. Lake, C. R. Miranda and Q. P. Nguyen, Stability of silica nanoparticle dispersions, J. Nanopart. Res., 2011, 13, 839 CrossRef CAS.
- H. Ramadan, A. Ghanem and H. El-Rassy, Mercury removal from aqueous solutions using silica, polyacrylamide and hybrid silica–polyacrylamide aerogels, Chem. Eng. J., 2010, 159, 107 CrossRef CAS PubMed.
- K. Nilles and P. Theato, Polymerisation of an activated ester monomer based on 4-vinylsulphonic acid and its polymer analogous reaction, Polym. Chem., 2011, 2, 376 RSC.
- S. B. Brijmohan, S. Swier, R. A. Weiss and M. T. Shaw, Synthesis and characterisation of cross-linked sulphonated polystyrene nanoparticles, Ind. Eng. Chem. Res., 2005, 44, 8039 CrossRef CAS.
- A. Cai, S. Zhang, A. Zhu and S. Dai, Synthesis and properties of polystyrene-g-mSiO2 filled polypropylene nanocomposites, Polym. Compos., 2010, 31, 807 CAS.
- C. Chen, R. S. Justice and W. S. Schaefer, Highly dispersed nanosilica–epoxy resins with enhanced mechanical properties, Polymer, 2008, 49, 3805 CrossRef CAS PubMed.
- S. Ahmed and F. R. Jones, A review of particulate reinforcement theories for polymer composites, J. Mater. Sci., 1990, 25, 4933 CrossRef CAS.
- C. D. Doyle, Estimating thermal stability of experimental polymers by empirical thermogravimetric analysis, Anal. Chem., 1961, 33, 77 CrossRef.
- J. R. Ebdon, B. J. Hunt, M. S. Jones and F. G. Thorpe, Chemical modification of polymers to improve flame retardance-I. The influence of silicone containing groups, Polym. Degrad. Stab., 1996, 54, 395 CrossRef CAS.
- J. D. Lichtenhan, Y. A. Otonari and M. J. Carr, Linear hybrid polymer building blocks: Methacrylate functionalised polyhedral oligomeric silsesquioxane monomers and polymers, Macromolecules, 1995, 28, 8435 CrossRef CAS.
- J. Choi, A. F. Yee and R. M. Laine, Organic/inorganic hybrid composites from cubic silsesquioxanes of octa(dimethylsiloxyethyl-cyclohexylepoxide)silsesquioxane, Macromolecules, 2003, 36, 5666 CrossRef CAS.
- J. Zhu, A. B. Morgan, F. J. Lamelas and C. A. Wilkie, Fire Properties of polystyrene–clay nanocomposites, Chem. Mater., 2001, 13, 3774 CrossRef CAS.
- H. Qin, Q. Su, S. Zhang, B. Zhao and M. Yan, Thermal stability and flammability of polyamide 66/montmorillonite nanocomposites, Polymer, 2003, 44, 7533 CrossRef CAS PubMed.
- A. Dorigato, A. Pegoretti and A. Frache, Thermal stability of high density polyethylene-fumed silica nanocomposites, J. Therm. Anal. Calorim., 2012, 109, 863 CrossRef CAS PubMed.
- T. Kashiwagi, J. W. Gilman, M. R. Nyden and S. M. Lomakin, Polymer combustion and new flame retardants, in Fire Retardancy of Polymers: The Use of Intumescence, ed. M. Le Bras, G. Camino, S. Bourbigot and R. Delobel, The Royal Society of Chemistry, Cambridge, UK, 1998, pp. 175–202 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |



